Thermal Cloud Point Fractionation of Poly(vinyl alcohol-co-vinyl acetate): Partition of Nanogels in the Fractions
Abstract
: Poly(vinyl acetate-co-vinyl alcohol) (PVA), well-known as emulsion stabilizers, are obtained by partial hydrolysis of poly(vinyl acetate) (PVAc). Their thermal cloud point fractionation was performed in aqueous medium between 40 and 75 °C. This fractionation was carried out in order to get an insight in the partition of the initially present nanogels in the different fractions. All the fractions were characterized by size exclusion chromatography (SEC), NMR and dynamic light scattering (DLS) giving access to average degree of polymerization , average degree of hydrolysis , average sequence lengths of vinyl acetate , volume fraction and average size diameter (Dv) of nanogels and “free PVA chains”. The polydispersity of the samples in , and could be confirmed. The nanogels characterized by the highest values of volume fraction and Dv, in the range of 40–43 nm, were separated in the first coacervate fraction, whereas the most soluble fraction with low VAc content does not contain nanogels but only “free chains” of a Dv value of around 7–8 nm. The nanogels in the various fractions could further be disaggregated into “free chains” by complex formation with sodium dodecyl sulfate (SDS).1. Introduction
Poly(vinyl alcohol-co-vinyl acetate) copolymers, currently referred as PVA, that are obtained by partial hydrolysis of poly(vinyl acetate) PVAc, are well known as stabilizers for the emulsion and suspension polymerization processes of various vinyl monomers such as styrene, vinyl acetate or vinyl chloride [1,2]. The molecular characteristics of PVA's, mainly their polymerization degrees , and the average degree of hydrolysis , in general in the range of 70 to 90 mol%, have a major influence on the monomer droplet size, the dispersion stability and on the properties of the final PVC resins [3,4].
An additional feature is that small colloidal particles, so-called nano- or microgels or “pseudo-micelles”, which are generally present in PVA's may have an influence on their emulsifying and stabilizing efficiency. As shown by different authors [5-8] these PVA nano- or microgels are formed by intermolecular hydrophobe-hydrophobe interactions for PVA's having values around 70–90 mol%. Furthermore, it should be mentioned that PVA's, used in industrial practice, are polydisperse in molar mass and in composition. For instance, Dawkins et al. [9] have clearly demonstrated by reversed phase liquid chromatography, that commercial PVA samples with an average value of 72 mol% contain in fact PVA chains with DH values ranging from 65 to 85 mol%. Zilberman et al. [10] came to a similar conclusion by cloud point fractionation, e.g., by temperature induced phase separation, of an aqueous PVA solution . According to these authors it turned out that the average sequence distribution of vinyl acetate (VAc) and vinyl alcohol (VOH) monomer units, usually designated by “blockiness”, has a major influence on the thermal phase separation and its compatibility with other water-soluble polymers. A typical example is that of hydroxypropyl methylcellulose, which is used in combination with PVA's in emulsifier formulations.
Another aspect of the PVA nanogels is their possibility to be disaggregated by complex formation with anionic surfactants; such as sodium dodecyl sulfate (SDS), as shown by Lewis and Robinson [6], Aladjoff et al. [11] and by Meehan et al. [12].
The aim of the present study was to take advantage of the cloud point fractionation technique, a process which does not need any additional solvent or precipitant, in order to check the “blockiness” index and the partition of the nanogels, especially their size and their relative volume fraction, in a fractioned PVA. In order to confirm the presence of nanogels in the different PVA fractions, this study will be completed by checking the influence of PVA/SDS complex formation on the disaggregation of these nanogels. These characteristics could be of interest for the optimization of the emulsifying efficiency of PVA's, by adjusting the saponification conditions of PVAc precursors.
2. Experimental Section
2.1. Materials
The PVAs examined in this study were supplied by Nippon Gohsei and Synthomer under the trade names KP08 and B72 respectively. These samples, hereafter used without further purification, are identified by their average and , as for instance PVA-73-650 and PVA-73-685 for sample B72 and KP08 respectively. The main characteristics of the PVA's, determined by 1H NMR and SEC, are summarized in Table 1.
