Polymer-Nanocrystal Hybrid Materials for Light Conversion Applications
Abstract
:1. General Introduction
1.1. Semiconductor Nanocrystals


1.2. Semiconductor NCs/Polymer Nanocomposites

2. Semiconductor NC Polymer Hybrid Materials Based on Non-Photoactive Polymers


3. Synthesis Approaches for NC Polymer Based Hybrid Materials
3.1. Integration of as Synthesized Semiconductor NCs into Polymers by Physical Mixing

3.2. In-Situ Synthesis of Semiconductor NCs in the Presence of Polymers
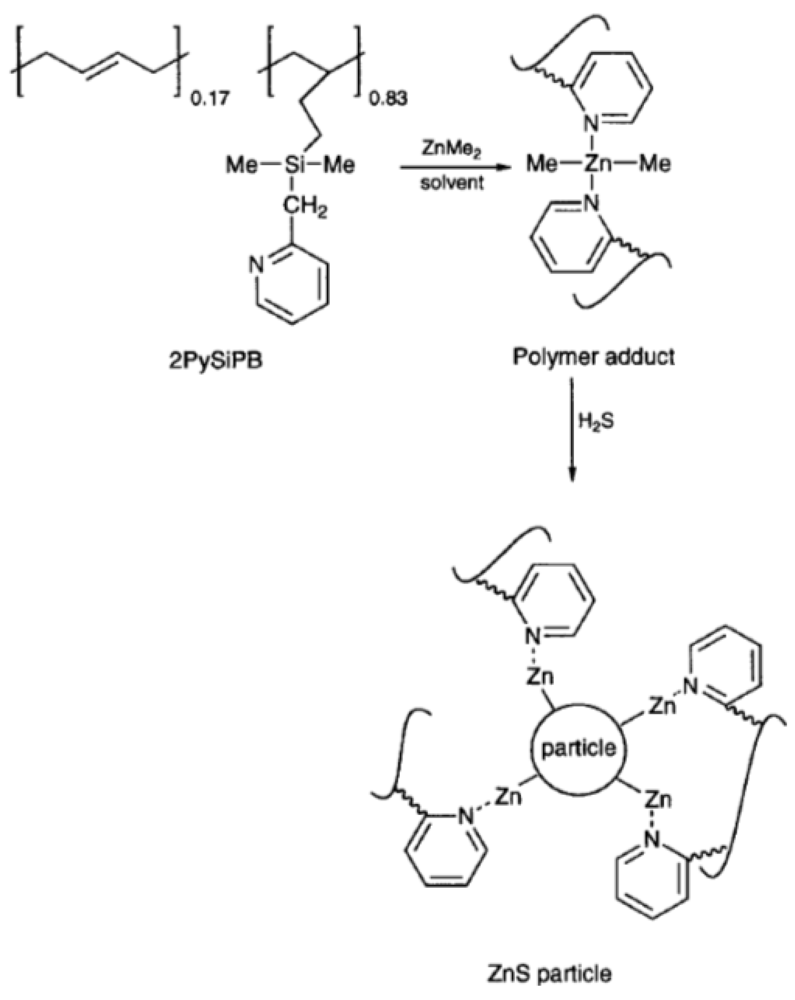
3.3. Polymerization Reaction in the Presence of Semiconductor NCs



4. Applications of Luminescent NC-Polymer Hybrid Materials
4.1. White Light Generation

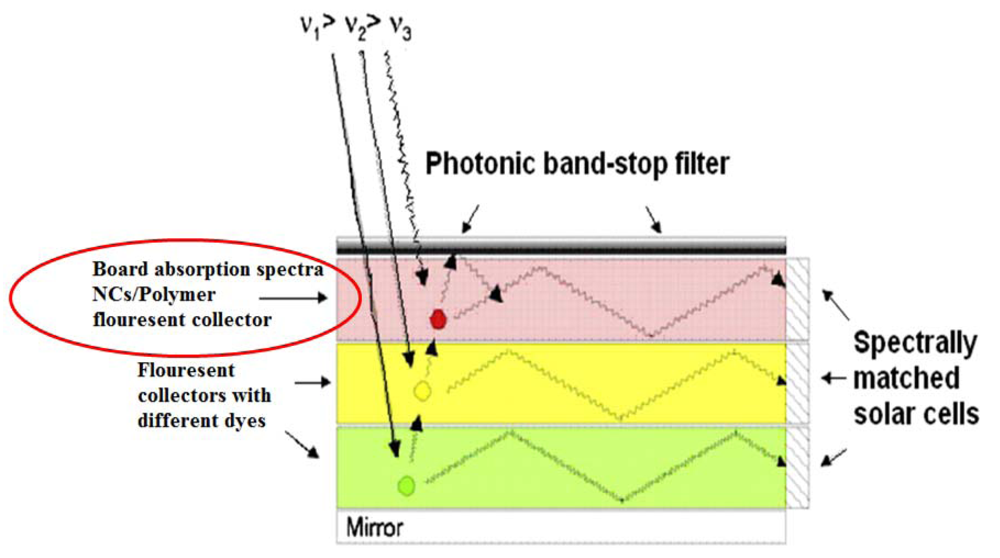
4.2. Light Conversion Layers in Concentrator Solar Cells
4.3. Light Conversion Layers for Enhanced Plant Growth to Increase Biofuel Production

