Synthesis and Optical Study of a New Oligophenylene
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Analysis
2.2.1. Spectroscopic Analysis
2.2.2. Gel permeation Chromatography (GPC) and Osmometry Analysis
2.2.3. Thermal Study
2.2.4. Photoluminescence Study
2.2.5. Electrochemical Techniques
2.3. Synthesis
2.3.1. OMPA Synthesis
2.3.2. Synthesis of (Z)-2,3-bis(4-methoxyphenyl)acrylonitrile (BMPA)
2.3.3. Oligo BMPA (OBMPA) Synthesis
3. Results and Discussion
3.1. Synthesis and Characterization




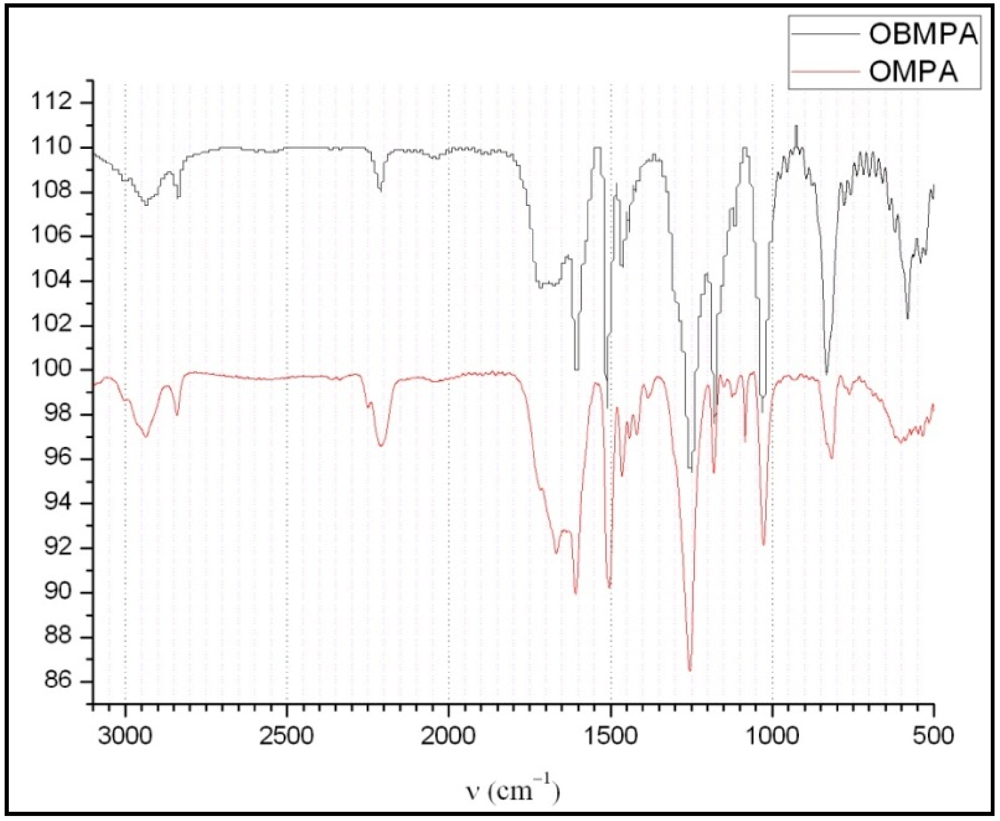
| Vibration | Aromatic ring C-H stretching | Methyl group C-H stretching | Nitrile C≡N stretching | Aromatic ring C=C stretching | Methoxy C-O-C |
|---|---|---|---|---|---|
| ν(cm−1) | 3,100 | 2,900 | 2,258 | 1,609, 1,512 ,1,470 | 1,250, 1,029 |