The average hydrolysis degree, with a precision of ±0.5 mol%, was determined using 1H NMR (Bruker AC-400F operating at 400 MHz) in dimethylsulfoxide (DMSO)-d6 at 70 ° C according to Van der Velden and Beulen [13]. These characteristics were confirmed by 13C NMR spectroscopy of the polymers solubilized in a 50/50 (v/v) D2O and deuterated acetone mixture. This technique gives in addition access to the average sequence lengths of vinyl acetate defined by Moritani and Fujiwara [14]. It may be noticed that sample PVA-73-685 has the highest VAc sequence length .
The SEC measurements were carried out with a Shimadzu LC-20AD liquid chromatograph equipped with two Varian PL gel 5 μm MIXED-C columns (column, injection and refractometer temperature: 30 °C; injection volume: 100 μL; solvent: tetrahydrofuran at 1 mL min−1) and a refractive index detector (Shimadzu RID-10A). The PVA samples were at first reacetylated as recommended by Bugada and Rudin [15] and the “universal calibration technique” with polystyrene standards was applied for the calculation of , and the polydispersity index .
Sodium dodecyl sulfate (SDS), obtained from Acros Organics with a purity of 99%, was used without any further purification.
2.2. Procedure for the Thermal Cloud Point Fractionation
The PVA solutions of 7.5 wt% were prepared by dissolving under agitation 7.50 g PVA powder in 92.50 g triple distilled and filtered (0.22 μm Millipore filter) water at room temperature for 24 hours. The obtained solutions were poured into a separating funnel and thermostated for 24 hours, the time required to reach an equilibrium situation corresponding to a constant fraction of phase separated coacervate at different fractionation temperatures. At the first fractionation temperature, 41 °C, two fractions were separated, a coacervate F1 and a completely transparent supernatant layer F1′. The coacervate F1 was removed from the funnel and the remaining supernatant layer F1′ was used for a second fractionation step at 50 ° C to give fractions F2 and F2′. The last two fractions, F3 and F4, were separated from the supernatant fraction F2′ at 75 °C.
Each isolated fraction was characterized by NMR, SEC and DLS.
2.3. Sample Preparation
The PVA solutions of 1 wt%, for the DLS measurements, were prepared by diluting under agitation the required amounts of each separated fraction in triple distilled and filtered (0.22 μm Millipore filter) water at room temperature for 24 hours.
The SDS solutions were prepared by dilution of a 1 wt % “stock solution”. To avoid the hydrolysis of SDS, all surfactant solutions were used within 24 hours.
For the preparation of PVA/SDS solutions, each PVA fraction was directly diluted in the aqueous SDS solution at the required concentration under agitation for 24 hours at room temperature.
Before use, all solutions have been filtrated over 0.45 μm Chromafil Xtra MV-45/25 filter.
2.4. Dynamic Light Scattering
DLS measurements were carried out on a Malvern Nano-ZS6 Zetasizer equipped with a 4 mW He-Ne laser operating at a wavelength of 532 nm. The measurements were made at a scattering angle θ = 173° at a fixed temperature of 20 °C. Quartz cuvettes were used for all the experiments. Data (Dv and volume fraction) were acquired with the Malvern's Dispersion Technology Software version 4.20.
To determine the diameter of the particles, the data were collected in automatic mode, typically requiring a measurement duration of 70 seconds. The “data quality report” incorporated in the software indicated “good quality” for all the obtained data. For each experiment at a given temperature, the average of 5 consecutive measurements is indicated in the tables and figures.
3. Results and Discussion
These two PVA samples, PVA-73-650 and PVA-73-685, having very similar characteristics, such as , but different values, as shown in Table 1, were selected for our study in order to check that the “blockiness”, e.g., the average VAc sequence length, could have an influence on the nanogel characteristics.
This aspect appears clearly in Figure 1, showing the particle size distribution of the initial PVA's at a concentration of 1 wt% in aqueous solution at 20 °C.
