Synthesis and Characterization of Novel Copper(II) 2D Coordination Polymers from a Fluorinated Flexible Ligand with Remarkable Clathration Ability
Abstract
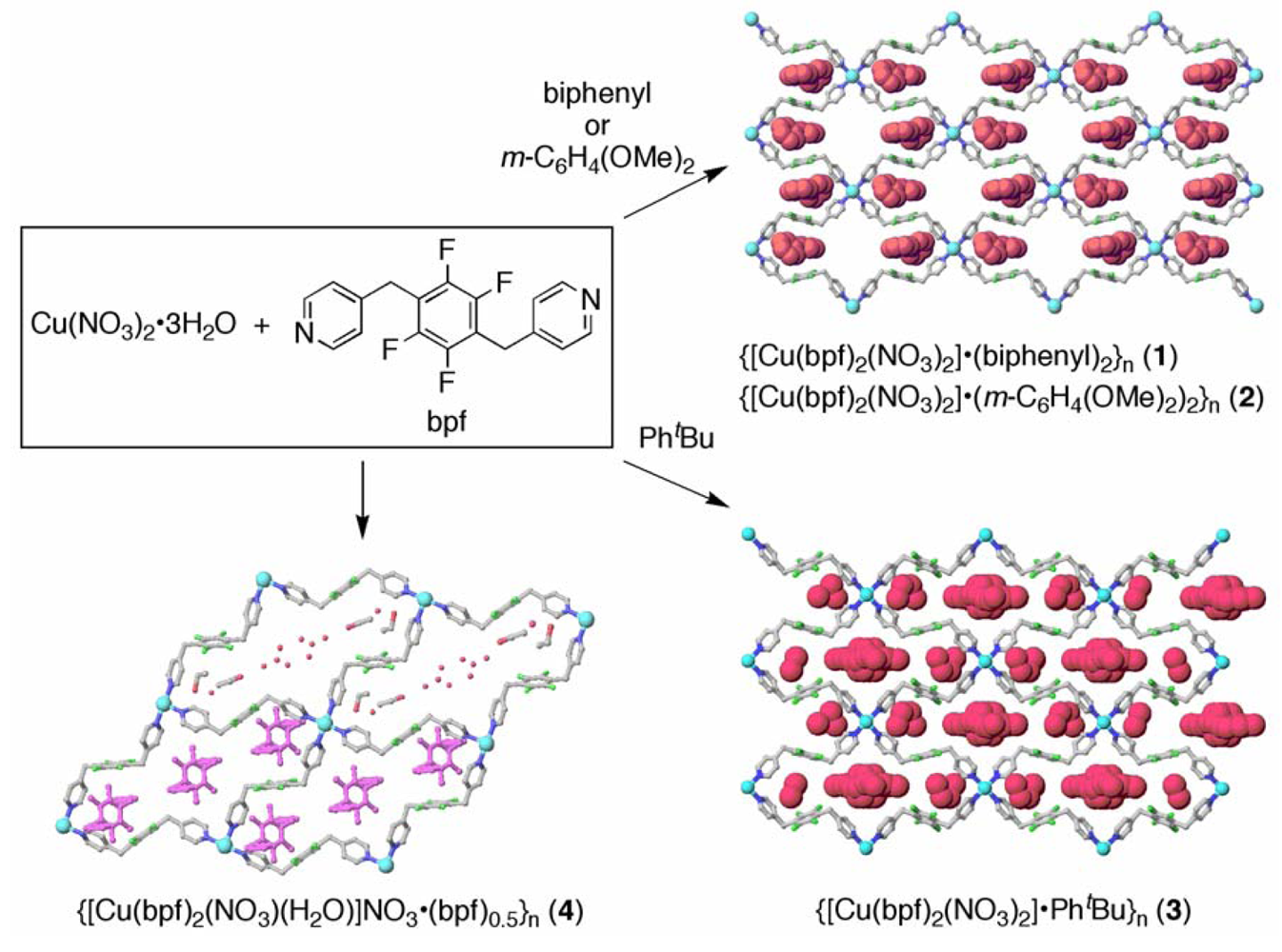
: Two-dimensional (2D) grid coordination polymers were prepared by the reaction of 1,4-bis(4-pyridylmethyl)tetrafluorobenzene (bpf) with Cu(NO3)2 in the presence of aromatic compounds. Crystal structures of {[Cu(bpf)2(NO3)2]·(biphenyl)2}n (1), {[Cu(bpf)2(NO3)2]·(m-C6H4(OMe)2)2}n (2), {[Cu(bpf)2(NO3)2]·PhtBu}n (3) and {[Cu(bpf)2(NO3)(H2O)]NO3·(bpf)0.5}n (4) were determined. The grid networks were held together by C–H⋯O and C–H⋯F hydrogen bonds via the NO3− anions and the tetrafluorophenylene rings of bpf, respectively. Biphenyl, m-dimethoxybenzene, t-butylbenzene, and bpf molecules were clathrated in cyclic cavities of the grid networks through arene-perfluoroarene interactions. These coordination networks have remarkable clathration ability for aromatic compounds.1. Introduction
Coordination networks with inner cavities and channels constructed from metal ions and bridging ligands [1-4] have attracted much attention owing to their potential functionalities such as molecular adsorption [5-8], ion-exchange [9,10], and heterogeneous catalysis [11,12]. Rational design of porous coordination networks with specific functions has been achieved by well-planned organic ligands and metal ions of various coordination geometries and oxidation states. We have reported the preparation of a