3.2. Electrochemical Study

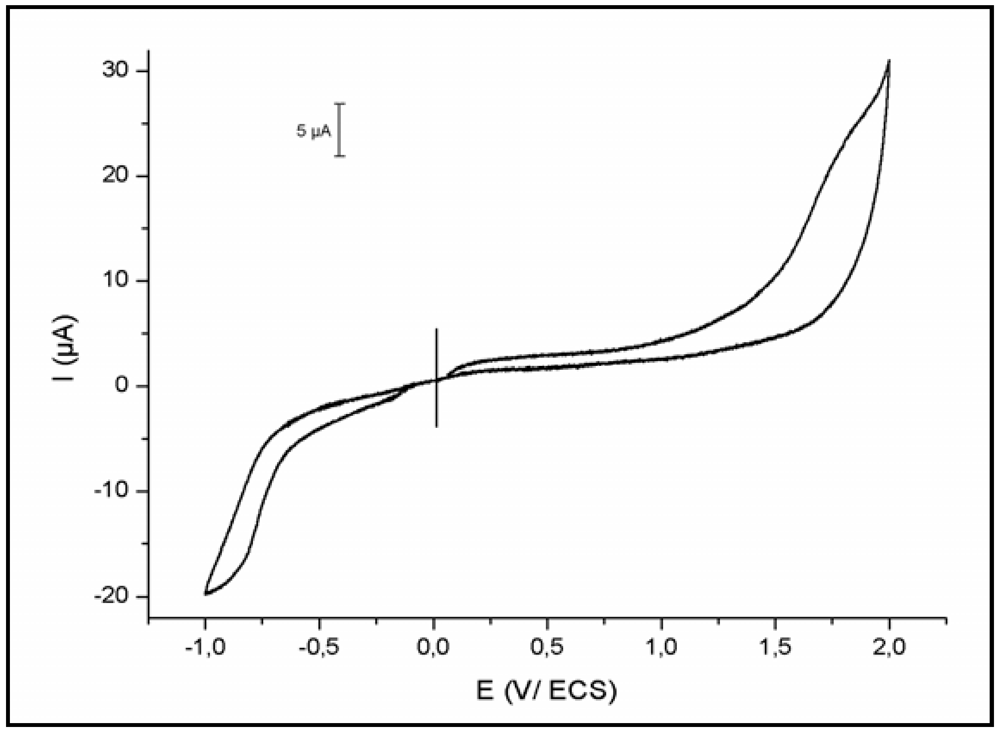
3.3. Thermal Study

3.4. Optical Properties
3.4.1. Optical Absorption
 ).
).
 ).
). 
3.4.2. Photoluminescence
Steady State PL

Time-Resolved Photoluminescence (TR-PL)


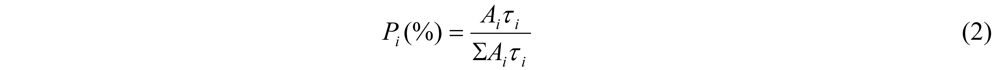


| Samples | τ1 (ns) P1% (A1) | τ2 (ns) P2% (A2) | τmean (ns) |
|---|---|---|---|
| OBMPA (solution) | 0.38 31 (9.5) | 2.22 68 (3.6) | 1.36 |
| OBMPA (film) | 0.034 56 (15.2) | 0.235 43 (1.67) | 0.11 |
| OMPA (solution) | 0.4 26 (8.7) | 2.32 74 (4.2) | 1.82 |
| OMPA (film) | 0.041 55 (14.6) | 0.25 45 (2) | 0.136 |
4. Conclusions
Acknowledgments
References
- Conjugated Polymers: Theory, Synthesis, Properties, and Characterization, 3rd; Skotheim, T.A.; Reynolds, J. (Eds.) CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007.