As previously shown, peak 1 and peak 2 correspond to “free PVA chains” and to nanogels respectively [8]. These so-called nanogels are formed by hydrophobic interactions between PVAc sequences as demonstrated by Lewis and Robinson [6] and by Aladjoff et al. [7].
The thermal cloud point fractionation process is schematically outlined in Figure 2:
Three temperature steps above the cloud point are involved in this process. A first separation is carried out at 41 °C. After separation of this fraction F1, the temperature of the supernatant fraction F1′ is increased to 50 °C. This operation leads to a coacervate F2 and a supernatant phase F2′ which is further fractionated at 75 °C. The final fractions are F3 and F4 respectively.
The different fractions were characterized by NMR, SEC and by gravimetry. For a given PVA sample, PVA-73-650 and PVA-73-685 respectively, it can be noticed that:
- -
the values of F1, F2 and F3 are lower than that of F0, the initial PVA sample; this is a direct evidence of a fractionation in composition; as expected, the VAc rich species, with a lower solubility, are predominant in the coacervate,
- -
the values of F4, the most soluble fraction, are coming close to 80 mol%,
- -
the values of F1 and F2 are higher than that of F0, with afterwards a decrease for F3 and F4; this is indicative of a fractionation in molar mass,
- -
the average VAc sequence length has a tendency to decrease from F1 to F4,
- -
F4 corresponds not only to fractions with lowest VAc content, but also to that of lower and respectively.
By taking into account the Wi values of the fraction, given as the weight % with respect to F0, it is of interest to notice that the mass balance of the complete fractions process are of 93.7 and 95.3, for PVA-73-650 and PVA-73-685 respectively. A similar observation could be made for , and balances.
In agreement with Zilberman et al. [10] and with Lerner and Alon [16] these results, obtained by thermal cloud point fractionation, are a direct evidence that the fractionation is influenced simultaneously by the polydispersity in composition, molar mass and sequence distribution of the VAc monomer units.
For a given PVA sample, the major differences in their characteristics, , and , which can be noticed between F1, the most insoluble fraction, and F4, the most soluble one, has a direct influence on their cloud points. In fact for sample PVA-73-650 the cloud point is shifted from 26.5 ° C for fraction F1 to 70.5 °C for fraction F4. For sample PVA-73-685 the corresponding shift is from 26.5 ° C to 70.5 °C. Furthermore, for the fractions F1 it is worth noting the 6% increase of Wi for the sample PVA-73-685 with respect to PVA-73-650 with the values of 4.5 and 4.0 respectively.
The next step consisted in the determination by DLS of the particle size distributions and the volume fractions of the nanogels and the “free chains”. An illustration of the bimodal size distribution of the non-fractionated sample PVA-73-650 (F0) is given by Figure 3. This figure shows in addition the size distribution of the corresponding fractions F1 and F4.
For F1 it appears clearly, as compared to F0, a size shift from 11.4 to 13.0 nm for the “free chains”, which is in agreement with the increase from 650 to 720. A similar trend is observed and for the nanogels, their size increase from 38.0 to 42.8 nm, with corresponding volume fraction of 18.0 and 24.2%. For fraction F4 an almost monomodal size distribution curve can be noticed with an average particle size Dv = 8.1 nm. This decrease in size can directly be correlated to the low of this fraction, e.g., 360 as compared to 720 for F1. If “free chains” are definitely predominant in fraction F4, a close inspection of the size distribution shows the presence of a small “tail” which might correspond to a minor residual amount of aggregates.
The detailed characteristics of the different fractions are given in Tables 2 and 3 for the sample PVA-73-650 and PVA-73-685 respectively.
As a general observation for both PVA samples it can be noticed that for the nanogels the values of the volume fraction and Dv corresponding to a coacervate phase (F1, F2, F3) are systematically higher that those present in the supernatant phase (F1′, F2′ and F3). This is a clear indication that the nanogels are preferentially located in the coacervate phase. Furthermore it appears that the volume fractions of nanogels in all coacervate phases are higher than the corresponding value of the starting PVA. Moreover, it is of interest to notice that for both PVA samples the size of nanogels present in the most insoluble fraction F1 is increased with respect to those existing in the initial fraction F0.