5. Outlook
Acknowledgments
References
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance raman spectra, and electronic spectra of CdS crystallites in aqueous solutions. J. Chem. Phys. 1983, 79, 1086–1087. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Zhou, Y.; Riehle, F.S.; Yuan, Y.; Schleiermacher, H.F.; Niggemann, M.; Urban, G.A.; Krueger, M. Improved efficiency of hybrid solar cells based on non-ligand exchanged CdSe quantum dots and poly (3-hexylthiophene). Appl. Phys. Lett. 2010, 96, 013304. [Google Scholar]
- Zhou, Y.; Eck, M.; Kruger, M. Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers. Energy Environ. Sci. 2010, 3, 1851–1864. [Google Scholar] [CrossRef]
- Klimov, V.I.; Mikhaelovsky, A.A.; Xu, S.; Malko, A.; Hollingsworth, J.A.; Leatherdale, C.A.; Eisler, H.-J.; Bawendi, M.D. Optical gain and stimulated emission in nanocrystal quantum dots. Science 2000, 290, 314–317. [Google Scholar] [CrossRef]
- Kazes, M.; Lewis, D.T.; Ebenstein, Y.; Mokari, T.; Banin, U. Lasing from semiconductor quantum rods in a cylindrical microcavity. Adv. Mater. 2002, 14, 317–321. [Google Scholar] [CrossRef]
- Colvin, V.L.; Schlamp, M.C.; Alivisatos, A.P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. [Google Scholar]
- Tessler, N.; Medvedev, V.; Kazes, M.; Kan, S.; Banin, Y. Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 2002, 295, 1506–1508. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef]
- Wang, R.Y.; Feser, J.P.; Lee, J.-S.; Talapin, D.V.; Segalman, R.; Majumdar, A. Enhanced thermopower in PbSe nanocrystal quantum dot superlattices. Nano Lett. 2008, 8, 2283–2288. [Google Scholar]
- Talapin, D.V.; Lee, J.; Kovalenko, M.V.; Schevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar]
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar]
- Chan, W.C.W.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Mitchell, P.G.; Mirkin, A.C.; Letsinger, L.R. Programmed assembly of DNA functionalized quantum dots. J. Am. Chem. Soc. 1999, 121, 8122–8123. [Google Scholar] [CrossRef]
- Peng, X.; Mingos, D.M.P. Semiconductor Nanocrytals and Silicate Nanoparticles; Springer: Berlin, Germany, 2005; pp. 59–119. [Google Scholar]
- Riehle, F.S.; Bienert, R.; Thomann, R.; Urban, G.A.; Krüger, M. Blue luminescence and superstructures from magic size clusters of CdSe. Nano Lett. 2009, 9, 514–518. [Google Scholar] [CrossRef]
- Yuan, Y.; Riehle, F.S.; Gu, H.; Thomann, R.; Urban, G.; Krueger, M. Critical parameters for the scale-up synthesis of quantum dots. J. Nanosci. Nanotech. 2010, 10, 6041–6045. [Google Scholar] [CrossRef]
- Yuan, Y.; Riehle, F.S.; Nitschke, R.; Krueger, M. Highly photoluminescent and photostable CdSe quantum dot-nylon hybrid composites for efficient light conversion applications. Mater. Sci. Eng. B 2012. [Google Scholar]
- Cao, X.; Li, C.; Bao, H.; Bao, Q.; Dong, H. Fabrication of strongly fluorescent quantum dot-polymercomposite in aqueous solution. Chem. Mater. 2007, 19, 3773–3779. [Google Scholar]
- Lee, J.; Sundar, V.C.; Heine, J.R.; Bawendi, M.G.; Jesen, K.F. Full color emission from II-VI semiconductor quantum dot-polymer composites. Adv. Mater. 2000, 12, 1102–1105. [Google Scholar] [CrossRef]
- Bomm, J.; Büchtemann, A.; Chatten, A.J.; Bose, R.; Farrell, D.J.; Chan, N.L.A.; Xiao, Y.; Slooff, L.H.; Meyer, H.; van Sark, W.G.J.H.M.; et al. Fabrication and full characterization of state-of-the-art quantum dot luminescent solar concentrators. Sol. Energy Mater. Sol. Cells 2011, 95, 2087–2094. [Google Scholar] [CrossRef]
