1. Introduction
Macromolecules with dendritic architecture have paid more and more attention over the past decade, and well-known polymers have gain interest by studying their dendritic analogues [
1,
2,
3]. This tendency is related to the advantages of dendrimers in comparison with the linear structure for modern nano- and biotechnology purposes which require a strong control of molecular properties at the nanoscale level [
2,
3]. In contrast to the linear polymers, dendrimers can be more easily controlled in their structure, molar mass, size, shape and periphery owing to the strategy of synthesis [
1]. Synthetic poly(aminoacids) and synthetic polypeptides are polymers of great importance for contemporary medicine, pharmacology, cosmetology and for biochemistry due to their biocompatibility. This class of biopolymers is also concerned by the previously mentioned general trends. Some successful attempts undertaken to design new dendritic polypeptide-like molecules as well as other water soluble dendrimers (for instance well-known poly(amido amine) (PAMAM)) had demonstrated the benefits of turning the shape and surface properties of such structurally ordered macromolecules [
2,
3,
4,
5].
Among the different classes of dendritic structures, dendrigraft polymers are the most recently discovered subset of dendrimer-like molecules whose solution properties have not been fully investigated. Synthesis of dendrigraft polymers is based on procedure of monomer units grafting onto a polymeric sub-structure using protect-deprotect steps [
1]. Dendrigraft polymers are characterized by high molecular weights attained in few synthetic steps. They are considered to stand at intermediate position between the fully controlled well determined dendrimers and the uncontrolled hyperbranched polymers [
1]. Within the class of macromolecules having dendritic architecture, dendrigraft polymers are expected to address the challenging issue regarding the industrial development of dendrimer-like structures, due to relatively low production cost while preserving the advantages belonging to dendritic structures.
The present work is devoted to the investigation of structure-properties relationships and to the study of molecular characteristics of dendrigraft poly-L-lysines (DGL,
Figure 1), the synthesis of which have been recently reported [
6,
7]. Contrary to previously described hyperbranched poly-L-lysine synthesis which was performed in organic solvent [
8,
9], DGL are synthesized by polycondensation of
N-epsilon-trifluoroacetyl-L-lysine-N-carboxyanhydride in water at pH 6.5 [
7]. The spontaneous precipitation of the growing
N-epsilon-trifluoroacetyl-protected polymer ensures fair control of the molar mass distribution. The subsequent deprotection of the trifluoroacetyl groups leads to water-soluble DGL that can be used as macroinitiator for the synthesis of the next generation. The unique reproducible structure of the DGL is thermodynamically controlled by precipitation in water, and kinetically controlled by steric hindrance during the polymerization. This new synthetic route leads to a straightforward easily scalable process, avoiding time-consuming multiple steps as described for the synthesis of lysine dendrimers [
7]. DGLs display an exponential growth of their molar mass with a 2.7 multiplying factor between generations (except between G1 and G2 for which a factor about 6 was obtained). They are non-immunogenic, biocompatible dendritic polypeptides that can be easily functionalized [
10]. They can be used for numerous applications, including drug carriers, gene delivery agents and antimicrobial agents [
10,
11].
Figure 1.
Schematic representation of the four first generations of dendrigraft poly-L-lysines (DGL) and typical chemical structure of DGL G3. Each dot represents a Lys residue. Chemical structure in red corresponds to G1. Chemical structure in red and blue corresponds to G2. The central part of the G3 structure is depicted in a cyclic conformation to facilitate the 2D-representation of the whole structure.
Figure 1.
Schematic representation of the four first generations of dendrigraft poly-L-lysines (DGL) and typical chemical structure of DGL G3. Each dot represents a Lys residue. Chemical structure in red corresponds to G1. Chemical structure in red and blue corresponds to G2. The central part of the G3 structure is depicted in a cyclic conformation to facilitate the 2D-representation of the whole structure.
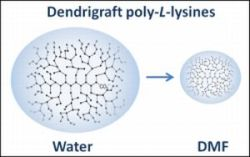
The goal of the present study was to compare the hydrodynamic characteristics of the first four generations of DGL in water (D2O + 0.2 M NaCl) and in dimethylformamide (DMF, aprotic solvent) using viscometry, isothermal translation diffusion and 1H NMR methodologies. A second objective was to shed more light on the structural and topological differences between dendrimers and dendrigraft poly-L-lysines. Dendrimers have a constant and maximal branching density value, while for dendrigraft poly-L-lysines the branching density is lower than for dendrimers and passes through a maximum for G4 generation.