On the opposite, the most soluble fraction F4 contains only “free chains” with a monomodal size distribution (see Figure 3). The corresponding Dv values of 8–9 nm are typical of the hydrodynamic diameter of “free PVA chains” having a of 360 [8].
From these fractionations tests, it turns finally out that the coacervate fraction F1 contains nanogels characterized by the highest size and volume fraction whereas in the fraction F4, the most soluble one, only “free chains” are present.
At this point, the proof has to be given that the nanogels initially present in F0, the starting PVA's, are in fact those distributed in the resulting fractions. In other words, the demonstration has to be provided that no additional nanogels might have been generated in the fractionation process operated at temperatures above the cloud point temperature. Moreover one has to take into account the fact that the characterization by DLS of the different fractions is carried out at 20 °C for PVA concentration of 1 wt%. In this respect, the thermo-reversibility of the PVA solutions at a concentration of 1 wt% could be assessed by different heating cycles at temperatures above the cloud point such as 41 °C and 50 °C, followed by subsequent cooling back to 20 °C. Within the experimental error limits no variations in size and volume fraction were observed with respect to the initial characteristics of the solution. Furthermore, it could be demonstrated that neither the volume fraction nor Dv of the PVA solutions are influenced by the dilution process [8].
A further proof that no additional nanogels have been generated in the fractionation process can be given by the size balance of nanogels by taking into account the weight % Wi of the different PVA fractions. In a first approximation this balance is such as ΣDv = ΣWiDvi/ΣWi. For PVA-73-650 this type of calculation leads to ΣDv of 40.5 nm, in fair agreement with the Dv of 39.6 nm corresponding to the non-fractionated PVA sample. A similar agreement was obtained for sample PVA-73-685 with ΣDv of 38.9 nm as compared with to initial Dv of 39.8 nm.
With the well-characterized PVA samples obtained by thermal cloud point fractionation process, it was of interest to study their behavior in presence of SDS. In fact, anionic surfactants, such as SDS, are known to form complexes with PVA, inducing the disaggregation of nanogels, as reported by different authors [6,8,11,12,17].
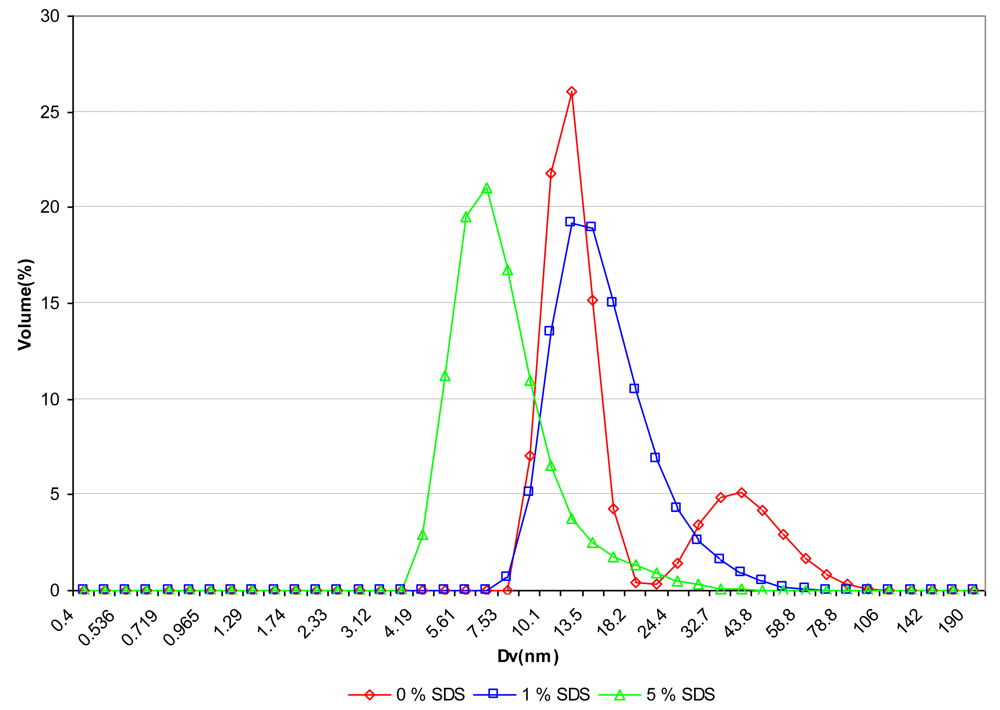
A typical example of the disaggregation effect of nanogels is displayed in Figure 4 for the fraction F1 of sample PVA-73-685:
From this figure, showing the size distribution of the fraction F1 of PVA-73-685 at two SDS concentrations, it can be noticed that by addition of SDS at a concentration of 1 wt% with respect to PVA, the peak attributed to nanogels has partially disappeared. The monomodal peak obtained at this SDS concentration shows a remaining “tail” attributed to a minor fraction of nanogels that are still present, however of lower size as the initial ones.