series of clathrate coordination networks with Cd(NO3)2 and fluorinated flexible ligands Py–CH2–X–CH2–Py (Py = 4-pyridyl; X = C6F4, C6F4C6F4 or C10F6) [13-16]. These networks have remarkable clathration ability for guest aromatic compounds whose structures and topologies differ significantly depending on the size, shape, and number of the guest to afford one-dimensional (1D) cyclic chains, two-dimensional (2D) grids, or three-dimensional (3D) diamond networks. Fluorine atoms contained in the flexible ligands are mainly responsible for the clathration ability through C–H···F and/or arene-perfluoroarene interactions [14]. The authors report here the structures and characterizations of a new number of this family with Cu(NO3)2, the flexible fluorinated ligand 1,4-bis(4-pyridylmethyl)tetrafluorobenzene (bpf) and guest aromatic molecules.
2. Results and Discussion
The reaction of Cu(NO3)2 and bpf in the presence of biphenyl, m-dimethoxybenzene and t-butylbenzene resulted in the clathrate 2D grid networks {[Cu(bpf)2(NO3)2]·(biphenyl)2}n (1), {[Cu(bpf)2(NO3)2]·(m-C6H4(OMe)2)2}n (2) and {[Cu(bpf)2(NO3)2]·PhtBu}n (3), respectively. The reaction of Cu(NO3)2 and bpf in the absence of aromatic molecules resulted in a similar 2D grid network, {[Cu(bpf)2(NO3)(H2O)]NO3·(bpf)0.5}n (4), in which bpf molecules were clathrated as guests (Scheme 1). Single crystal X-ray analysis of 1–4 confirms that these are 2D grid networks with [4,4] topology based on distorted octahedral Cu(II) centers.