3. Results and Discussion
The linear dependences between dispersion σ
2 of boundary curve and diffusion time
t obtained by isothermal translational diffusion method are displayed in
Figure 2. The diffusion coefficient
D of DGL molecules were determined from the slope of these dependences and converted to
dh (see Equation (1) in the experimental part).
Table 1 contains the experimental data on hydrodynamic diameters of DGL samples obtained by means of isothermal translational diffusion in DMF and by
1H NMR in D
2O + 0.2 M NaCl. DGL are cationic highly ramified polyelectrolytes since their amine groups are protonated in aqueous conditions (see schematic representation of DGL topology in
Figure 1). The previous investigation of DGL hydrodynamic behavior by Taylor Dispersion Analysis in phosphate buffers at pH 4.5 and 7 (0.61 M and 0.306 M ionic strength respectively) demonstrated an absence of any significant influence of pH on the DGL dimensions [
6]. The present study confirms these results since DGL dimensions determined by
1H NMR in 0.2 M NaCl deuterated water were found to be in good agreement with the previously published values (see
Table 1) [
6]. Good agreement of DGL molecular size determinations derived from different methods in aqueous solvents may be also considered as an evidence of DGL samples homogeneity.
Nevertheless, according to the data presented in the third column of
Table 1, the hydrodynamic diameters
dh of DGL in DMF determined by isothermal translation diffusion strongly differ from those obtained in aqueous solvents. The dimension of DGL is about twice smaller (or compact) in DMF in comparison with water, except for G1 which is only 1.4 smaller in DMF than in water (see the ratio in the last column of
Table 1). An obvious reason for the lower DGL dimension in DMF is that DMF is aprotic solvent that should significantly reduce the degree of protonation of the DGL, thus decreasing the repulsion between monomers and between branches that constitute the DGL. Another possible explanation (or mechanism) of DGL compacting in DMF may be related to the conformational state of the linear central backbone constituted of 8 Lys residues (G1). Indeed, in the uncharged state, linear poly-L-lysine tends to adopt a helical conformation. Possible twisting of the central part of DGLs in DMF can contribute to the decrease of overall dendrigraft molecule dimensions.
A peculiar tendency of dendimers bearing charged terminal groups to change their conformations with the variation of charge density was theoretically predicted for dendrimers [
17]. In particular, molecular modeling by means of different techniques predicted an essential dependence of size of PAMAM dendrimers on pH media conditions [
18,
19]. But experimental methods did not confirm this tendency. For well known dendrimers such as diaminobutane dendrimer (DAB) or PAMAM, there was no detected significant change of the molecular hydrodynamic size with pH or in different solvents. For instance, the invariance of PAMAM dendrimers under variable pH conditions was asserted from small angle neutron scattering data [
20,
21]. Furthermore, the PAMAM dendrimer dimension was found to vary in the range of 5 to 10%, depending on the solvent quality [
22]. One possible reason of such PAMAM behavior under variable pH conditions have been explained in [
23]. By means of atomistic molecular dynamics simulation on the fourth generation, it has been shown that the charge density distribution inside the dendrimer can change with the pH conditions, without any swelling.
DGLs may be similar to classical dendrimers regarding their pH behavior since their size just slightly depends on pH in aqueous media [
6], but at the same time, DGLs are dendritic macromolecules that behave differently from DAB and PAMAM and display a strong dependence of their size on thermodynamic quality of the solvent, similarly to flexible linear polymer chains [
24]. As discussed earlier, this difference can comes from the linear multifunctional core of the DGL compared to the point-like core of PAMAM and DAB dendrimers [
1]. In addition, the charged amine end-groups can be situated not only on the outer part of the DGL, but also inside the dendritic structure, in contrast to DAB and PAMAM for which amine end-groups are located only at the periphery. This means that DGL can attract the counter ions into the interior part of the dendritic structure, which is impossible for DAB or PAMAM dendrimers. The transfer of counter ions inside DGL in aqueous buffers (high ionization state) may be additionally responsible for a higher DGL dimension compared to DMF (low ionization state). It can be mentioned also, that the hydrodynamic size of DGL molecules in DMF (
Table 1) are close to the size of the same generation number PAMAM in methanol even though molar mass of corresponding DGL generation is more than twice higher [
22,
25].