On further addition of SDS, such as a concentration of 5 wt%, the whole distribution curve is shifted to lower size values, corresponding to the “free chains”/SDS complex. For this fraction it can be admitted from DLS distribution curves that the nanogels are disaggregated at a SDS concentration around 5 wt% with respect to PVA.
The size characteristic of the different fractions of PVA-73-650 and PVA-73-685 at SDS concentrations of 1 and 5 wt% with respect to PVA are summarized in Table 4:
From this table it is worth noting that for both PVA samples at a SDS concentration of 1 wt% with respect to PVA, the Dv values are decreasing from fraction F1 to fraction F4. For these fractions, this tendency can be correlated to the corresponding decrease of vinyl acetate content and of .
However, it can be noticed that a SDS concentration of 1 wt% with respect to PVA is not sufficient to disaggregate completely the nanogels present in fractions F1, F2 and F3. A complete disaggregation is only reached at higher SDS concentrations, such as 5 wt%, with a possible formation of “free chains”/SDS complex having a Dv value of around 7 nm.
A particular behavior can be observed for the most soluble fraction F4 of lower and VAc content. Moreover, this fraction does not contain nanogels, as shown in Tables 2 and 3, and the monomodal distribution can be considered as “free chains” with a Dv values between 8–9 nm. As no size change is observed in the presence of a SDS concentration of 1 wt% with respect to PVA, it can be concluded that the possible complex formation with SDS has no major influence on their size.
Further work is in progress in order to investigate the direct thermal cloud point fractionation of PVA/SDS complexes and preliminary tests have already shown that the cloud point of such systems are shifted to higher temperatures by addition of SDS. As a consequence the fractionation of “nanogels-free” systems would have to be performed at temperatures of around 80–85 °C.
4. Conclusions
In addition to various fractionation techniques, such as SEC and HPLC published up to now for PVA's, it could be confirmed that the thermal cloud point fractionation is a valuable alternative procedure to give an insight in the polydispersity characteristics of this type of polymer.
Two PVA samples, with same overall and values but having different average sequence lengths of vinyl acetate , were fractionated in three temperature steps between 40 and 75 °C.
The nanogels with highest values of volume fraction and Dv were separated in the most insoluble fraction F1 whereas the most soluble fraction F4 no longer contains nanogels, only “free chains”.
In the presence of an SDS concentration of 1 wt% with respect to PVA a partial disaggregation of nanogels present in fractions F1, F2 and F3 was noticed. A complete disaggregation is only reached at higher SDS concentrations, such as 5 wt%, with formation of “free chains”/SDS complex having a Dv value of around 7 nm. In the presence of SDS, no size change is observed for the fraction F4 with higher values and lower “blockiness”.



