1. Introduction
When a material is introduced into a living organism and is exposed to circulating blood, its surface is quickly covered with proteins. The subsequent events depend primarily on the relative size of the material with respect to the sizes of blood vessels and phagocytic cells. Large particles for therapeutic embolisation injected via a catheter are designed to intentionally block vessels. These particles are then covered by a clot which isolates the material from direct blood contact. When smaller particles are transiently present within blood vessels, their fate depends on their size when compared with the diameter of the smallest blood vessels. Sub-millimetric particles or agglomerates of smaller particles are retained in lung capillaries that act as sieves, at least in humans. Sub-micronic particles,
i.e., nanoparticles (NP), which, as a result of their small size, may be able to circulate, are rapidly taken up by phagocytic cells. In fact, NPs are foreign bodies that trigger the defense systems. Thus, NPs are usually eliminated from bloodstream and sequestered within the organs of the Mononuclear Phagocytic System (MPS), mainly the liver and spleen. If these NPs are loaded with a drug aimed at treating liver cancer, this is a convenient way to deliver the drug directly to the target. If the drug has, for example, cardiotoxic properties, this is an elegant way to decrease side effects. However, delaying or avoiding this uptake is necessary if the aim is to obtain NPs as a drug delivery system with long-circulating properties or able to target another tissue or organ. A detailed review about nanocarriers can be found in Vauthier and Labarre [
1].
Nanoparticles can be composed of materials from various origins. In contrast to macromolecules of natural origin which can induce an immunogenic response, synthetic polymeric materials are usually not recognized as antigenic per se. However, such materials are foreign bodies which have to be quickly removed from the blood by the action of non-specific defense systems. In case of non-antigenic NPs, the protein system which is involved is usually the alternative pathway (AP) of the complement system (
Figure 1). The complement system is a surface-active system which plays a vital role in self-protection against “invaders” [
2]. The complement system per se is not able to stop and destroy “powerful invaders”, but it acts as a border guard. Its main roles are tagging the surface of the invader, recruiting and stimulating the phagocytic leukocytes.
An essential feature of the AP is the fact that this non-specific system remains permanently active at a background level. The key protein of the AP is C3, which is endowed with two active sites normally hidden or in an inactive conformation in circulating blood. However, a very small proportion of C3 is in a surface-active conformation. This molecule bearing a reactive site can bind to an accessible nucleophilic group, or is randomly deposited on any surface which it comes into contact with, including host cells, or is inactivated by water. Healthy cells are normally protected from developments of further complement activity as a result of interactions between receptors present on their surface and circulating control proteins such as protein H. On the other hand, foreign bodies devoid of such receptors are usually not protected. Thus an amplification process can be initiated from the site created by the randomly deposited surface-active protein. The presence of Mg2+ ions is required to start the amplification process, named the amplifying C3-convertase loop, which leads to an amplified conversion of C3 into the active fragments C3a and nascent C3b*. While the small fragment C3a remains into blood, the very transiently active large fragment C3b* can bind to the surface in the immediate vicinity, or is inactivated by water.
Figure 1.
The alternative pathway of complement. Binding of Mg2+ and B results in amplified C3 cleaving, whereas binding of H results in escape.
Figure 1.
The alternative pathway of complement. Binding of Mg2+ and B results in amplified C3 cleaving, whereas binding of H results in escape.
Some detailed mechanisms of complement activation as a function of the material surface chemistry are still being debated. However, it is clear that the surface of the foreign body is decorated by some large fragments such as C3b and its derivatives. The fragments C3a generated during conversion of C3, and also C5a generated by the subsequent conversion of C5 are able to recruit and to activate phagocytic cells, even at a very low concentration. The activated phagocytes are then able to adhere to the tagged surface and start to engulf the foreign body by phagocytosis. It should be noted that macrophages are themselves able to secrete C3 even in a “complement-depleted” medium.
In this paper, we examine some strategies aimed to avoid fast phagocytosis of NPs by MPS. Complement activation and deposition of other proteins can be modulated by changing the nature and structure of the hydrogel covering the surface.