Thus, the results of the study of translation diffusion in DMF allow us to conclude that DGL may be considered as one of the most flexible dendritic macromolecules among those currently used or studied for biological applications. This unique property of contraction/swelling of DGL dimension may be advantageously utilized for encapsulation/decapsulation processes.
Figure 4 displays the dependencies of translation diffusion coefficients
D and intrinsic viscosity
[η] vs. DGL molar mass
M in a double logarithmic scale in water and DMF. Scaling relationships of translation diffusion coefficient for DGL that derived from the plots are the following:
D = 4.12 × 10−5 M−0.30 ± 0.07 (DMF) (3a)
D = 2.24 × 10−5 M−0.35 ± 0.03 (D2O + 0.2 M NaCl) (3b)
The slopes of the log
D = f(log
M) dependencies are closed to each other for the two solvents, taking into account the experimental error values. The exponent values in relationships Equations (3a) and (3b) are close to those previously determined in phosphate buffers (0.36–0.37) [
6]. The fact that the exponent does not significantly change in the two solvents suggests an homothetic change of the DGL size in DMF and in D
2O + 0.2 M NaCl. The non-variation of this exponent with the solvent, with the ionic strength and with the pH is in great contrast to linear polymers. In the latter case, the scaling exponents are very sensitive to thermodynamic quality of the solvent [
24]. Compared to other dendritic structures, such as PAMAM in methanol [
1,
25], carbohydrate coated PAMAM [
26] and DAB modified by lactose-end groups [
27] in aqueous buffers, similar exponents from 0.25 to 0.37 were obtained. Indeed, diffusion scaling indexes for dendrimers and dendrimer-like structures are in the vicinity of 1/3. The latter diffusion coefficient scaling index value was theoretically predicted for homogeneous impenetrable spheres [
24]. Thus, in translation diffusion process, DGL behave themselves similar to dense highly ramified structures as for classical dendrimers.
Figure 4.
The dependencies of diffusion coefficient
D and intrinsic viscosity
[η] with the molar mass of DGL in different conditions. Experimental conditions: D
2O containing 0.2 M NaCl, phosphate buffer pH 4.5 (0.59 M ionic strength) [
6] and DMF.
Figure 4.
The dependencies of diffusion coefficient
D and intrinsic viscosity
[η] with the molar mass of DGL in different conditions. Experimental conditions: D
2O containing 0.2 M NaCl, phosphate buffer pH 4.5 (0.59 M ionic strength) [
6] and DMF.
On the other hand, according to the theoretical predictions molecules which hydrodynamic properties correspond to the behavior of homogeneous impenetrable spheres, should have a scaling index
a = 0 in Mark-Houwink-Kuhn relationship
[η] ~
Ma [
24]. In other words, the intrinsic viscosity that is directly inversely proportional to the density in the macromolecule, becomes independent of the molar mass for impenetrable spheres. In reality,
a is not equal to zero for many dendrimers and theirs derivatives and the dependence of
[η] with
M is often non-monotonous [
1]. For DGL in D
2O + 0.2 M NaCl, the following Mark-Houwink-Kuhn equation has been experimentally established for generations 1 to 4:
[η] = 2.01 × M0.12 ± 0.01 (4)
A clear contradiction appears between the translation diffusion of dendrimers whose behavior can be described by impenetrable spheres (nanoparticles), and the intrinsic viscosity data that does not follow the hard sphere model. This discrepancy is often considered as the manifestation of the dendrimer flexibility and as the evidence that dendrimers are rather macromolecules than nanoparticles [
1]. To conclude on that part of the study, it may be asserted that hydrodynamic properties of DGL are similar to charged high molar mass dendrimers, excepted that the DGL dimension is much more affected by the solvent than other dendrimers such as PAMAM. DGL can be therefore considered as soft dendritic structures.