One-Dimensional Helical Homochiral Metal-Organic Framework Built from 2,2′-Dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic Acid
Abstract
: A homochiral metal-organic framework (MOF) based on enantiopure (R)-2,2′-dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic acid was synthesized. X-ray crystal diffraction studies revealed that the MOF adopts a one-dimensional infinite right-handed helical tubular structure along the a-axis, which serves as a host for the inclusion of guest dimethylformamide (DMF) molecules.1. Introduction
The field of metal-organic frameworks (MOFs) has grown explosively in recent years [1-8]; numerous studies have been reported owing to the potential applications of MOFs in gas storage [9-15], separation [16-23], luminescent materials [24-32], and heterogeneous catalysis [33-41]. While several MOFs have been discovered so far, only a few examples of chiral MOFs for enantiomer separations or heterogeneous asymmetric catalysis have been investigated [42]. We recently reported the synthesis of a novel two-dimensional homochiral MOF, (R)-MOF-1, from (R)-2,2′-dihydroxy-1,1′-binaphthyl-5,5′-dicarboxylic acid (1) (Scheme 1) and its application as an effective catalyst for the asymmetric ring-opening reaction of epoxide with amine [43] and the alcoholytic kinetic resolution of styrene oxide under heterogeneous conditions [44]. The helical structures of MOFs have also attracted considerable attention because of not only their intriguing structures, but also their potential applications in chiral recognition, nonlinear optical materials, and asymmetric catalysis. Over the past two decades, several MOFs containing single-, double-, and multi-stranded helices have been constructed and recently reviewed [45]. For example, one-dimensional helical metal-organic framework built from a chiral octahydrobinaphthalene-derived dicarboxylic acid showed the intense broad photoluminescence emission in the solid state [46]. Tridentate chiral Schiff base ligands has been found to form 1D helical framework which allow highly enantioselective separation of racemic secondary alcohols by inclusion crystallization [47]. Chiral binaphthylbisbipyridine-based copper (I) coordination polymer gels for use as catalysts in 1,3-dipolar Huisgen cycloaddition reactions are also reported [48]. Herein, we report the synthesis and X-ray crystal structure of the one-dimensional helical homochiral MOF, (R)-MOF-2, constructed from (R)-2,2′-dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic acid (2) (Scheme 2).
2. Experimental Section
General
1H-NMR spectra were recorded on a JEOL JNM-GSX 400 spectrometer with tetramethylsilane (TMS) as the internal standard. IR spectra were recorded with a JASCO FT-IR 4100 spectrometer. Thermogravimetric (TG) analyses were performed on a Rigaku TG8120 instrument. Solid-state circular dichroism (CD) spectra were recorded as KBr pellets on a JASCO J-820 CD system.
Synthesis of enantiopure 2,2′-dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic acid (2)
(R)- and (S)-2,2′-dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic acid (2) were synthesized according to the procedure previously reported by D. J. Cram et al. [49].
Synthesis of [Mn2((R)-1)2(DMF)4(H2O)4]·2DMF
A mixture of (R)-2,2′-dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic acid (2) (78 mg, 0.2 mmol) and MnCl2·4H2O (40 mg, 0.2 mmol) was dissolved in DMF (1 mL) and H2O (2 mL), and then pyridine (1 mL) was added to the solution. The solution was stirred for 30 min at room temperature and then left for 3 days. Pale yellow prisms were obtained, filtered, and dried at room temperature to give (R)-MOF-2 (132 mg). IR (KBr pellet, cm−1): 3,402, 2,931, 1,655, 1,559, 1,505, 1,457, 1,392, 1,336, 1,309, 1,242, 1,101, 932, 874, 810, 755, 702.
X-ray analysis
X-ray single-crystal diffraction data for (R)-MOF-2 were collected on a Rigaku RAXIS RAPID imaging plate diffractometer using Cu Kα radiation. Crystal data: Formula C62H74Mn2N6O22, Formula weight 1365.17, Space group P21(# = 10.9585(3), b = 25.2165(8), c = 11.8505(9) Å, β = 96.629(7)°, V = 3252.8(3) Å3, Z = 2, ρ = 1.394 g/cm3, 2θmax = 136.4°, R1 = 0.0514 (for 8372 reflections with I > 2σ(I)), wR2 = 0.1315 (for 11,652 reflections), GOF = 0.985, Flack parameter = 0.009(4) (calculated using 5,571 Friedel pairs). The structure was solved by SHELXS97 and refined by SHELXL97. The absolute structure was deduced from the Flack parameter.