2.1. Description of Crystal Structure of {[Cu(bpf)2(NO3)2]·(biphenyl)2}n (1)
The asymmetric unit of 1, as illustrated by the labeled unprimed atoms of Figure 1(a), consists of half of Cu(NO3)2, a bpf molecule with trans conformation, a biphenyl molecule, and half of the EtOH solvent molecule. The copper ion adopts a distorted octahedral geometry with four bpf pyridine rings at the equatorial positions and two monodentate NO3− anions at the axial positions. Some selected bond lengths and angles around the Cu center are listed in Table 1. The extended structure forms a 2D rhombus grid and the shape of the cavity is an accurate rhombus. The diagonal-to-diagonal distances are 16.05 × 28.10 Å, corresponding to the dimensions of the b and c axes, and the grid dimensions are 16.18 Å. Each cavity is surrounded by four Cu(II) ions and four ligands in which two guest molecules of biphenyl are clathrated [Figure 1(b)]. Each guest molecule is packed between the perfluoroaromatic rings of the host framework with arene-perfluoroarene face-to-face interactions [17-20]. The shortest intermolecular contact C10⋯C27 is 3.530 Å [Figure 1(c)]. A side view of the stacking of 1 is shown in Figure 1(d). Interestingly, the adjacent sheets are enantiomers of each other and stacked alternately. The same enantiomers are eclipsed whereas the opposite enantiomers are staggered with an interplane distance of 6.2 Å. Each layer is linked by two types of hydrogen bonds [Figure 1(e)]. An oxygen atom of the nitrate anion binds to both the methylene group and pyridine ring of the neighboring layer (N3–O3⋯H4A (pyridine): 2.376 Å; N3–O3⋯H6B (CH2): 2.518 Å), whereas fluorine atoms of the perfluorophenylene ring bind to pyridine rings of the neighboring layer (H1A⋯F2: 2.698 Å; H3A⋯F4: 2.435 Å; H15A⋯F3: 2.474 Å) [18,21]. Moreover, the guest molecules are linked by a C–H⋯F hydrogen bond with the neighboring layer (H27A⋯F4: 2.772 Å).
2.2. Description of Crystal Structure of {[Cu(bpf)2(NO3)2]·(m-C6H4(OMe)2)2}n (2)
The structure of 2 is identical to that of 1 with the same space group C2/c and similar unit cell dimensions. The diagonal-to-diagonal distances of the cyclic cavity are 15.16 × 28.48 Å, corresponding to the dimensions of the b and c axes, and the grid dimensions are 16.13 Å. Two guest molecules are clathrated in a cavity through arene-perfluoroarene interactions with the shortest intermolecular contact C7⋯C24 of 3.462 Å.
2.3. Description of Crystal Structure of {[Cu(bpf)2(NO3)2]·PhtBu}n (3)
When t-butylbenzene is used, which contains a bulky group, only one guest molecule is clathrated in each cavity to afford 3. Figure 2(a) shows that the disordered t-butylbenzene molecules are clathrated at the cavity centers in the 2D grid network. The cavity is not exactly a rhombus but a quadrangle in which the two pairs of adjacent sides are the same (15.80 and 16.12 Å), and the diagonal-to-diagonal distances are 15.00 × 28.18 Å. This 2D sheet is relatively flat, and is made up of layers stacked on each other with an interplane distance of 5.8 Å. These layers are linked by two types of hydrogen bonds [Figure 2(b)]. An oxygen atom of the nitrate anion binds to both the methylene group and pyridine ring of the neighboring layer (N3–O2⋯H6A (pyridine): 2.446 Å), whereas fluorine atoms of the perfluorophenylene ring bind to pyridine rings of the neighboring layer (H1A⋯F1: 2.547 Å; H10A⋯F3: 2.468 Å). Consequently, microchannels still remain in the solid along the a axis [Figure 2(c)].
2.4. Description of Crystal Structure of {[Cu(bpf)2(NO3)(H2O)]NO3·(bpf)0.5}n (4)
The coordination environment of 4 is different from those of the other networks. Instead of an uncoordinated NO3− anion bonding to a water molecule through a hydrogen bond, the oxygen atom of a water molecule coordinates to the copper ion, while the other NO3− anion coordinates to the copper ion in a monodentate mode [Figure 3(a)]. Some selected bond lengths and angles around the Cu center are listed in Table 2. The grid sheet structure of 4 is shown in Figure 3(b). There are two cavities with alternate linking of alignments: cavity A clathrates three molecules of bpf, while cavity B apparently contains water and EtOH molecules. While these cavities are accurately classified as rhomboids, the shape is almost a rhombus. The diagonal-to-diagonal distances are 15.31 × 28.20 and 15.46 × 28.07 Å and the grid dimensions are 16.16 × 15.93 and 16.16 × 15.88 Å in cavities A and B, respectively. In cavity A, pyridine rings of bpf molecules are packed between perfluoroaromatic rings of the host framework with arene-perfluoroarene face-to-face interactions at both ends of the cavity. The shortest intermolecular contact C34⋯C38 is 3.404 Å [Figure 3(c)]. The middle bpf molecule is held in place by edge-to-face interactions between guest F atoms and host pyridine rings with F⋯centroid distances of 3.417 and 3.441 Å. This 2D sheet is relatively flat, and is made up into layers stacked and eclipsed on each other along the a axis with an interplane distance of 5.9 Å [Figure 3(d)]. It is clear that the bpf molecules in the cavities (colored with pink) are clathrated across the three sheets. These layers are linked by C–H⋯F interactions. The distances of H1A⋯F5, H18A⋯F1, H20A···F4, and H28A⋯F7 are 2.392, 2.568, 2.627, and 2.597 Å, respectively [Figure 3(e)]. Consequently, microchannels are formed along the a axis [Figure 3(f)].
