Synthesis and Characterization of Poly(3-hexylthiophene)-b-Polystyrene for Photovoltaic Application
Abstract
: Poly(3-hexylthiophene)-block-polystyrene (P3HT-b-PS) was synthesized by Suzuki coupling reaction between P3HT and PS, prepared by Grignard metathesis polymerization and atom transfer radical polymerization (ATRP), respectively. The formation of block copolymer was confirmed by gel permeation chromatography (GPC) and NMR. Differential scanning calorimetry (DSC) thermogram of block copolymers showed glass transition of PS block and melting/crystallization of P3HT block, suggesting a microphase separated structure, which was also confirmed by atomic force microscopy (AFM) images and UV-vis absorption spectra. The annealing effect on the morphology of the composite films consisting of P3HT-b-PS and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) was investigated. Photovoltaic cells fabricated using P3HT-b-PS and PCBM were evaluated.1. Introduction
Organic solar cells, based on poly(3-hexylthiophene) (P3HT) in combination with a fullerene derivative, [6,6]-phenyl-C61-butyric acid methyl ester (PCBM), have been intensively investigated to show high conversion efficiency of around 5% [1-5]. High device performance in such a blend system is considered to be achieved by suitable phase-separated morphology, known as bulk heterojunction. The scale of these structures lies in the order of tens of nanometer, which allows photo-generated excitons to reach the interface between p- and n-type semiconductors effectively [6]. Therefore precise control in morphology of the active layer is a key to improve the conversion efficiency of polymer solar cells, and it is reported that thermal [7-10] and/or solvent vapor [11] annealing treatments afford the phase-separated morphology resulting in significant improvement of device performance. However the phase-separated structure observed in the composite films is assumed to be thermodynamically unstable due to the nature of polymer/small molecule blend, and it is difficult to control the size and orientation of each domain.
Utilization of block copolymers where one block contains p-type electron donor and the other block contains n-type electron acceptor as active materials, seems promising since such block copolymers afforded thermodynamically stable microphase-separated structure, which is regularly sized and oriented. Several attempts have been carried out in order to obtain ideal morphology and high device performance. For example, poly(3-alkylthiophene) based block copolymers containing a polyacrylate block with perylene bisimide pendant groups were investigated [12,13]. However conversion efficiencies were still lower than that of the conventional P3HT/PCBM composite indicating that further investigations, including molecular design and process for film fabrication, are required for improving performance. Synthesis of P3HT based block copolymers with fullerene containing blocks and morphological studies were also reported [14-16].
An alternative approach to control the phase-separated morphology in the active layer is to use block copolymers consisting of p-type block (e.g., P3HT) and electrically inert block. We anticipate that it is possible to control the domain size in the composite film consisting of block copolymer and n-type small molecule (PCMB), via self-assembly of the block copolymer, and it also assists the regular alignment of each domain. Previously we reported that the p-type diblock copolymer, Poly(3-hexylthiophene)-b-poly(ethylene oxide) (P3HT-b-PEO) was synthesized by coupling between regioregular P3HT and PEO homopolymers, and that the regular morphology with small size can be obtained in the thin films of P3HT-b-PEO blended with PCBM [17]. A similar strategy using P3HT-b-poly(4-vinylpyridine) was reported independently [18].
In this paper, we synthesized a block copolymer, poly(3-hexylthiophene)-block-polystyrene (P3HT-b-PS). Since PS is hydrophobic and shows high miscibility to PCBM compared with PEO, different morphologies are expected. Several studies have been carried out for the preparation of similar block copolymers. The synthetic routes are mainly categorized into two types. One is a macroinitiator method where one or both terminal(s) of P3HT were modified to initiating end groups followed by living polymerizations such as atom transfer radical polymerization (ATRP) [19], nitroxide-mediated polymerization (NMP) [20,21], and reversible addition fragmentation chain transfer polymerization (RAFT) [21]. The other is a coupling method which is based on the coupling reaction between living polystyryl anion and end-functionalized P3HT [22,23]. Herein we present the new synthetic route based on Suzuki coupling reaction between bromo-terminated P3HT and PS with boronic acid ester moiety as an end group. Both homopolymers, P3HT and PS were separately prepared by Grignard metathesis and atom transfer radical polymerizations, respectively. In this method, each homopolymer can be well characterized before coupling reaction making a thorough molecular characterization of resulting block copolymer. Since the coupling reactions do not require severe conditions, it is possible to introduce a variety of non-P3HT building blocks [23]. Since Grignard metathesis polymerization of 2-bromo-3-hexylthiophene exclusively affords polymer chains terminated with a proton at one end and a bromine at the other end [24], no further chemical modification is necessary for P3HT to obtain diblock copolymers.