CCDC
838075. See http://www.rsc.org/suppdata/cc/….….…./ for crystallographic data in cif or other electric formats.
3. Results and Discussion
3.1. Synthesis of Chiral MOF
Chiral ligand (R)-2,2′-dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic acid (2) was prepared in good yield by the diastereomeric complexation of rac-2 with L-(+)-leucine methyl ester (Scheme 3). The CD spectra of (R)-(+)- and (S)-(−)-2 in CHCl3 are shown in Figure 1.
New homochiral (R)-MOF-2 [Mn2((R)-1)2(DMF)4(H2O)4]·2DMF was synthesized by the reaction of (R)-(+)-2 and MnCl2·4H2O in the presence of pyridine in DMF at room temperature. The product was characterized by IR spectroscopy, CD spectroscopy, thermogravimetric analysis (TGA), and X-ray analysis. The IR spectra of (R)-MOF-2 exhibited peaks of νOH and νCO2− at 3,401 and 1,559 cm−1, respectively. TGA showed that (R)-MOF-2 loses 34.3% of its total weight in the range of 26–300 °C, which is ascribed to the loss of six DMF and four water molecules per formula unit (calculated at 37.4% of the total weight) (Figure 2).
3.2. Crystal Structure of (R)-MOF-2
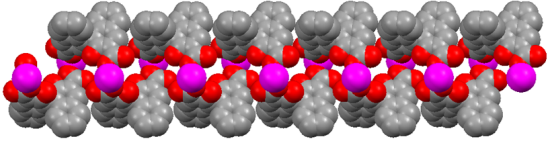
X-ray diffraction measurement revealed that (R)-MOF-2 crystallizes in a chiral space group of P21. An asymmetric unit of (R)-MOF-2 contains two Mn2+ ions, two (R)-22−groups, four DMF molecules, four water molecules, and two DMF guest molecules, as shown in Figure 3. The Mn2+ ion is coordinated by two (R)-22-groups, two DMF molecules, and two water molecules. The sixth coordination site of Mn1, although vacant in Figure 3, is occupied by O11 of the (R)-2 group lying in the next unit cell in the direction of a-axis. A helical chain composed of –Mn–(R)-2–Mn–(R)-2– is thus formed in the right-handed form and extends along the a-axis as shown in Figure 4. The guest molecules are bound to the water molecules by the hydrogen bonds of O21–H…O18 and O22–H…O19.
We also prepared (S)-MOF-2 using (S)-2 as the chiral ligand. As shown in Figure 5, the solid-state CD spectra of (R)- and (S)-MOF-2 synthesized from (R)- and (S)-2, respectively, are mirror images of each other, thus indicating that the helices built from (R)- and (S)-2 are enantiomeric.
4. Conclusions
We have synthesized a one-dimensional helical homochiral MOF (MOF-2) using MnCl2 and C2 symmetric chiral ligands (R)- and (S)-2 as the building blocks. We are currently studying its potential applications in heterogeneous asymmetric catalysis and enantioselective separations.








Acknowledgments
We thank JASCO Corporation Tokyo Japan for help with solid-state CD spectral data collection.