3. Experimental Section
3.1. Materials
Ligand bpf was prepared in a similar manner as described in the literature [14]. Coordination networks were prepared in air. Cu(NO3)2·3H2O and biphenyl were purchased from Kanto Chemical Co., Ltd. m-Dimethoxybenzene and t-butylbenzene were purchased from Tokyo Chemical Industry Co., Ltd.
Synthesis of {[Cu(bpf)2(NO3)2]·(biphenyl)2}n (1)
A solution of bpf (80 mg, 0.24 mmol) in ethanol (16 mL) was added to a solution of Cu(NO3)2·3H2O (29 mg, 0.12 mmol) in H2O (4 mL) with stirring. After filtration, a saturated ethanol solution of biphenyl (2 mL) was added slowly. The mixture was allowed to stand for 48 h at room temperature to give blue prism shaped crystals (yield: 111 mg, 77%). Anal. Calcd for C62H50O7N6CuF8 {[Cu(bpf)2(NO3)2] · (biphenyl)2·EtOH}: C, 61.71; H, 4.18; N, 76.96. Found: C, 61.88; H, 4.18; N, 7.19%.
Synthesis of {[Cu(bpf)2(NO3)2]·(m-C6H4(OMe)2)2}n (2)
A solution of bpf (20 mg, 0.06 mmol) in ethanol (4 mL) was added to a solution of Cu(NO3)2·3H2O (7.2 mg, 0.03 mmol) in H2O (1 mL) with stirring. After filtration, m-dimethoxybenzene (0.25 mL, 1.9 mmol) was added slowly. The mixture was allowed to stand for 48 h at room temperature to give blue prism shaped crystals (yield: 7.2 mg, 20%). Anal. Calcd for C54H54O13N6CuF8 {[Cu(bpf)2(NO3)2] · (m-C6H4(OMe)2)2· (EtOH) · (H2O)2}: C, 53.58; H, 4.50; N, 6.94. Found: C, 53.42; H, 4.36; N, 7.06%.
Synthesis of {[Cu(bpf)2(NO3)2]·PhtBu}n (3)
A solution of bpf (20 mg, 0.06 mmol) in ethanol (4 mL) was added to a solution of Cu(NO3)2·3H2O (7.2 mg, 0.03 mmol) in H2O (1 mL) with stirring. After filtration, t-butylbenzene (0.29 mL, 1.9 mmol) was added slowly. After standing for 48 h at room temperature, the title compound was obtained as blue prism shaped crystals (yield: 13 mg, 42%). Anal. Calcd for C47H45O8.5N6CuF8 {[Cu(bpf)2(NO3)2] ·PhtBu·(EtOH)0.5· (H2O)2}: C, 54.00; H, 4.34; N, 8.04. Found: C, 54.12; H, 4.24; N, 8.21%.