The microphase-separated structure in these thin films was investigated by differential scanning calorimetry (DSC), UV, and atomic force microscopy (AFM) analyses. Annealing by heating or immersing in solvent vapor atmosphere was carried out on the active layer of photovoltaic devices fabricated with P3HT or P3HT-b-PS blended with PCBM. The I-V characteristics were evaluated for the devices, and the correlation between the morphology and the device performance was discussed.
2. Results and Discussion
2.1. Synthesis of Diblock Copolymer P3HT-b-PS
Block copolymer consisting of P3HT and PS segments were prepared by coupling between each homopolymer as shown in Scheme 1.
Polystyrene with tert-butyl ester as an end functional group (1) was synthesized by ATRP using CuBr/CuBr2/PMDETA system as reported elsewhere [25]. 1H NMR signals for the initiating end group (CH3CH–, and –C(CH3)3) for PS resonated at 0.9 and 1.35 ppm in CDCl3, respectively, and the degree of polymerization (DP) was determined to be 25 based on the relative intensity of the former signal to aromatic protons. The number average molecular weight (Mn) and the polydispersity (PDI) were determined as 2,300 and 1.23 by gel permeation chromatography (GPC), respectively. The glass transition temperature (Tg) determined from a differential scanning calorimetry (DSC) thermogram was approximately 65 °C.
Elimination of tert-butyl group was confirmed by the disappearance of tert-butyl resonance at 1.35 ppm in 1H NMR spectrum, and by the decrease of the doublet absorption observed in 1,350–1,400 cm−1 (CH3 deformation) in IR spectrum for 2. After the esterification reaction with phenol containing boronic ester group, the absorption at 1,350–1,400 cm−1 for branched alkyl groups increased again.
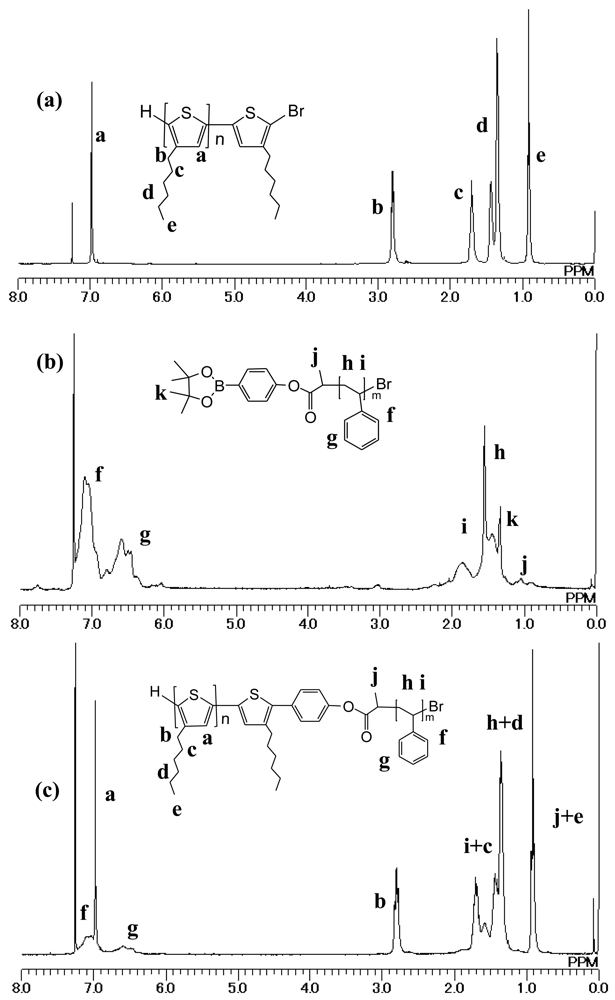
2-Bromo-3-hexylthiophene (4) was synthesized by the bromination of 3-hexylthiophene and the product was consistently a mixture of raw 3-hexylthiophene, 2-bromo-3-hexylthiophene, and 2,5-dibromo-3-hexylthiophene (purity: 89.8% checked by HPLC). In order to obtain P3HT with a well defined structure including both end groups, it is necessary to use highly pure monomer. After the column chromatography, purity of monobromo thiophene increased to 99.3%. P3HT with a bromo terminal (P3HT-Br; 5) was synthesized by Grignard metathesis polymerization according to reference [24]. The Mn and PDI were determined by GPC as 8,100 and 1.49, respectively. End group analysis by 1H NMR as reported by Iovu et al. [26] revealed that the degree of polymerization was circa. 50. Melting and crystallization were observed at 220 and 204 ° C in heating and cooling scan, respectively.