References
- Yaghi, O.M.; Li, H.; Davis, C.; Richardson, D.; Groy, T.L. Synthetic Strategies, Structure Patterns, and Emerging Properties in the Chemistry of Modular Porous Solids. Acc. Chem. Res. 1998, 31, 474–484. [Google Scholar]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O'Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal-Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar]
- Yaghi, O.M.; O'Keeffe, M.; Ockwig, N.M.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar]
- Zhao, X.; Xiao, B.; Fletcher, A.J.; Thomas, K.M.; Bradshaw, D.; Rosseinsky, M.J. Hysteretic Adsorption and Desorption of Hydrogen by Nanoporous Metal-Organic Frameworks. Science 2004, 306, 1012–1015. [Google Scholar]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. Crystallized Frameworks with Giant Pores: Are There Limits to the Possible? Acc. Chem. Res. 2005, 38, 217–225. [Google Scholar]
- Hill, R.J.; Long, D.L.; Champness, N.R.; Hubberstey, P.; Schroder, M. New Approaches to the Analysis of High Connectivity Materials: Design Frameworks Based upon 44- and 63-Subnet Tectons. Acc. Chem. Res. 2005, 38, 335–348. [Google Scholar]
- Wang, Z.; Chen, G.; Ding, K. Self-Supported Catalysts. Chem. Rev. 2009, 109, 322–359. [Google Scholar]
- Long, J.R.; Yaghi, O.M. The Pervasive Chemistry of Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O'Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular Metal-Organic Frameworks and Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar]
- Seki, K.; Mori, W. Syntheses and Characterization of Microporous Coordination Polymers with Open Frameworks. J. Phys. Chem. B 2002, 106, 1380–1385. [Google Scholar]
- Rowsell, J.L.C.; Millward, A.R.; Park, K.S.; Yaghi, O.M. Hydrogen Sorption in Functionalized Metal-Organic Frameworks. J. Am. Chem. Soc. 2004, 126, 5666–5667. [Google Scholar]
- Matsuda, R.; Kitaura, R.; Kitagawa, S.; Kubota, Y.; Belosludov, R.V.; Kobayashi, T.C.; Sakamoto, H.; Chiba, T.; Takata, M.; Kawazoe, Y.; Mita, Y. Highly Controlled Acetylene Accommodation in a Metal-Organic Microporous Material. Nature 2005, 436, 238–241. [Google Scholar]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 Saturation Uptake in Microporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar]
- Xiang, S.; Zhou, W.; Gallegos, J.M.; Liu, Y.; Chen, B.; Xiang, S.; Zhou, W.; Gallegos, J.M.; Liu, Y.; Chen, B. Methane Storage in Porous Metal-Organic Frameworks: Current Records and Future Perspectives. J. Am. Chem. Soc. 2009, 131, 12415–12419. [Google Scholar]
- Chen, Z.; Xiang, S.; Arman, H.D.; Li, P.; Tidrow, S.; Zhao, D.; Chen, B. A Microporous Metal-Organic Framework with Immobilized −OH Functional Groups within the Pore Surfaces for Selective Gas Sorption. Eur. J. Inorg. Chem. 2010, 2010, 3745–3749. [Google Scholar]
- Min, K.S.; Suh, M.P. Self-Assembly of 3-D Open-Framework Solids from Macrocyclic Complexes as Trifunctional Metal Building Block and Selective Guest Binding. Chem. Eur. J. 2001, 7, 303–313. [Google Scholar]
- Uemura, K.; Kitagawa, S.; Kondo, M.; Fukui, K.; Kitaura, R.; Chang, H.C.; Mizutani, T. Novel Flexible Frameworks of Porous Cobalt(II) Coordination Polymers Which Show Selective Guest Adsorption Based on Switching of Hydrogen Bond Pairs of Amide Groups. Chem. Eur. J. 2002, 8, 3586–3600. [Google Scholar]
- Bradshaw, D.; Prior, T.J.; Cussen, E.J.; Claridge, J.B.; Rosseinsky, M.J. Permanent Microporosity and Enantioselective Sorption in a Chiral Open Framework. J. Am. Chem. Soc. 2004, 126, 6106–6114. [Google Scholar]