Synthesis of {[Cu(bpf)2(NO3)(H2O)]NO3·(bpf)0.5}n (4)
A solution of bpf (25 mg, 0.075 mmol) in ethanol (4 mL) was added to a solution of Cu(NO3)2·3H2O (7.2 mg, 0.03 mmol) in H2O (1 mL) with stirring. After filtration and standing for 48 h at room temperature, the title compound was obtained as blue prism shaped crystals (yield: 11 mg, 32%). Anal. Calcd for C48H43O9.5N7CuF10 {[Cu(bpf)2(NO3)(H2O)]NO3· (bpf)0.5· (EtOH)1.5· (H2O)1.5}: C, 50.91; H, 3.92; N, 8.66. Found: C, 50.79; H, 4.02; N, 8.57%.
3.2. Crystallographic Data Collection and Refinement
Crystal data for all the structures were collected with a Bruker SMART/CCD diffractometer (MoKa radiation, λ = 0.71073 Å) by the ω-2 θ scan technique using frames of 0.3° oscillation (1.92 ≤ 2θ ≤ 28.01° for 1; 2.54 ≤ 2θ ≤ 28.02° for 2; 1.90 ≤ 2θ ≤ 27.56° for 3; and 2.02 ≤ 2θ ≤ 28.02° for 4). An empirical absorption correction was applied using the SADABS program. The structures were solved by the direct method (SIR 97 [22] for 1, 2, and 4 and SHELXS 97 [23] for 3) and refined by full-matrix least-squares against F2 of all data using the SHELXL 97 program package [24]. The positions of the hydrogen atoms were generated geometrically, assigned isotropic thermal parameters, and allowed to ride on their respective parent atoms before the final cycle of least-squares refinements. Crystallographic data for the four structures are listed in Table 3. CCDC-282151–CCDC-282154 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
4. Conclusions
Assembly of Cu(II) with flexible fluorinated ligand bpf in the presence of aromatic compounds affords 2D grid coordination networks. The guest molecules are clathrated in cyclic cavities of the grid networks. The grid networks are held together by C–H⋯O and C–H⋯F hydrogen bonds via the NO3− anions and tetrafluorophenylene rings of bpf, respectively. The guest aromatic rings are held in the cavity by arene-perfluoroarene interactions. Even in the absence of aromatic compounds, the combination of Cu(NO3)2 and bpf can afford a 2D grid network which clathrates the ligand as a guest molecule. These coordination networks have remarkable clathration ability for aromatic compounds.




| Cu1–N1 | 2.026(3) | Cu1–N2 | 2.011(3) |
| Cu1–O1 | 2.538(4) | ||
| N1–Cu1–N1* | 88.45(19) | N1–Cu1–N2* | 90.11(13) |
| N1–Cu1–N2** | 176.98(15) | N1*–Cu1–N2** | 90.11(13) |
| N1*–Cu1–N2* | 176.98(15) | N2*–Cu1–N2** | 91.45(19) |
| N1–Cu1–O1 | 89.33(14) | N1*–Cu1–O1 | 87.82(13) |
| N2*–Cu1–O1 | 89.51(13) | N2**–Cu1–O1 | 93.26(13) |
| Cu1–N1 | 2.037(4) | Cu1–N2* | 2.025(4) |
| Cu1–N3 | 2.034(4) | Cu1–N4 | 2.021(4) |
| Cu1–O1 | 2.571(4) | Cu1–O9 | 2.327(4) |
| N1–Cu1–N2* | 175.54(18) | N1–Cu1–N3 | 89.74(16) |
| N1–Cu1–N4 | 89.75(17) | N2*–Cu1–N3 | 91.03(17) |
| N2*–Cu1–N4 | 89.21(17) | N3–Cu1–N4 | 176.51(19) |
| N1–Cu1–O1 | 84.48(16) | N2*–Cu1–O1 | 91.21(16) |
| N3–Cu1–O1 | 84.73(15) | N4–Cu1–O1 | 91.79(15) |
| N1–Cu1–O9 | 92.59(16) | N2*–Cu1–O9 | 91.81(16) |
| N3–Cu1–O9 | 89.60(15) | N4–Cu1–O9 | 93.87(16) |
| O1–Cu1–O9 | 173.62(13) |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Formula | C62H50CuF8N6O7 | C54H54CuF8N6O13 | C47H45CuF8N6O8.5 | C48H44CuF10N7O10 |