Finally, 5 was coupled with 3 via Suzuki coupling reaction to obtain diblock copolymer (6). Figure 1 shows GPC profile for 6 accompanied with those of homopolymer 3 and 5. The increase in Mn of 6 with a unimodal profile in the GPC chromatogram (Mn: 10,000, PDI: 1.99) also indicated the formation of block copolymer without any trace of polystyrene homopolymer.
In 1H NMR spectrum of 6, the signals in aromatic region assignable to the protons on the phenyl ring of PS can be observed at 7.0 and 6.7 ppm as shown in Figure 2. In addition, the disappearance of the small triplet signal at 2.56 ppm was observed as shown in Figure 3, which is assigned as the signal of methylene proton on the first carbon of hexyl group substituted on the bromo-terminal unit [26]. These results indicate that PS block was successfully introduced at the end of 5. By comparing the integrations for alkyl protons of P3HT and aromatic protons of PS, the molar ratio of PS to P3HT segments was found to be 25:50, in almost the same as the theoretical chemical composition.
2.2. Characterization of P3HT-b-PS in Solid State
Figure 4 shows DSC thermogram for 6. The glass transition of PS block and melting of P3HT block were observed, and those transitions occurred at almost the same temperatures as those of both homopolymers, indicating the microphase separation.
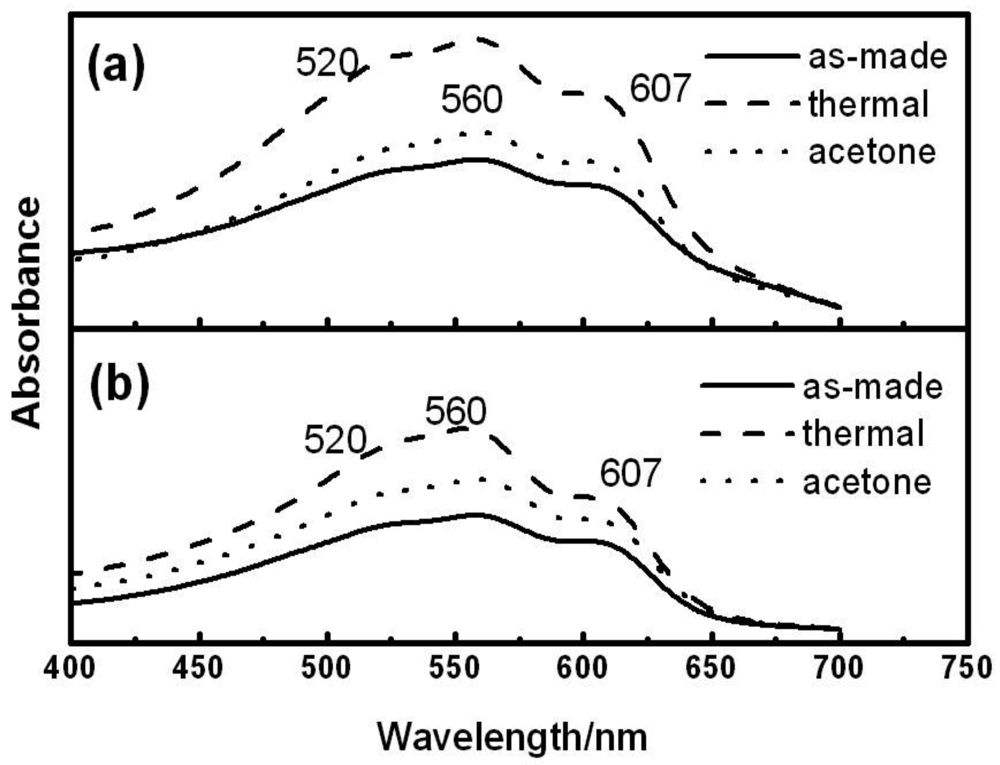
Aggregation state of P3HT chains can be evaluated by UV-vis spectroscopy (Figure 5). Thin films of 5 and 6 were prepared by spin coating from chloroform solution on glass substrates at room temperature. One set of films were thermally annealed at 150 °C for 20 min (thermal annealing), and the others were transferred into a jar filled with acetone vapor, exposed for 5 h (solvent annealing). All the spectra exhibit similar profile with maximum absorption wavelength at 560 nm. Both thermal and solvent annealing treatments provide a slight increase in absorbance at 607 nm, derived from the strong intermolecular interaction indicating the high crystallizability of the P3HT chains [27]. Therefore, the author can conclude that the the presence of PS block does not interfere with the stacking of the P3HT block . Furthermore, it is considered that microphase separation makes the P3HT block form a more densely stacked structure.