- Yang, J.; Dave, S.R.; Gao, X. Quantum dot nanobarcodes: Epitaxial assembly of nanoparticle-polymer complexes in homogeneous solution. J. Am. Chem. Soc. 2008, 130, 5286–5292. [Google Scholar] [CrossRef]
- Liu, S.; Ke, D.; Zeng, J.; Zhou, J.; Peng, T.; Zhang, L. Construction of inorganic nanoparticles by micro-nanoporous structure of cellulose matrix. Cellulose 2011, 18, 945–956. [Google Scholar] [CrossRef]
- Schlamp, M.C.; Peng, X.; Alivisatos, A.P. Improved efficiencies in light emitting diodes made with CdSe.CdS.core/shell type nanocrystals and a semiconducting polymer. J. Appl. Phys. 1997, 82, 5837–5842. [Google Scholar] [CrossRef]
- Wood, V.; Panzer, M.J.; Chen, J.; Bradley, M.S.; Halpert, J.E.; Bawendi, M.G.; Bulovic, V. Inkjet-printed quantum dot-polymer composites for full-color AC-driven displays. Adv. Mater. 2009, 21, 2151–2155. [Google Scholar] [CrossRef]
- Kim, T.; Cho, K.; Lee, E.K.; Lee, S.J.; Chae, J.; Kim, J.W.; Kim, D.H.; Kwon, J.; Amaratunga, G.; Lee, S.Y.; et al. Full-color quantum dot displays fabricated by transfer printing. Nat. Photonic 2011, 5, 176–182. [Google Scholar] [CrossRef]
- Chung, W.; Park, K.; Yu, H.J.; Kim, B.; Kim, S.H. White emission using mixtures of CdSe quantum dots and PMMA as a phosphor. Opt. Mater. 2010, 32, 515–521. [Google Scholar] [CrossRef]
- Menkara, H.; Gilstrap, R.A., Jr.; Morris, T.; Minkara, M.; Wagner, B.K.; Summers, C.J. Develoment of nanophosphors for light emitting diodes. Opt. Express 2011, 19, 972–981. [Google Scholar]
- Song, H.; Lee, S. Red light emitting solid state hybrid quantum dot-near-UV GaN LED devices. Nanotechnology 2007. [Google Scholar]
- Nizamoglu, S.; Zengin, G.; Demir, H.V. Color-converting combination of nanocrystal emittrts for warm-white light generation with high color rendering index. Appl. Phys. Lett. 2008. [Google Scholar]
- Schreuder, M.A.; Gosnell, J.D.; Smith, N.J.; Warnement, M.R.; Weiss, S.M.; Rosenthal, S.J. Encapsulated white-light CdSe nanocrystals as nanophosphors for solid-state lighting. J. Chem. Mater. 2008, 18, 970–975. [Google Scholar] [CrossRef]
- Bomm, J.; Buechtemann, A.; Fiore, A.; Manna, L.; Nelson, J.H.; Hill, D.; Sark, W.G.J.H.M.V. Fabrication and spectroscopic studies on highly luminescent CdSe/CdS nanorod polymer composites. Beilstein J. Nanotechnol. 2010, 1, 94–100. [Google Scholar] [CrossRef]
- Ziegler, J.; Xu, S.; Kucur, E.; Meister, F.; Batentschuk, M.; Gindele, F.; Nann, T. Silica-coated InP/ZnS nanocrystals as converter material in white leds. Adv. Mater. 2008, 20, 4068–4073. [Google Scholar] [CrossRef]
- Rogach, A.L. Semiconductor Nanocrystal quantum Dots: Synthesis, Assembly, Spectroscopy and Application; Springer Verlag: Berlin, Germany, 2008; pp. 173–176. [Google Scholar]
- Haggata, S.W.; Li, X.; Cole-Hamilton, D.J.; Fryer, J.R. Synthesis and characterization of II-VI semiconductor nanoparticulates by the reaction of a metal alkyl polymer adduct with hydrogen sulfide. J. Chem. Mater. 1996, 6, 1771–1780. [Google Scholar] [CrossRef]
- Haggata, S.W.; Cole-Hamilton, D.J.; Fryer, J.R. Control of average size and size distribution in as-grown nanoparticle polymer composites of MSe (M=Cd or Zn). J. Mater. Chem. 1997, 7, 1969–1975. [Google Scholar] [CrossRef]
- Firth, A.V.; Haggata, S.W.; Khanna, P.K.; Williams, S.J.; Allen, J.W.; Magennis, S.W.; Samuel, I.D.W.; Cole-Hamilton, D.J. Production and luminescent properties of CdSe and CdS nanoparticle polymer composites. J. Lumin. 2004, 109, 163–172. [Google Scholar]
