Templated Formation of Hydroxyapatite Nanoparticles from Self-Assembled Nanogels Containing Tricarboxylate Groups
Abstract
:1. Introduction

2. Experimental Section
2.1. General
2.2. Synthesis of CHPAc3

2.3. Preparation of the Nanogel Suspension
2.4 Mineralization of Calcium Phosphate by the pH Gradient Method
2.5. Characterization of the Nanomaterials
3. Results and Discussion
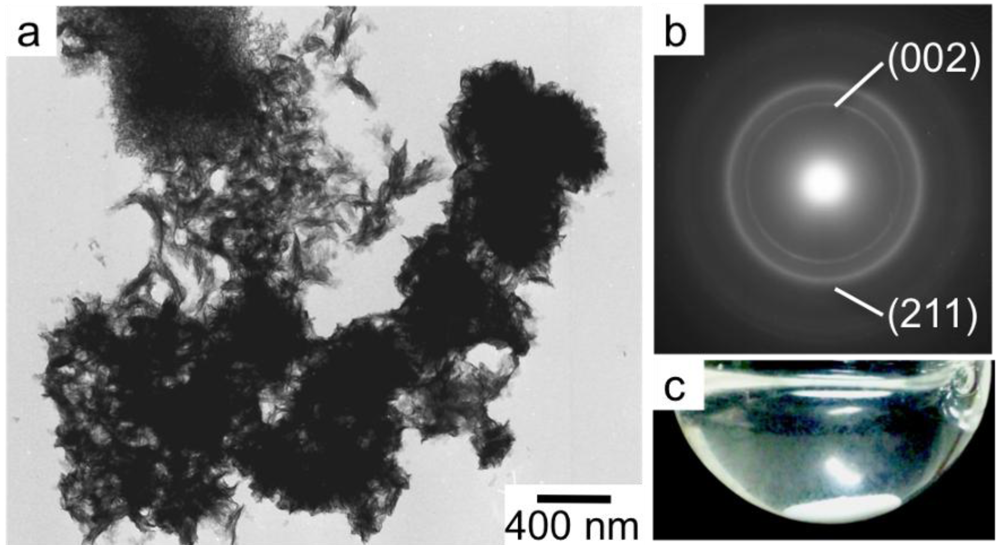
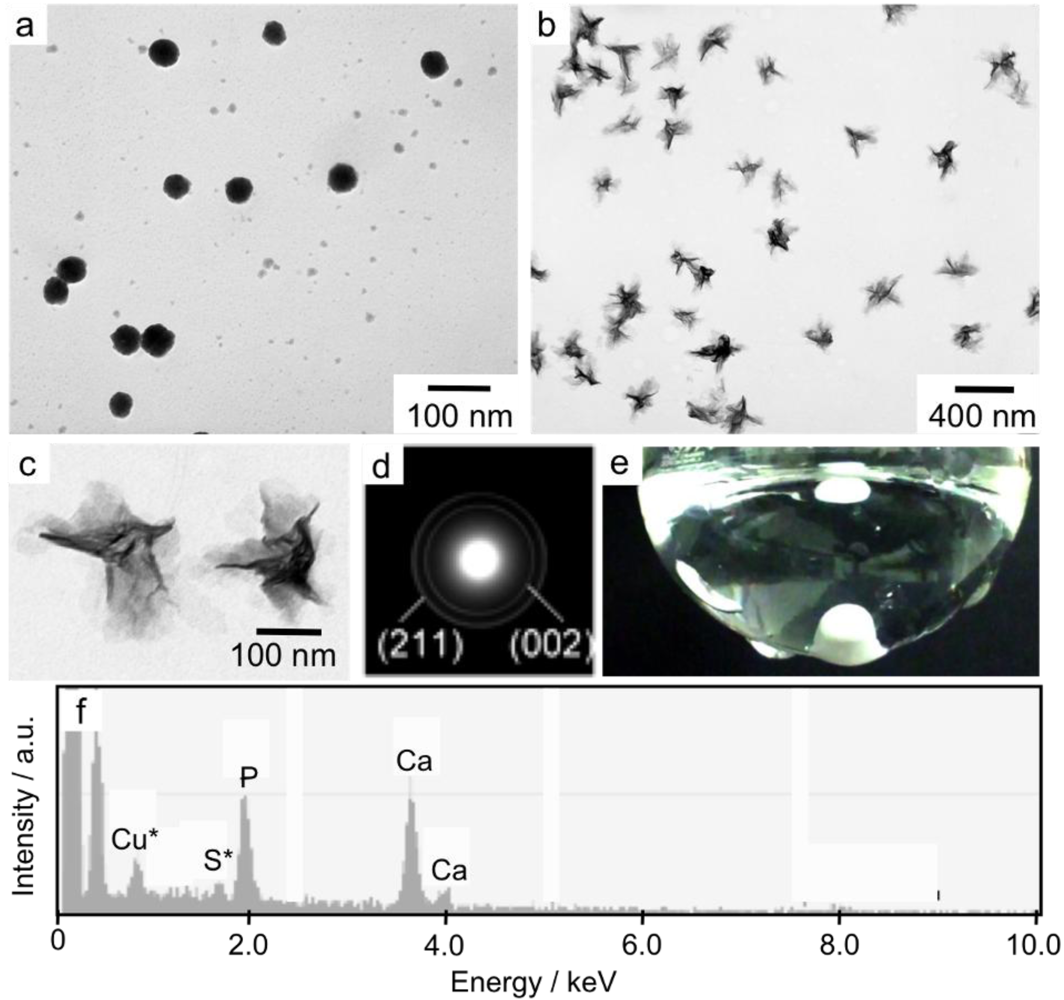
4. Conclusions
Acknowledgments
References
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar]
- Vallet-Regi, M.; Colilla, M.; Gonzalez, B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar]
- Habraken, W.; Wolke, J.G.C.; Jansen, J.A. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 234–248. [Google Scholar]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar]
- Tari, N.E.; Motlagh, M.K.M.; Sohrabi, B. Synthesis of hydroxyapatite particles in cationic mixed surfactants template. Mater. Chem. Phys. 2011, 131, 132–135. [Google Scholar]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar]
- Chu, M.Q.; Liu, G.J. Preparation and characterization of hydroxyapatite/liposome core-shell nanocomposites. Nanotechnology 2005, 16, 1208–1212. [Google Scholar]
- Sugawara, A.; Yamane, S.; Akiyoshi, K. Nanogel-templated mineralization: Polymer-calcium phosphate hybrid nanomaterials. Macromol. Rapid Commun. 2006, 27, 441–446. [Google Scholar]
- Yamane, S.; Sugawara, A.; Sasaki, Y.; Akiyoshi, K. Nanogel-calcium phosphate hybrid nanoparticles with negative or positive charges for potential biomedical applications. Bull. Chem. Soc. Jpn. 2009, 82, 416–418. [Google Scholar]
- Yamane, S.; Sugawara, A.; Watanabe, A.; Akiyoshi, K. Hybrid nanoapatite by polysaccharide nanogel-templated mineralization. J. Bioact. Biocomp. Polym. 2009, 24, 151–168. [Google Scholar]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar]