- Suslick, K.S.; Bhyrappa, P.; Chou, J.H.; Kosal, M.E.; Nakagaki, S.; Smithenry, D.W.; Wilson, S.R. Microporous Porphyrin Solids. Acc. Chem. Res. 2005, 38, 283–291. [Google Scholar]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastre, J. Metal-Organic Frameworks-Prospective Industrial Applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar]
- Pan, L.; Olson, D.H.; Ciemnolonski, L.R.; Heddy, R.; Li, J. Separation of Hydrocarbons with a Microporous Metal-Organic Framework. Angew. Chem. Int. Ed. 2006, 45, 616–619. [Google Scholar]
- Horike, S.; Tanaka, D.; Nakagawa, K.; Kitagawa, S. Selective Guest Sorption in Interdigitated Porous Framework with Hydrophobic Pore Surfaces. Chem. Commun. 2007. [Google Scholar] [CrossRef]
- Couck, S.; Denayer, J.F.M.; Baron, G.V.; Remy, T.; Gascon, J.; Kapteijin, F. An Amine-Functionalized MIL-53 Metal-Organic Framework with Large Separation Power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar]
- Schlichte, K.; Kratzka, T.; Kaskel, S. Improved Synthesis, Thermal Stability and Catalytic Properties of the Metal Organic Framework Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar]
- Wu, C.; Hu, A.; Zhang, L.; Lin, W. A Homochiral Porous Metal-Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941. [Google Scholar]
- Alaerts, L.; Seguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; De Vos, D.E. Probing the Lewis Acidity and Catalytic Activity of the Metal-Organic Framework [Cu3(btc)2]. Chem. Eur. J. 2006, 12, 7353–7363. [Google Scholar]
- Hasegawa, S.; Horike, S.; Furukawa, S.; Mochizuki, K.; Kinoshita, Y.; Kitagawa, S. A Three Dimensional Porous Coordination Polymer Functionalized with Amide Groups Based on Tridentate Ligand: Selective Sorption and Catalysis. J. Am. Chem. Soc. 2007, 129, 2607–2614. [Google Scholar]
- Proch, S.; Hermansdorfer, J.; Kempe, R.; Kern, C.; Jess, A.; Seyfarth, L.; Senker, J. Pt@MOF-177: Synthesis, Room-Temperature Hydrogen Storage and Oxidation Catalysis. Chem. Eur. J. 2008, 14, 8204–8212. [Google Scholar]
- Ravon, U.; Domine, M.E.; Gaudillere, C.; Desmartin-Chomel, A.; Farrusseng, D. MOFs as Acid Catalysts with Shape Selectivity Properties. New. J. Chem. 2008, 32, 937–940. [Google Scholar]
- Horike, S.; Dinca, M.; Tamaki, K.; Long, J.R. Size-Selective Lewis-Acid Catalysis in a Microporous Metal-Organic Framework with Exposed Mn2+ Coordination Sites. J. Am. Chem. Soc. 2008, 130, 5854–5855. [Google Scholar]
- Henschel, A.; Gedrich, K.; Kraehnert, R.; Kaskel, S. Catalytic Properties of MIL-101. Chem. Commun. 2008. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar]
- Evans, O.R.; Ngo, H.L.; Lin, W. Chiral Porous Solids Based on Lamellar Lanthanide Phosphonates. J. Am. Chem. Soc. 2001, 123, 10395–10396. [Google Scholar]
- Cui, Y.; Evance, O.R.; Ngo, L.H.; White, P.S.; Lin, W. Rational Design of Homochiral Solids Based on 2D Metal Carboxylates. Angew. Chem. Int. Ed. 2002, 41, 1159–1162. [Google Scholar]
- Bradshaw, D.; Prior, T.J.; Cussen, E.J.; Claridge, J.B.; Rossensky, M.J. Permanent Microporosity and Enantioselective Sorption in a Chiral Open Framework. J. Am. Chem. Soc. 2004, 126, 6106–6114. [Google Scholar]
- Wu, C.D.; Hu, A.; Zhang, L.; Lin, W. A Homochiral Porous Metal-Organic Framework for Highly Enantioselective Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2005, 127, 8940–8941. [Google Scholar]