- Wallace, G.G.; Sprinks, G.M.; Kane-Maguire, L.A.P.; Teasdale, P.R. Conductive Electroactive Polymers: Intelligent Materials Systems, 2nd ed; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly(thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Higgins, S.J. Conjugated polymers incorporating pendant functional groups: Synthesis and characterization. Chem. Soc. Rev. 1997, 26, 247–257. [Google Scholar] [CrossRef]
- Leclerc, M.; Faid, K. Electrical and optical properties of processable polythiophene derivatives: Structure-property relationships. Adv. Mater. 1997, 9, 1087–1094. [Google Scholar] [CrossRef]
- Swager, T.M. The molecular wire approach to sensory signal amplification. Acc. Chem. Res. 1998, 31, 201–207. [Google Scholar] [CrossRef]
- Cravino, A.; Zerza, G.; Maggini, M.; Bucell, S.; Svensson, M.; Andersson, M.R.; Neugebauer, H.; Sariciftci, N.S. A novel polythiophene with pendant fullerenes: Toward donor/acceptor double-cable polymers. Chem. Commun. 2000, 24, 2487–2488. [Google Scholar]
- Cihaner, A.; Önal, A.M. Synthesis of a regular polymer containing pseudo-polyether cages. Synth. Met. 2005, 150, 39–45. [Google Scholar] [CrossRef]
- Roncali, J. Synthetic principles for bandgap control in linear π-conjugated systems. Chem. Rev. 1997, 97, 173–206. [Google Scholar] [CrossRef]
- Wang, H.-J.; Chan, L.-H.; Chen, C.-P.; Lin, S.-L.; Lee, R.-H.; Jeng, R.-J. Bulky side-chain density effect on the photophysical, electrochemical and photovoltaic properties of polythiophene derivatives. Polymer. 2011, 52, 326–338. [Google Scholar]
- Chen, J.-C.; Liu, Y.-C.; Ju, J.-J.; Chiang, C.-J.; Chern, Y.-T. Synthesis, characterization and hydrolysis of aromatic polyazomethines containing non-coplanar biphenyl structures. Polymer 2011, 52, 954–964. [Google Scholar] [CrossRef]
- Karsten, B.P.; Bijleveld, J.C.; Viani, L.; Cornil, J.; Gierschner, J.; Janssen, R.A.J. Electronic structure of small band gap oligomers based on cyclopentadithiophenes and acceptor units. J. Mater. Chem. 2009, 19, 5343–5350. [Google Scholar]
- Paul-Roth, C.; Rault-Berthelot, J.; Simonneaux, G.; Poriel, C.; Abdalilah, M.; Letessier, J. Electroactive films of poly(tetraphenylporphyrins) with reduced bandgap. J. Electroanal. Chem. 2006, 597, 19–27. [Google Scholar] [CrossRef]
- Rault-Berthelot, J.; Paul-Roth, C.; Poriel, C.; Juillard, S.; Ballut, S.; Drouet, S.; Simonneaux, G. Comparative behaviour of the anodic oxidation of mono-, di- and tetra-arylporphyrins: Towards new electroactive materials with variable bandgaps. J. Electroanal. Chem. 2008, 623, 204–214. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Müllen, K. Oligomers and polymers based on bridged phenylenes as electronic materials. Macromol. Rapid. Commun. 2007, 28, 1676–1702. [Google Scholar] [CrossRef]
- Poriel, C.; Liang, J.-J.; Rault-Berthelot, J.; Barrière, F.; Cocherel, N.; Slawin, A.M.Z.; Horhant, D.; Virboul, M.; Alcaraz, G.; Audebrand, N.; Vignau, L.; Huby, N.; Wantz, G.; Hirsch, L. Dispirofluorene—indenofluorene derivatives as new building blocks for blue organic electroluminescent devices and electroactive polymers. Chem. Eur. J. 2007, 13, 10055–10069. [Google Scholar]
- Yildirim, A.; Tarkuc, S.; Aka, M.; Toppare, L. Syntheses of electroactive layers based on functionalized anthracene for electrochromic applications. Electrochim Acta. 2008, 53, 4875–4882. [Google Scholar] [CrossRef]