Morphology of film surface was observed by AFM. The phase images of the thin films of 6 were shown in Figure 6. The thin films were also subjected to thermal annealing at 150 °C for 20 min or solvent annealing by acetone for 5 h. Before annealing, a flat surface without any distinctive structure was observed in the AFM image (Figure 5(a)). After thermal annealing, a phase-separated structure appeared with a domain size of about 150 nm in 6. Fur thermore, the block polymer showed a clearer phase separated structure after solvent annealing. Since acetone is partially a good solvent for PS, the PS chains can change the conformation by swelling with acetone. Therefore, the PS segment reconstructed a thermodynamically stable structure in the thin film under acetone vapor, which induced a more densely stacked structure of P3HT. Other common solvents such as chloroform and toluene were also used for solvent annealing. However, no clear phase-separated morphology was observed for these thin films because the dewetting of the films probably occurred by use of the better solvents for both P3HT and PS segments.
2.3. Characterization of Composite Consisting of P3HT-b-PS and PCBM
Morphology of film surface was observed by atomic force microscopy (AFM). The phase images of the thin films of 5/PCBM and 6/PCBM (1:1 weight ratio) are shown in Figure 7. The composite films were subjected to thermal annealing at 150 °C for 20 min or solvent annealing by acetone for 5 h.
Before annealing, a flat surface without any distinct structure was observed in the AFM images for 6/PCBM (Figure 7(d)). Phase-separated structure appeared with domain size of about 30–40 nm in film of 6/PCBM after thermal annealing (Figure 7(e)), whereas the domain size observed in the film of 5/PCBM was less than 10 nm (Figure 7(b)). It is considered that the self-assembly of 6 promoted the phase separation and aggregation of PCBM. The phase separation in the film of 6/PCBM after the solvent annealing became more distinct compared with the thermal annealing (Figure 7(f)). Since acetone is a good solvent for PCBM, it accelerated the aggregation of PCBM. From UV-vis spectrum, both annealing processes induced a more densely stacked structure of P3HT chain.
Performance of photovoltaic cells based on 5 or 6/PCBM in 1:1 weight ratio was evaluated. The device structure is described as Al/LiF/composite/PEDOT: PSS/ITO. The I-V curves before and after annealing are shown in Figure 8. Table 1 lists the device performance. For the as-made film, the conversion efficiency of devices using 6 was 0.42%. After thermal annealing process, the efficiency increased up to 1.93%, which is slightly higher than that observed in homopolymer-based device. This is probably due to the highly segregated structure of 6/PCBM as shown in Figure 7. High open-circuit voltage and fill factor were also observed in this device. The solvent annealing with acetone provided an efficiency of 0.84% for 6/PCBM device. It is considered that the domain size is too large for photo-generated excitons to reach the interface between p- and n-type phases in the solvent-annealed device.
3. Experimental Section
3.1. Materials
Tetrahydrofunan (THF) was dried by distilling over calcium hydride, and stored under nitrogen atmosphere. Styrene was distilled under vacuum. The other reagents and solvents were obtained commercially and were used as received.