- Sasaki, Y.; Akiyoshi, K. Development of an artificial chaperone system based on cyclodextrin. Curr. Pharm. Biotechnol. 2010, 11, 300–305. [Google Scholar]
- Hasegawa, U.; Sawada, S.; Shimizu, T.; Kishida, T.; Otsuji, E.; Mazda, O.; Akiyoshi, K. Raspberry-like assembly of cross-linked nanogels for protein delivery. J. Control Release 2009, 140, 312–317. [Google Scholar]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control Release 2010, 142, 483–489. [Google Scholar]
- Ikuta, Y.; Katayama, N.; Wang, L.; Okugawa, T.; Takahashi, Y.; Schmitt, M.; Gu, X.; Watanabe, M.; Akiyoshi, K.; Nakamura, H.; Kuribayashi, K.; Sunamoto, J.; Shiku, H. Presentation of a major histocompatibility complex class 1-binding peptide by monocyte-derived dendritic cells incorporating hydrophobized polysaccharide-truncated HER2 protein complex: implications for a polyvalent immuno-cell therapy. Blood 2002, 99, 3717–3724. [Google Scholar]
- Kageyama, S.; Kitano, S.; Hirayama, M.; Nagata, Y.; Imai, H.; Shiraishi, T.; Akiyoshi, K.; Scott, A.M.; Murphy, R.; Hoffman, E.W.; Old, L.J.; Katayama, N.; Shiku, H. Humoral immune responses in patients vaccinated with 1–146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008, 99, 601–607. [Google Scholar] [CrossRef]
- Nochi, T.; Yuki, Y.; Takahashi, H.; Sawada, S.; Mejima, M.; Kohda, T.; Harada, N.; Kong, I.G.; Sato, A.; Kataoka, N.; Tokuhara, D.; Kurokawa, S.; Takahashi, Y.; Tsukada, H.; Kozaki, S.; Akiyoshi, K.; Kiyono, H. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccine. Nat. Mater. 2010, 9, 572–578. [Google Scholar]
- Boanini, E.; Fini, M.; Gazzano, M.; Bigi, A. Hydroxyapatite nanocrystals modified with acidic amino acids. Eur. J. Inorg. Chem. 2006, 23, 4821–4826. [Google Scholar]
- Jiang, H.D.; Liu, X.Y.; Zhang, G.; Li, Y. Kinetics and template nucleation of self-assembled hydroxyapatite nanocrystallites by chondroitin sulfate. J. Biol. Chem. 2005, 280, 42061–42066. [Google Scholar]