| PVA's | (mol%) | (g mol−1) | (g mol−1) | PI | CP* (°C) | ||
|---|---|---|---|---|---|---|---|
| PVA-73-650 | 73.2 | 36,400 | 15,100 | 650 ± 20 | 2.41 ± 0.05 | 3.6 ± 0.1 | 28.5 ± 0.5 |
| PVA-73-685 | 72.5 | 37,700 | 18,400 | 685 ± 35 | 2.05 ± 0.03 | 4.3 ± 0.1 | 27.5 ± 0.5 |
*Cloud Point.
| PVA-73-650 | Nanogels | Free-chains | ||
|---|---|---|---|---|
| Volume fraction (%) | Dv (nm) | Volume fraction (%) | Dv (nm) | |
| F0 | 18.0 | 38.0 | 82.0 | 11.4 |
| F1* | 24.2 | 42.8 | 75.8 | 13.0 |
| F1′** | 15.5 | 32.4 | 84.5 | 9.7 |
| F2* | 23.1 | 41.2 | 76.9 | 14.2 |
| F2′** | 10.1 | 27.9 | 89.9 | 9.1 |
| F3* | 20.9 | 32.7 | 79.1 | 9.1 |
| F4** | - | - | 100 | 8.1 |
*coacervate phase.**supernatant phase.
| PVA-73-685 | Nanogels | Free-chains | ||
|---|---|---|---|---|
| Volume fraction (%) | Dv (nm) | Volume fraction (%) | Dv (nm) | |
| F0 | 22.9 | 39.8 | 77.1 | 14.2 |
| F1* | 29.5 | 40.7 | 70.5 | 11.6 |
| F1′** | 15.8 | 36.1 | 84.2 | 12.3 |
| F2* | 26.7 | 39.2 | 73.3 | 12.1 |
| F2′** | 20.2 | 22.1 | 79.8 | 7.5 |
| F3* | 27.0 | 32.3 | 73.0 | 10.7 |
| F4** | - | - | 100 | 9.0 |
*coacervate phase.**supernatant phase.
| Fractions | Dv (nm) | |||
|---|---|---|---|---|
| PVA-73-650 | PVA-73-685 | |||
| 1% SDS | 5% SDS | 1% SDS | 5% SDS | |
| F1 | 19.3 ± 0.9 | 7.6 ± 0.7 | 16.6 ± 0.3 | 7.1 ± 0.5 |
| F2 | 16.2 ± 0.7 | 7.4 ± 0.5 | 13.8 ± 0.7 | 6.9 ± 0.6 |
| F3 | 14.7 ± 0.4 | 6.9 ± 0.3 | 14.1 ± 0.7 | 7.7 ± 0.3 |
| F4 | 8.3 ± 0.3 | 7.8 ± 0.3 | 9.3 ± 0.2 | 7.6 ± 0.3 |
References
- Ramirez, J.C.; Herrera-Ordonez, J.; Gonzalez, V.A. Kinetics of styrene miniemulsion polymerization using a mixture PVA-SDS as stabilizer. Polymer 2006, 47, 3336–3343. [Google Scholar]
- Saeki, Y.; Emura, T. Technical progress for PVC production. Prog. Polym. Sci. 2002, 27, 2055–2131. [Google Scholar]
- Hong, S.; Albu, R.; Labbe, C.; Lasuye, T.; Stasik, B.; Riess, G. Preparation and characterization of colloidal dispersions of vinyl alcohol-vinyl acetate copolymers: Application as stabilizers for vinyl chloride suspension polymerization. Polym. Int. 2006, 55, 1426–1434. [Google Scholar]
- Boscher, V.; Helleboid, R.; Lasuye, T.; Stasik, B.; Riess, G. On-line acoustic attenuation spectroscopy of emulsions stabilized by vinyl alcohol-vinyl acetate copolymers: A model system for the suspension polymerization of vinyl chloride. Polym. Int. 2009, 58, 1209–1216. [Google Scholar]
- Crowther, N.J.; Eagland, D.J. The volumetric behaviour of poly(vinyl alcohol) in aqueous solution. J. Chem. Soc. Faraday Trans. 1986, 82, 2791–2799. [Google Scholar]
- Lewis, K.E.; Robinson, C.P. The interaction of sodium dodecyl sulfate with methyl cellulose and polyvinyl alcohol. J. Colloid Interface Sci. 1970, 32, 539–546. [Google Scholar]
- Aladjoff, I.; Nilsson, H.; Silvegren, C.; Tornell, B. Poly(vinyl alcohol) polymers with a low degree of hydrolysis. I. Formation and dissociation of multimers in aqueous solution. Acta Chem. Scand. 1982, 36, 259–266. [Google Scholar]