3.2. Preparation of Homopolymer of PS (1) with End Group of t-Bu Ester
2-Bromopropionic acid tert-butyl ester (0.146 mL, 0.875 mmol), styrene (20 mL, 0.175 mmol), anisole (4.7 mL, 0.0438 mol), CuBr (0.125 g, 0.875 mmol), CuBr2 (0.01 g, 0.0438 mmol), and N,N′,N′,N″,N″-pentamethyldiethylenetriamine (0.185 mL, 0.875 mmol) were added into a flask equipped with a stopcock. The mixture was subjected to freeze-and-thaw cycles to eliminate air, then heated for 24 h at 90 °C under nitrogen. The reaction mixture was poured into methanol to precipitate the product. Mn: 2,300 (GPC); Yield: 4.06 g (20%).
3.3. Preparation of PS with End Group of Carboxylic Acid (2)
Polystyrene 1 (3.6 g, 2.0 mmol), p-toluenesulfonic acid (3.44 g, 20 mmol), dioxane (anhydrous, 9 mL, 10 mmol) were placed into a flask equipped with a condenser, and the mixture was stirred for 24 h at 95 °C under nitrogen. The product was precipitated in methanol. Yield: 2.1 g (61.2%). Mn: 2,300 (GPC), PDI: 1.23.
3.4. Preparation of PS with End Group of Boronic Acid Ester (3)
Homopolymer 2 (0.36 g, 0.2 mmol), CH2Cl2 (5 mL), 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenol (0.155 g, 0.7 mmol), dicyclohexylcarbodiimide (0.076 g, 0.6 mmol), N,N-dimethylaminopyridine (0.123 g, 0.6 mmol) were placed into a flask, then the mixture was stirred for 24 h at room temperature under nitrogen. After reaction a few drops of water was added. Precipitate was removed by filtration and the product was precipitated in methanol. Yield: 0.287 g (71.8%).
3.5. Preparation of 2-Bromo-3-Hexylthiophene (4)
3-Hexylthiophene (5.00 g, 29.7 mmol) and THF (50 mL) were placed to a flask under nitrogen. N-bromosuccinimide (5.43 g, 30.5 mmol) was separated into 5 parts, and each part was added every 3 min at 0 °C. After two hours reaction at room temperature, the product was extracted with chloroform, purified into 99% by the column chromatography (silica/hexane). Yield: 6.76g (92%).
3.6. Preparation of Poly(3-hexylthiophene) (5)
Regioregular poly(3-hexylthiophene) with bromo group at a terminal (P3HT-Br, 5) were prepared by the modified method previously reported [24]. A flask equipped with a stopcock was charged with 130 mL of distilled THF and cooled down to −78 °C. Diisopropylamine (3.72 mL, 26.3 mmol), n-butyllithium (2.6 mol/L solution, 9.61 mL, 25.0 mmol) were successively added, and then the temperature was increased up to room temperature. After stirring for 5 min. the temperature was returned to −78 °C, and then 2-bromo-3-hexylthiophene (4) (6.50 g, 26.3 mmol) was added. After stirring for 5 min, anhydrous ZnCl2 (3.76 g, 27.6 mmol) was added. The reaction mixture was stirred for 2 h, and then was warmed up to 0 °C . After adding 91 mg (0.169 mmol) of bis(diphenylphosphino) propanedichloronickel(II) (Ni(dppp)Cl2) and warming to room temperature, the stirring was continued for 30 min. The mixture was poured into methanol to precipitate the product which was extracted by Soxhlet with hexane, dichloromethane, and THF in turn. THF fraction was characterized and used in the remaining experiments. Yield: 0.734 g (17%), Mn (GPC): 8,100, PDI: 1.49.
3.7. Preparation of Block Copolymer P3HT-b-PS (6)
Pd(PPh3)4 (0.023 g, 0.02 mmol), 3 M K2CO3 (aqueous solution, 0.35 ml), PS 3 (0.108 g, 0.0415 mmol) and P3HT-Br 5 (0.104 g, 0.0125 mmol), toluene (6.7 mL) were placed into a flask equipped with a condenser, followed by freeze-and-thaw cycles to eliminate air in the mixture. Then the mixture was stirred for 24 h at 100 °C. The product was reprecipitated in methanol twice and in acetone twice. Yield: 0.084 g (61.5%).