2. Results and Discussion
2.1. Modulating the Events Occurring at the Blood/Polymer Interface by PEGylation
Trying to control the events occurring on the surface of a material in contact with blood
in vivo is a real challenge, since a large number of processes are involved. Adsorption of proteins on the surface is the predominant phenomenon. This fast, spontaneous and dynamic process was first described by Vroman [
3]. Conformational changes may follow adsorption, especially when large proteins or protein complexes are involved. These changes can occur immediately after adsorption on the material surface, but also on proteins already deposited on the surface of the material [
4]. Demasking of active enzymatic sites normally hidden or inactive in the liquid phase can result from such conformational changes on surfaces.
Adsorption of proteins at the interface cannot be completely prevented, but it has been known for a long time that increasing hydrophilicity of the solid phase results in more reversibility of proteins adsorption.
In vitro, this strategy has been widely used in order to separate and recover proteins in chromatographic systems.
In vivo, coating the surface of intravenously injected hydrophobic polystyrene NPs with non ionic surfactants such as Poloxamers, resulted in a slight increase in their circulation time [
5]. Poloxamers are amphiphilic copolymers made of poly(ethylene glycol) (PEG) as hydrophilic segments and poly(propylene glycol) as hydrophobic segments. The hydrophobic part of Poloxamers could stick to polystyrene while the hydrophilicity of the outermost surface of the NPs was increased. However, such coatings present on the surface of biodegradable NPs suitable for drug delivery were too rapidly displaced by blood proteins [
6]. Thus it became obvious that covalent binding of hydrophilic moieties on the surface was required, but some early experimental results were disappointing [
7].
2.2. The Steric-Hindrance Concept Illustrated by PEO/PEG Brushes
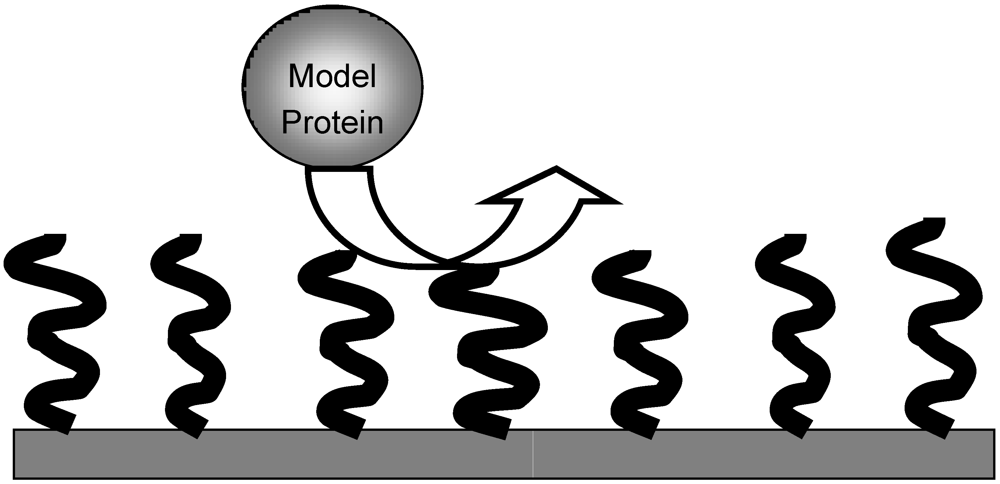
In the early 90s, a model of hydrophilic brush able to repel proteins was described [
8]. In this model system, molecules of hydrated poly(ethylene oxide) (PEO) of similar length were bound by one end to a solid surface with a given surface density. Providing that surface density and length of PEO were sufficient, it was calculated that large model proteins could be efficiently repelled (
Figure 2).
Experimental data have shown that the model was relevant
in vitro and
in vivo, at least in case of NPs. NPs obtained from block copolymers polylactides-block-poly(ethylene oxide) methyl ether (PLA-PEO) were able to circulate in the bloodstream for several hours [
9,
10]. This unique feature was due to the fact that PLA-PEO block copolymers were able to self-organize in aqueous medium as large micelles of polymeric surfactants. PEO blocks were organized on the surface of NP as a hydrated brush and linked to the hydrophobic core of PLA. Such long-circulating NP activated neither the complement system [
11], nor the coagulation system [
12].
Figure 2.