| M | 1,206.62 | 1,210.57 | 1,045.43 | 1,132.44 |
| Crystal system | monoclinic | monoclinic | monoclinic | triclinic |
| Space group | C2/c (No. 15) | C2/c (No. 15) | P2/c (No. 13) | P1(No. 2) |
| a (Å) | 28.104(3) | 28.476(6) | 10.7067(15) | 10.1918(8) |
| b (Å) | 16.0488(16) | 15.156(2) | 15.089(2) | 11.6258(9) |
| c (Å) | 14.1585(14) | 15.346(2) | 15.475(2) | 22.3500(19) |
| α(°) | 84.796(2) | |||
| β(°) | 116.884(2) | 121.965(4) | 98.769(4) | 83.948(2) |
| γ(°) | 84.632(2) | |||
| V (Å3) | 5,695.8(10) | 5,618.5(16) | 2,470.8(6) | 2,613.1(4) |
| Z | 4 | 4 | 2 | 2 |
| T (K) | 153(2) | 153(2) | 200(2) | 153(2) |
| Dc (g cm−3) | 1.407 | 1.431 | 1.405 | 1.439 |
| μ (mm−1) | 0.469 | 0.483 | 0.531 | 0.516 |
| F(000) | 2,484 | 2,500 | 1,076 | 1,160 |
| Reflections collected | 13,835 | 10,182 | 22,146 | 17,746 |
| Unique reflections (Rint) | 6,682 (0.0512) | 5,675 (0.0914) | 5,624 (0.0368) | 12,237 (0.0523) |
| Parameters | 393 | 434 | 407 | 723 |
| goodness-of-fit | 0.950 | 0.864 | 1.005 | 0.889 |
| R1 indices [I > 2σ(I)] | 0.0710 (3,418) | 0.0679 (1,901) | 0.0556 (4,076) | 0.0732 (4,299) |
| wR2 (all data) | 0.2318 | 0.2040 | 0.1626 | 0.2113 |
Acknowledgments
The author gratefully acknowledges Makoto Fujita of The University of Tokyo for single crystal X-ray diffraction studies and valuable discussions.
References
- Zaworotko, M.J. Superstructural Diversity in Two Dimensions: Crystal Engineering of Laminated Solids. Chem. Commun. 2001. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O'Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A Highly Porous Metal-Organic Framework with Unusual Guest-Dependent Dynamic Behavior. Angew. Chem. Int. Ed. 2004, 43, 5033–5036. [Google Scholar]
- Maury, O.; Le Bozec, H. Molecular Engineering of Octupolar NLO Molecules and Materials Based on Bipyridyl Metal Complexes. Acc. Chem. Res. 2005, 38, 691–704. [Google Scholar]
- Kondo, M.; Yoshitomi, T.; Seki, K.; Matsuzaka, H.; Kitagawa, S. Three-Dimensional Framework with Channeling Cavities for Small Molecules: {[M2(4, 4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn). Angew. Chem. Int. Ed. Engl. 1997, 36, 1725–1727. [Google Scholar]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O'Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar]
- Takamizawa, S.; Nakata, E.; Yokoyama, H.; Mochizuki, K.; Mori, W. Carbon Dioxide Inclusion Phases of a Transformable 1D Coordination Polymer Host [Rh2(O2CPh)4(pyz)]n. Angew. Chem. Int. Ed. 2003, 42, 4331–4334. [Google Scholar]
- Ohmori, O.; Kawano, M.; Fujita, M. A Two-in-One Crystal Uptake of Two Different Guests into Two Distinct Channels of a Biporous Coordination Network. Angew. Chem. Int. Ed. 2005, 44, 1962–1964. [Google Scholar]
- Min, K.S.; Suh, M.P. Silver(I)-Polynitrile Network Solids for Anion Exchange: Anion-Induced Transformation of Supramolecular Structure in the Crystalline State. J. Am. Chem. Soc. 2000, 122, 6834–6840. [Google Scholar]
- Zhang, F.; Yajima, T.; Li, Y.-Z.; Xu, G.-Z.; Chen, H.-L.; Liu, Q.-T.; Yamauchi, O. Iodine-Assisted Assembly of Helical Coordination Polymers of Cucurbituril and Asymmetric Copper(II) Complexes. Angew. Chem. Int. Ed. 2005, 44, 3402–3407. [Google Scholar]