3.7. Measurements
1H and 13C NMR spectra were obtained on a JEOL AL300 instrument at 300 and 75 MHz, respectively. Deutrated chloroform was used as a solvent with tetramethylsilane as an internal standard. Number- and weight-average molecular weights (Mn and Mw) were determined by gel permeation chromatography (GPC) analysis with a JASCO RI-2031 detector eluted with chloroform at a flow rate of 1.0 mL min−1 and calibrated by standard polystyrene samples. Differential scanning calorimetry (DSC) analyses were performed on a Rigaku DSC-8230 under a nitrogen atmosphere at heating and cooling rates of 10 °C/min. Atomic force microscopy (AFM) measurements were performed on a JEOL JSPM-4200 system in tapping mode (phase and topographic modes) with an MPP-11100-10 silicon probe (resonant frequency: 300 kHz, force constant: 40 N/m). All thin films of polymers were spin-cast onto glass slide by a MIKASA 1H-D7 spin coater from chlorobenzene solutions at 1,500 rpm for 30 s.
3.6. Photovoltaic Device Evaluation
All the devices were manufactured with a structure of ITO/PEDOT: PSS(30 nm)/active layer/LiF(0.5 nm)/Al(100 nm). Prior to preparation of devices, a glass slide with indium tin oxide (ITO) patterns was washed by an alkaline cleaner under sonication and rinsed with deionized water. The substrate was subsequently washed by 2-propanol under sonication, rinsed with clean 2-propanol, and dried with nitrogen. PEDOT: PSS with 30 nm of thickness was spin-coated on the substrate at 2,500 rpm for 30 sec from the dispersion in water filtered by 0.2 μm of membrane filter, followed by annealing at 200 °C for 1 h. Polymer blend layer was laminated on PEDOT:PSS by spin-coating at 1,000 rpm for 30 sec from chlorobenzene solution (10 mg/mL) filtered by 0.2 μm of membrane filter. After annealing process, lithium fluoride with 0.5 nm of thickness followed by aluminum with 100 nm of thickness was vacuum-deposited on the polymer layer at a rate of 0.1 Å/sec and a rate of 4.5 Å/sec using tantalum and tungsten boats, respectively. A typical size of the photo-active area was 4 mm2. The photocurrent-voltage characteristic was measured upon the exposure of the light by a xenon lump with 100 mW/cm2. Annealing was carried out as follows. The substrates were transferred inside a nitrogen-filled glove box under atmospheric pressure, annealed at 150 °C for 20 min, or under solvent vapor (acetone) at room temperature for 5 h.
4. Conclusions
In conclusion, the p-type conducting block copolymer poly(3-hexylthiophene)-block-polystyrene (P3HT-b-PS) was synthesized by Suzuki coupling between P3HT and PS homopolymers. During thermal and solvent annealing processes, block copolymer assisted the segregation of PCBM through microphase separation. Photovoltaic cell was fabricated using P3HT-b-PS and PCBM, and the device provided conversion efficiency of 1.93% after thermal annealing and 0.84% after solvent annealing. It is possible to control morphologies in the composite films by the utilization of the block copolymer. Further investigation on the optimization of block copolymer (PS content, molecular weight, and so on) and on the relationship between photovoltaic performance and morphologies of the active layer is ongoing.







| Polymer | Anneal | Voc (V) | Jsc (mA/cm2) | Efficiency (%) | Fill Factor (%) |
|---|---|---|---|---|---|
| 5 | none | 0.40 | 3.00 | 0.38 | 0.31 |
| thermal | 0.60 | 7.04 | 1.38 | 0.33 | |
| solvent | 0.48 | 4.47 | 0.89 | 0.42 | |
| 6 | none | 0.44 | 2.68 | 0.42 | 0.35 |
| thermal | 0.58 | 7.90 | 1.93 | 0.42 | |
| solvent | 0.58 | 3.61 | 0.86 | 0.40 |
Thermal: 150 °C for 20 min; solvent: under acetone vapor at room temperature for 5 h.