- Woelfle, C.; Claus, R.O. Transparent and flexible quantum dot-polymer composites using an ionic liquid as compatible polymerization medium. Nanotechnology 2007, 18, 025402. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Z.; Wang, Y.; Zhang, K.; Ji, X.; Lü, C.; Yang, B.; Gao, M. From water-soluble CdTe nanocrystals to fluorescent nanocrystal-polymer transparent composites using polymerizable surfactants. Adv. Mater. 2003, 15, 777–780. [Google Scholar] [CrossRef]
- Krames, M.R.; Shchekin, O.B.; Mueller-Mach, R.; Zhou, L.; Harbers, G.; Craford, M.G. Novel color-sequential transflective liquid crystal displays. J. Display Technol. 2007, 3, 2–8. [Google Scholar] [CrossRef]
- Tsao, J.Y. Solid-state lighting: Lamps, chips, and materials for tomorrow. IEEE Circuits Devices Mag. 2004, 20, 28–37. [Google Scholar] [CrossRef]
- Nizamoglu, S.; Erdem, T.; Sun, X.W.; Demir, H.V. Warm-whiter light-emitting diodes integrated with colloidal quantum dots for high luminous efficacy and color redering. Opt. Lett. 2010, 35, 3372–3374. [Google Scholar]
- Gosnell, J.D.; Rosenthal, S.J.; Weiss, S.M. White light emission characteristics of polymer-encapsulated CdSe nanocrystal films. IEEE Photon. Technol. Lett. 2010, 22, 541–543. [Google Scholar] [CrossRef]
- Chandramohan, S.; Ryu, B.D.; Kim, H.K.; Hong, C.; Suh, E. Trap-state-assisted white light emission from a CdSe nanocrystal integrated hybrid light-emitting diode. Opt. Lett. 2011, 36, 802–804. [Google Scholar] [CrossRef]
- Chung, W.; Jung, H.; Kim, S.H. Application of CuInS2-ZnS Nanocrystals Red Light Converter for white LED. In Proceedings of the 2011 Spring Meeting, Symposium G, Nice, France, 9-13 May 2011.
- Weaver, J.; Zakeri, R.; Aouadi, S.; Kohli, P. Synthesis and characterization of quantum dot-polymer composites. J. Mater. Chem. 2009, 19, 3198–3206. [Google Scholar] [CrossRef]
- Yu, H.; Chung, W.; Kim, S.H. White Light Emission from Blue InGaN LED with Hybrid Phosphor. In Proceedings of the 10th IEEE Conference on Nanotechnology (IEEE-NANO), Seoul, Korea, 17-20 August 2010; pp. 958–961.
- Goldschmidt, J.C.; Peters, M.; Bösch, A.; Henning, H.; Dimroth, F.; Glunz, S.W.; Willeke, G. Increasing the efficiency of fluorescent concentrator systems. Sol. Energy Mater. Sol. Cells 2009, 93, 176–182. [Google Scholar] [CrossRef]
- Shcherbatyuk, G.V.; Inman, R.H.; Wang, C.; Winston, R.; Ghosh, S. Viability of using near infrared PbS quantum dots as active materials in luminescent solar concentrators. Appl. Phys. Lett. 2010, 96, 191901. [Google Scholar]
- Image by Aushulz. Licensed: GNU. Available online: http://en.wikipedia.org/wiki/File:Chlorophyll_ab_spectra2.PNG (accessed on 22 December 2011).
- Anh, T.; Benalloul, P.; Barthou, C.; Giang, L.T.; Vu, N.; Minh, L. Luminescrnce, energy transfer and uoconversion machanisms of Y2O3 nanomat rials doped with Eu3+, Tb3+, Tm3+, and, Yb3+ ions. J. Nanomater. 2007, 10, 48247. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yuan, Y.; Krüger, M. Polymer-Nanocrystal Hybrid Materials for Light Conversion Applications. Polymers 2012, 4, 1-19. https://doi.org/10.3390/polym4010001
Yuan Y, Krüger M. Polymer-Nanocrystal Hybrid Materials for Light Conversion Applications. Polymers. 2012; 4(1):1-19. https://doi.org/10.3390/polym4010001
Chicago/Turabian StyleYuan, Ying, and Michael Krüger. 2012. "Polymer-Nanocrystal Hybrid Materials for Light Conversion Applications" Polymers 4, no. 1: 1-19. https://doi.org/10.3390/polym4010001