- Wu, C.; Lin, W. A Chiral Porous 3D Metal-Organic Framework with an Unprecdented 4-Connected Network Topology. Chem. Commun. 2005. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Nuzhdin, A.L.; Chun, H.; Bryliakov, K.P.; Talsi, E.P.; Fedin, V.P.; Kim, K. A Homochiral Metal-Organic Material with Permanent Porosity, Enantioselective Sorption Properties, and Catalytic Activity. Angew. Chem. Int. Ed. 2006, 45, 916–920. [Google Scholar]
- Cho, S.; Ma, B.; Nguyen, S.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. A Metal-Organic Framework Material that Functions as an Enantioselective Catalyst for Olefin Epoxidation. Chem. Commun. 2006. [Google Scholar] [CrossRef]
- Ingleson, M.J.; Barrio, J.P.; Bacsa, J.; Dickinson, C.; Park, H.; Rosseinsky, M.J. Generation of a Solid Brønsted Acid Site in a Chiral Framework. Chem. Commun. 2008. [Google Scholar] [CrossRef]
- Liao, T.; Ling, Y.; Chen, Z.; Zhao, Y.; Weng, L. A Rutile-Type Porous Zinc (II)-Phosphonocarboxylate Framework: Local Proton Transfer and Size-Selected Catalysis. Chem. Commun. 2010, 46, 1100–1102. [Google Scholar]
- Ma, L.; Abney, C.; Lin, W. Enantioselective Catalysis with Homochiral Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar]
- Tanaka, K.; Oda, S.; Shiro, M. A Novel Chiral Porous Metal-Organic Framework: Asymmetric Ring Opening Reaction of Epoxide with Amine in the Chiral Open Space. Chem. Commun. 2008. [Google Scholar] [CrossRef]
- Tanaka, K.; Otani, K. Asymmetric Alcoholytic Kinetic Resolution of Styrene Oxide Catalysed by Chiral Metal-Organic Framework Crystals. New J. Chem. 2010, 34, 2389–2391. [Google Scholar]
- Leong, W.L.; Vittal, J.J. One-Dimensional Coordination Polymers: Complexity and Diversity in Structures, Properties, and Applications. Chem. Rev. 2011, 111, 688–764. [Google Scholar]
- Ouyang, X.; Chen, Z.; liu, X.; Yang, Y.; Deng, M.; Weng, L.; Zhou, Y.; Jia, Y. One-Dimensional (1D) Helical and 2D Homochiral Metal-Organic Frameworks Built from A New Chiral Octahydrobinaphthalene-Derived Dicarboxylic Acid. Inorg. Chem. Commun. 2008, 11, 948–950. [Google Scholar]
- Yuan, G.; Zhu, C.; Xuan, W.; Cui, Y. Enantioselective Recognition and Separation by a Homochiral Porous Lamellar Solid Based on Unsymmetrical Schiff Base Metal Complexes. Chem. Eur. J. 2009, 15, 6428–6434. [Google Scholar]
- He, Y.; Bian, Z.; Kang, C.; Cheng, Y.; Gao, L. Chiral Binaphthylbisbipyridine-Based Copper (I) Coordination Polymer Gels as Supramolecular Catalysts. Chem. Commun. 2010, 46, 3532–3534. [Google Scholar]
- Cram, D.J.; Helgeson, R.C.; Peacock, S.C.; Kaplan, L.J.; Domeier, L.A.; Moreau, P.; Koga, K.; Mayer, J.M.; Chao, Y.; Siegel, M.G.; Hoffman, D.H.; Sogah, G.D.Y. Host-Guest Complexation. 8. Macrocyclic Polyethers Shaped by Two Rigid Substituted Dinaphthyl or Ditetralyl Units. J. Org. Chem. 1978, 43, 1930–1946. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tanaka, K.; Kikumoto, Y.; Shiro, M. One-Dimensional Helical Homochiral Metal-Organic Framework Built from 2,2′-Dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic Acid. Polymers 2011, 3, 1866-1874. https://doi.org/10.3390/polym3041866
Tanaka K, Kikumoto Y, Shiro M. One-Dimensional Helical Homochiral Metal-Organic Framework Built from 2,2′-Dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic Acid. Polymers. 2011; 3(4):1866-1874. https://doi.org/10.3390/polym3041866
Chicago/Turabian StyleTanaka, Koichi, Yuki Kikumoto, and Motoo Shiro. 2011. "One-Dimensional Helical Homochiral Metal-Organic Framework Built from 2,2′-Dihydroxy-1,1′-binaphthyl-3,3′-dicarboxylic Acid" Polymers 3, no. 4: 1866-1874. https://doi.org/10.3390/polym3041866