References
- Li, G.; Shrotriya, V.; Huang, J.S.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar]
- Ma, W.L.; Yang, C.Y.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar]
- Shrotriya, V.; Yao, Y.; Li, G.; Yang, Y. Effect of self-organization in polymer/fullerene bulk heterojunctions on solar cell performance. Appl. Phys. Lett. 2006, 89, 063505. [Google Scholar]
- Campoy-Quiles, M.; Ferenczi, T.; Agostinelli, T.; Etchegoin, P.G.; Kim, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; Bradley, D.D.C.; Nelson, J. Morphology evolution via self-organization and lateral and vertical diffusion in polymer: fullerene solar cell blends. Nat. Mater. 2008, 7, 158–164. [Google Scholar]
- Huang, W.Y.; Huang, P.T.; Han, Y.K.; Lee, C.C.; Hsieh, T.L.; Chang, M.Y. Aggregation and gelation effects on the performance of poly(3-hexylthiophene)/fullerene solar cells. Macromolecules 2008, 41, 7485–7489. [Google Scholar]
- Li, G.; Shrotriya, V.; Yao, Y.; Huang, J.S.; Yang, Y. Manipulating regioregular poly(3-hexylthiophene): [6,6]-phenyl-C61-butyric acid methyl ester blends—route towards high efficiency polymer solar cells. J. Mater. Chem. 2007, 17, 3126–3140. [Google Scholar]
- Kim, Y.; Choulis, S.A.; Nelson, J.; Bradley, D.D.C.; Cook, S.; Durrant, J.R. Device annealing effect in organic solar cells with blends of regioregular poly(3-hexylthiophene) and soluble fullerene. Appl. Phys. Lett. 2005, 86, 063502. [Google Scholar]
- Li, G.; Shrotriya, V.; Yao, Y.; Yang, Y. Investigation of annealing effects and film thickness dependence of polymer solar cells based on poly(3-hexylthiophene). J. Appl. Phys. 2005, 98, 043704. [Google Scholar]
- Reyes-Reyes, M.; Kim, K.; Carroll, D.L. High-efficiency photovoltaic devices based on annealed poly(3-hexylthiophene) and 1-(3-methoxycarbonyl)-propyl-1-phenyl-(6,6)C61 blends. Appl. Phys. Lett. 2005, 87, 083506. [Google Scholar]
- Ko, C.J.; Lin, Y.K.; Chen, F.C. Microwave annealing of polymer photovoltaic devices. Adv. Mater. 2007, 19, 3520–3523. [Google Scholar]
- Li, G.; Yao, Y.; Yang, H.; Shrotriya, V.; Yang, G.; Yang, Y. “Solvent annealing” effect in polymer solar cells based on poly(3-hexylthiophene) and methanofullerenes. Adv. Funct. Mater. 2007, 17, 1636–1644. [Google Scholar]
- Sommer, M.; Lang, A.S.; Thelakkat, M. Crystalline-crystalline donor-acceptor block copolymers. Angew. Chem. Int. Ed. 2008, 47, 7901–7904. [Google Scholar]
- Zhang, Q.L.; Cirpan, A.; Russell, T.P.; Emrick, T. Donor-acceptor poly(thiophene-block-perylene diimide) copolymers: Synthesis and solar cell fabrication. Macromolecules 2009, 42, 1079–1082. [Google Scholar]
- Sivula, K.; Ball, Z.T.; Watanabe, N.; Fréchet, J.M.J. Amphiphilic diblock copolymer compatibilizers and their effect on the morphology and performance of polythiophene: Fullerene solar cells. Adv. Mater. 2006, 18, 206–210. [Google Scholar]
- Dante, M.; Yang, C.; Walker, B.; Wudl, F.; Nguyen, T.-Q. Self-Assembly and charge-transport properties of a polythiophene-fullerene triblock copolymer. Adv. Mater. 2010, 22, 1835–1839. [Google Scholar]
- Hiorns, R.C.; Cloutet, E.; Ibarboure, E.; Khoukh, A.; Bejbouji, H.; Vignau, L.; Cramail, H. Synthesis of donor-acceptor multiblock copolymers incorporating fullerene backbone repeat units. Macromolecules 2010, 43, 6033–6044. [Google Scholar]
- Gu, Z.; Kanto, T.; Tsuchiya, K.; Ogino, K. Synthesis of poly(3-hexylthiophene)-b-poly(ethylene oxide) for application to photovoltaic device. J. Photopolym. Sci. Technol. 2010, 23, 405–406. [Google Scholar]