Model of repelling brush according to Jeon
et al. [
8]. Model hydrated poly(ethylene oxide) (PEO) chains of similar length are bound to the surface of the material by one end with a given density. A large model protein is repelled by the water-swollen brush.
Figure 2.
Model of repelling brush according to Jeon
et al. [
8]. Model hydrated poly(ethylene oxide) (PEO) chains of similar length are bound to the surface of the material by one end with a given density. A large model protein is repelled by the water-swollen brush.
Plasma protein deposition was influenced by density and length of PEO [
13]. It was also shown that NPs on which PEG was bound by both ends as loops (
i.e., side-on) were also poor activators of complement [
14]. Thus it could be concluded that both types of structure, brush or loops, could efficiently repel large proteins, at least in case of PEG/PEO.
Attempts were made to obtain macroscopic surfaces covered with such coatings. It was shown that binding PEG/PEO or dextran “end-on” or “side-on” was efficient at decreasing adsorption of a large protein such as fibrinogen [
15]. However, there was no experimental approach to what could happen in the presence of the amplified defense systems in blood. In fact, obtaining such a dense and well organized coating on the surface of a large piece of material is not easy, in contrast to NPs which are self-organized systems. If a defect in the coating occurs, the amplified defense systems can be triggered [
16,
17].
2.3. Towards Biomimetic Strategies
As already mentioned, healthy cells are protected against self-attack by the complement system. Sialic acid present on the surface of human cells in contact with blood is able to inhibit the amplifying loop of human complement by interacting with circulating regulatory proteins such as protein H [
18,
19]. Strategies of camouflage have evolved over time in some pathogens which are covered by sialic acid, allowing them to escape recognition by complement and as a result making them very dangerous for humans [
20,
21].
This strategy of camouflage can be adapted to polymer surfaces by covering them with entities able to cooperate with circulating control proteins. Sialic acid and poly(sialic acid) were obvious candidates for this application, and have been shown to be efficient in case of liposomes. However, a major problem in the development of poly(sialic acid)-coated drug delivery systems can be the difficulty of producing the camouflaging material.
As mentioned above, poly(sialic acids) are extracted from highly pathogenic bacteria strains. Thus this development is not devoid of risks for humans. Heparin is also a candidate, as it has been shown to cooperate with protein H to block C3 activation [
22,
23]. In addition, it has been used as a drug in humans for years. Inhibition of complement activation by polymer surfaces bearing heparin was obtained, especially when heparin was bound by one end [
24,
25]. Long-circulating and very low complement activating NPs were also obtained from block copolymers composed of heparin and acrylic polymers (Hep-PMMA) [
26,
27]. It was also observed that despite a rather high amount of heparin bound to the NPs and injected to mice, no bleeding was observed and long circulation into blood was obtained, showing that the effects were localized to the surface of the NPs.
2.4. Effects Resulting from the Structure of Polysaccharidic Surfaces
Control NPs covered with dextran were prepared using a similar synthetic route (Dex-PMMA). Surprisingly, these NPs were also low complement activators and circulated for a long time, whereas uncovered PMMA NPs were strong activators and were eliminated within a few minutes from bloodstream. Similarly, NPs obtained by anionic polymerization of isobutylcyanoacrylate (IBCA) in the presence of dextran were taken up within a few minutes by macrophages and stored in the organs of MPS [
28].
It was already known that Sephadex
®,
i.e., crosslinked dextran, was a strong activator of complement [
29]. It has been shown that activation was linked to the presence of many hydroxyl groups and these groups were also present on the surface of the Dex-PMMA NPs.