- Atanase, L.I.; Riess, G. Poly(vinyl alcohol-co-vinyl acetate) complex formation with anionic surfactants particle size of nanogels and their disaggregation with sodium dodecyl sulfate. Colloid. Surface. A Physicochem. Eng. Aspects 2010, 355, 29–36. [Google Scholar]
- Dawkins, J.V.; Nicholson, T.A.; Handley, A.J.; Meehan, E.; Nevin, A.; Shaw, P.L. Compositional heterogeneity in partially hydrolysed poly(vinyl alcohol) by reversed phase liquid chromatography. Polymer 1999, 40, 7331–7339. [Google Scholar]
- Zilberman, E.N.; Lerner, F.; Joseph, H.M.; Grossman, D.; Alon, M. Fractional composition of partly hydrolysed polyvinyl acetate and its compatibility with hydroxypropyl methylcellulose in aqueous solutions. Polym. Int. 1993, 30, 89–92. [Google Scholar]
- Aladjoff, I.; Nilsson, H.; Silvegren, C.; Tornell, B. Poly(vinyl alcohol) polymers with a low degree of hydrolysis. II. Complex formation with ammonium laurate and sodium lauryl sulfate. Acta Chem. Scand. 1982, 36, 267–272. [Google Scholar]
- Meehan, E.; Warner, F.P.; Reid, S.P.; Patterson, M.; Dawkins, J.V. Characterization of poly(vinyl alcohol) by liquid chromatographic techniques. Int. J. Polym. Anal. Char. 1995, 2, 71–82. [Google Scholar]
- van der Velden, G.; Beulen, J. 300-MHz 1H NMR and 25-MHz 13C NMR Investigations of sequence distributions in vinyl alcohol-vinyl acetate copolymers. Macromolecules 1982, 15, 1071–1075. [Google Scholar]
- Moritani, T.; Fujiwara, Y. 13C- and 1H NMR Investigations of sequence distributions in vinyl alcohol-vinyl acetate copolymers. Macromolecules 1977, 10, 532–535. [Google Scholar]
- Bugada, D.C.; Rudin, A. Characterization of poly(vinyl alcohol). J. Appl. Polym. Sci. 1985, 30, 4137–4147. [Google Scholar]
- Lerner, F.; Alon, M. Fractionation of partly hydrolyzed polyvinyl acetate. J. Polym. Sci. A Polym. Chem. 1987, 25, 181–189. [Google Scholar]
- Atanase, L.I.; Lasuye, T.; Stasik, B.; Riess, G. Colloidal characteristics of vinyl alcohol-vinyl acetate copolymers by complex formation with sodium dodecyl sulfate. Rev. Roum. Chim. 2009, 54, 577–581. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Atanase, L.I.; Riess, G. Thermal Cloud Point Fractionation of Poly(vinyl alcohol-co-vinyl acetate): Partition of Nanogels in the Fractions. Polymers 2011, 3, 1065-1075. https://doi.org/10.3390/polym3031065
Atanase LI, Riess G. Thermal Cloud Point Fractionation of Poly(vinyl alcohol-co-vinyl acetate): Partition of Nanogels in the Fractions. Polymers. 2011; 3(3):1065-1075. https://doi.org/10.3390/polym3031065
Chicago/Turabian StyleAtanase, Leonard I., and Gérard Riess. 2011. "Thermal Cloud Point Fractionation of Poly(vinyl alcohol-co-vinyl acetate): Partition of Nanogels in the Fractions" Polymers 3, no. 3: 1065-1075. https://doi.org/10.3390/polym3031065