- Sary, N.; Richard, F.; Brochon, C.; Leclerc, N.; Lévêque, P.; Audinot, J.-N.; Berson, S.; Heiser, T.; Hadziioannou, G.; Mezzenga, R. A new supramolecular route for using rod-coil block copolymers in photovoltaic applications. Adv. Mater. 2010, 22, 763–768. [Google Scholar]
- Iovu, M.C.; Jeffries-El, M.; Zhang, R.; Kowalewski, T.; McCullough, R.D. Conducting block copolymer nanowires containing regioregular poly(3-hexylthiophene) and polystyrene. J. Macromol. Sci. A Pure Appl. Chem. 2006, 43, 1991–2000. [Google Scholar]
- Kaul, E.; Senkovskyy, V.; Tkachov, R.; Bocharova, V.; Komber, H.; Stamm, M.; Kiriy, A. Synthesis of a bifunctional initiator for controlled Kumada catalyst-transfer polycondensation/nitroxide-mediated polymerization and preparation of poly(3-hexylthiophene)-polystyrene block copolymer therefrom. Macromolecules 2010, 43, 77–81. [Google Scholar]
- Iovu, M.C.; Craley, C.R.; Jeffries-EL, M.; Krankowski, A.B.; Zhang, R.; Kowalewski, T.; McCullough, R.D. Conducting regioregular polythiophene block copolymer nanofibrils synthesized by reversible addition fragmentation chain transfer polymerization (RAFT) and nitroxide mediated polymerization (NMP). Macromolecules 2007, 40, 4733–4735. [Google Scholar]
- Higashihara, T.; Ohshimizu, K.; Hirao, A.; Ueda, M. Facile Synthesis of ABA triblock copolymer containing regioregular poly(3-hexylthiophene) and polystyrene segments via linking reaction of poly(styryl)lithium. Macromolecules 2008, 41, 9505–9507. [Google Scholar]
- Lim, H.; Huang, K.T.; Su, W.F.; Chao, C.Y. Facile syntheses, morphologies, and optical absorptions of P3HT coil-rod-coil triblock copolymers. J. Polym. Sci. A Polym. Chem. 2010, 15, 3311–3322. [Google Scholar]
- Liu, J.; McCullough, R.D. End group modification of regioregular polythiophene through postpolymerization functionalization. Macromolecules 2002, 35, 9882–9889. [Google Scholar]
- Maeda, Y.; Shimoi, Y.; Ogino, K. Fabrication of microporous films utilizing amphiphilic block copolymers and their use as templates in poly(aniline) preparation. Polym. Bull. 2005, 53, 315–321. [Google Scholar]
- Iovu, M.C.; Sheina, E.E.; Gil, R.R.; McCullough, R.D. Experimental evidence for the quasi-“living” nature of the Grignard metathesis method for the synthesis of regioregular poly(3-alkylthiophenes). Macromolecule 2005, 38, 8649–8656. [Google Scholar]
- Yamamoto, T.; Komarudin, D.; Arai, M.; Lee, B.L.; Suganuma, H.; Asakawa, N.; Inoue, Y.; Kubota, K.; Sasaki, S.; Fukuda, T.; Matsuda, H. Extensive studies on π-stacking of poly(3-alkylthiophene-2,5-diyl)s and poly(4-alkylthiophene-2,5-diyl)s by optical spectroscopy, NMR analysis, light scattering analysis, and X-ray crystallography. J. Am. Chem. Soc. 1998, 120, 2047–2058. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gu, Z.; Tan, Y.; Tsuchiya, K.; Shimomura, T.; Ogino, K. Synthesis and Characterization of Poly(3-hexylthiophene)-b-Polystyrene for Photovoltaic Application. Polymers 2011, 3, 558-570. https://doi.org/10.3390/polym3010558
Gu Z, Tan Y, Tsuchiya K, Shimomura T, Ogino K. Synthesis and Characterization of Poly(3-hexylthiophene)-b-Polystyrene for Photovoltaic Application. Polymers. 2011; 3(1):558-570. https://doi.org/10.3390/polym3010558
Chicago/Turabian StyleGu, Zhijie, Ying Tan, Kousuke Tsuchiya, Takeshi Shimomura, and Kenji Ogino. 2011. "Synthesis and Characterization of Poly(3-hexylthiophene)-b-Polystyrene for Photovoltaic Application" Polymers 3, no. 1: 558-570. https://doi.org/10.3390/polym3010558