- Manhart, S.A.; Adachi, A.; Sakamaki, K.; Okita, K.; Ohshita, J.; Ohno, T.; Hamaguchi, T.; Kunai, A.; Kido, J. Synthesis and properties of organosilicon polymers containing 9,10-diethynylanthracene units with highly hole-transporting properties. J. Organomet. Chem. 1999, 592, 52–60. [Google Scholar]
- Killian, J.G.; Gofer, Y.; Sarker, H.; Poehler, T.O.; Searson, P.C. Electrochemical synthesis and characterization of a series of fluoro-substituted phenylene-2-thienyl polymers. Chem. Mater. 1999, 11, 1075–1082. [Google Scholar] [CrossRef]
- Lu, Y.; Chena, H.; Houa, X.; Hub, X.; Ng, S.-C. A novel low band gap conjugated polymer based on N-substituted dithieno[3,2-b:2′,3′-d]pyrrole. Synth. Met. 2010, 160, 1438–1441. [Google Scholar] [CrossRef]
- Koyuncua, F.B.; Koyuncub, S.; Ozdemir, E.A. A Novel donor-acceptor polymeric electrochromic material containing carbazole and 1,8-naphtalimide as subunit. Electrochim. Acta. 2010, 55, 4935–4941. [Google Scholar] [CrossRef]
- Pan, X.Y.; Liu, S.P.; Chan, H.S.O.; Ng, S.C. Novel fluorescent carbazolyl-pyridinyl alternating copoloymers: Synthesis, characterization, and properties. Macromolecules 2005, 38, 7629–7635. [Google Scholar] [CrossRef]
- Janietz, S.; Bradley, D.D.C.; Grell, M.; Gieberler, C.; Inbasekaran, M. Electrochemical determination of the ionization potential and electron affinity of poly(9,9-dioctylfluorene). Appl. Phys. Lett. 1998, 73, 2453–2455. [Google Scholar] [CrossRef]
- Bredas, J.L.; Silbey, R.; Boudreaux, D.S.; Chance, R.R. Chain-length dependence of electronic and electrochemical properties of conjugated systems: Polyacetylene, polyphenylene, polythiophene, and polypyrrole. J. Am. Chem. Soc. 1983, 105, 6555–6559. [Google Scholar]
- Ben Amor, S.; Haj Said, A.; Chemek, M.; Ayachi, S.; Massuyeau, F.; Wéry, J.; Alimi, K.; Roudesli, S. Electrosynthesis and characterization of oligophenylene deriving from 4-(methoxyphenyl) acetonitrile. J. Mol. Struct. 2012. [Google Scholar] [CrossRef]
- Massuyeau, F.; Faulques, E.; Lefrant, S.; Majdoub, M.; Ghedira, M.; Alimi, K.; Wéry, J. Photoluminescence properties of new PPV derivatives. J. Luminesc. 2011, 131, 1541–1544. [Google Scholar] [CrossRef]
- Haj Said, A.; Ayachi, S.; Issaoui, C.; Wéry, J.; Alimi, K. Optical and vibrational studies on single walled carbon nanotubes/short oligo-para-methoxy-toluene composite. J. Appl Polym. Sci. 2011, 122, 1889–1897. [Google Scholar] [CrossRef]
- Chemli, M.; Haj Said, A.; Jaballah, N.; Fave, J.-L.; Majdoub, M. Synthesis and characterization of new electroluminescent poly(p-phenylene) derivative. Synt. Met. 2011, 161, 1463–1468. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amor, S.B.; Said, A.H.; Chemek, M.; Massuyeau, F.; Wéry, J.; Faulques, E.; Alimi, K.; Roudesli, S. Synthesis and Optical Study of a New Oligophenylene. Polymers 2012, 4, 1226-1241. https://doi.org/10.3390/polym4021226
Amor SB, Said AH, Chemek M, Massuyeau F, Wéry J, Faulques E, Alimi K, Roudesli S. Synthesis and Optical Study of a New Oligophenylene. Polymers. 2012; 4(2):1226-1241. https://doi.org/10.3390/polym4021226
Chicago/Turabian StyleAmor, Sarra Ben, Ayoub Haj Said, Mourad Chemek, Florian Massuyeau, Jany Wéry, Eric Faulques, Kamel Alimi, and Sadok Roudesli. 2012. "Synthesis and Optical Study of a New Oligophenylene" Polymers 4, no. 2: 1226-1241. https://doi.org/10.3390/polym4021226