- Kawai, T.; Ohtsuki, C.; Kamitakahara, M.; Hosoya, K.; Tanihara, M.; Miyazaki, T.; Sakaguchi, Y.; Konagaya, S. In vitro apatite formation on polyamide containing carboxyl groups modified with silanol groups. J. Mater. Sci. Mater. Med. 2007, 18, 1037–1042. [Google Scholar]
- Liu, Q.; Ding, J.; Mante, F.K.; Wunder, S.L.; Baran, G.R. The role of surface functional groups in calcium phosphate nucleation on titanium foil: A self-assembled monolayer technique. Biomaterials 2002, 23, 3103–3111. [Google Scholar]
- Wong, A.T.C.; Czernuszka, J.T. Transformation behaviour of calcium phosphate 2. Effects of various phosphorylated amino acids. Colloid Surf. A Physicochem. Eng. Asp. 2005, 103, 23–36. [Google Scholar]
- Granja, P.L.; Barbosa, M.A.; Pouysegu, L.; De Jeso, B.; Rouais, F.; Baquey, C. Cellulose phosphates as biomaterials. Mineralization of chemically modified regenerated cellulose hydrogels. J. Mater. Sci. 2001, 36, 2163–2172. [Google Scholar] [CrossRef]
- Akiyoshi, K.; Deguchi, S.; Moriguchi, N.; Yamaguchi, S.; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sasaki, Y.; Yamane, S.; Kurosu, K.; Sawada, S.-I.; Akiyoshi, K. Templated Formation of Hydroxyapatite Nanoparticles from Self-Assembled Nanogels Containing Tricarboxylate Groups. Polymers 2012, 4, 1056-1064. https://doi.org/10.3390/polym4021056
Sasaki Y, Yamane S, Kurosu K, Sawada S-I, Akiyoshi K. Templated Formation of Hydroxyapatite Nanoparticles from Self-Assembled Nanogels Containing Tricarboxylate Groups. Polymers. 2012; 4(2):1056-1064. https://doi.org/10.3390/polym4021056
Chicago/Turabian StyleSasaki, Yoshihiro, Setsuko Yamane, Kei Kurosu, Shin-Ichi Sawada, and Kazunari Akiyoshi. 2012. "Templated Formation of Hydroxyapatite Nanoparticles from Self-Assembled Nanogels Containing Tricarboxylate Groups" Polymers 4, no. 2: 1056-1064. https://doi.org/10.3390/polym4021056