- Fujita, M.; Kwon, J.Y.; Washizu, S.; Ogura, K. Preparation, Clathration Ability, and Catalysis of a Two-Dimensional Square Network Material Composed of Cadmium(II) and 4,4′-Bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A Homochiral Metal-Organic Porous Material for Enantioselective Separation and Catalysis. Nature 2000, 404, 982–986. [Google Scholar]
- Kasai, K.; Aoyagi, M.; Fujita, M. Flexible Coordination Networks with Fluorinated Backbones. Remarkable Ability for Induced-Fit Enclathration of Organic Molecules. J. Am. Chem. Soc. 2000, 122, 2140–2141. [Google Scholar]
- Kasai, K.; Fujita, M. Guest-Dependent Flexible Coordination Networks with Fluorinated Ligands. Chem. Eur. J. 2007, 13, 3089–3105. [Google Scholar]
- Debata, N.B.; Tripathy, D.; Ramkumar, V.; Chand, D.K. Coordination-Driven Self-Assembly in a Single Pot. Tetrahedron Lett. 2010, 51, 4449–4451. [Google Scholar]
- Yamashita, K.; Sato, K.; Kawano, M.; Fujita, M. Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II)–py Bond. New J. Chem. 2009, 33, 264–270. [Google Scholar]
- Williams, J.H. The Molecular Electric Quadrupole Moment and Solid-State Architecture. Acc. Chem. Res. 1993, 26, 593–598. [Google Scholar]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chem. Int. Ed. 2003, 42, 1210–1250. [Google Scholar]
- Reichenbächer, K.; Süss, H.I.; Hulliger, J. Fluorine in Crystal Engineering—“The Little Atom That Could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar]
- Hori, A.; Shinohe, A.; Yamasaki, M.; Nishibori, E.; Aoyagi, S.; Sakata, M. 1:1 Cross-Assembly of Two β-Diketonate Complexes through Arene-Perfluoroarene Interactions. Angew. Chem. Int. Ed. 2007, 46, 7617–7620. [Google Scholar]
- Thalladi, V.R.; Weiss, H.-C.; Bläser, D.; Boese, R.; Nangia, A.; Desiraju, G. C–H⋯F Interactions in the Crystal Structures of Some Fluorobenzenes. J. Am. Chem. Soc. 1998, 120, 8702–8710. [Google Scholar]
- Cascarano, G.; Altomare, A.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Siliqi, D.; Burla, M.C.; Polidori, G.; Camalli, M. SIRWARE. Acta Crystallogr. 1996, A52. C-79. [Google Scholar]
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Crystallogr. 1990, 32, 467–473. [Google Scholar]
- Sheldrick, G.M. Program for the Solution of Crystal Structure; SHELXL 97; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Beni, T.; Kasai, K. Synthesis and Characterization of Novel Copper(II) 2D Coordination Polymers from a Fluorinated Flexible Ligand with Remarkable Clathration Ability. Polymers 2011, 3, 1934-1943. https://doi.org/10.3390/polym3041934
Beni T, Kasai K. Synthesis and Characterization of Novel Copper(II) 2D Coordination Polymers from a Fluorinated Flexible Ligand with Remarkable Clathration Ability. Polymers. 2011; 3(4):1934-1943. https://doi.org/10.3390/polym3041934
Chicago/Turabian StyleBeni, Tomohiro, and Kayoko Kasai. 2011. "Synthesis and Characterization of Novel Copper(II) 2D Coordination Polymers from a Fluorinated Flexible Ligand with Remarkable Clathration Ability" Polymers 3, no. 4: 1934-1943. https://doi.org/10.3390/polym3041934