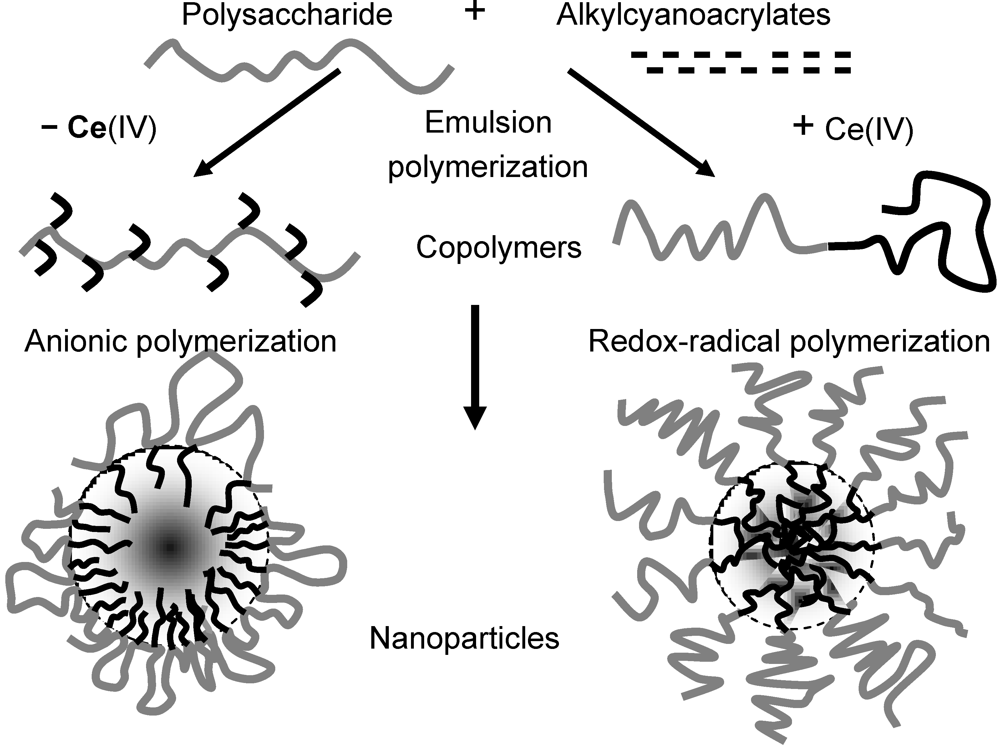
In order to understand the reasons of these apparent inconsistencies, we investigated the polymers obtained by reactions of polymerization of IBCA initiated by dextran or other polysaccharides in the presence or absence of Ce(IV) ions in acidic aqueous medium. By using a monomer which was able to polymerize in the presence of dextran either through a redox-radical initiation mechanism or through an anionic mechanism, we were able to compare some properties of NPs with a similar composition but with different surface structures. The details of these emulsion polymerizations can be found in Bertholon
et al. [
30]. Briefly, in such an acidic aqueous medium, the rate of the anionic polymerization initiated by the hydroxyl groups in the absence of Ce(IV) was slowed down, whereas the polysaccharidic chain was irreversibly cleaved when Ce(IV) ions were present, transiently creating an active radical site at the cleavage site. Thus biblock copolymers were obtained through redox-radical polymerization and graft copolymers through anionic polymerization. NPs spontaneously forming in the aqueous medium were endowed with a core-shell structure. In both cases, the shell was polysaccharidic, but a brush was probably obtained from the biblock copolymers and loops from the graft copolymers (
Figure 3).
Activation of complement was tested in human serum supplemented with divalent ions, in the presence of NPs covered either with dextran or with chitosan of different length and surface structure. The detailed results can be found in Bertholon
et al. [
31]. As briefly shown in
Table 1, complement was moderately activated when the polysaccharides were short, whatever their structure. Complement activation decreased to control level in the presence of both NPs when increasing molecular weight of polysaccharides in the form of brush, whereas it increased in case of both NPs with increasing molecular weight of polysaccharides in the form of loops. The results emphasize the prime importance of the configuration of the polysaccharidic chains in the surface-coating layer in modulating the reaction of the OH groups with complement proteins. Thus, depending on the configuration, polysaccharide chains with the same molecular weight and containing the same amount of OH groups may or may not induce complement activation. These opposing effects were attributed to the combined effects of the repelling brush of the polysaccharide and to the availability of OH groups on the polysaccharide to the complement protein C3 in the loop configuration of the polysaccharide chains.
Adsorption of proteins from citrated human plasma on NPs covered with long (71 × 10
3 Da) or short (15 × 10
3 Da) dextran as a brush or loops was tested after five minutes of contact. As shown in
Table 2, the capacity to adsorb proteins was reduced on NPs bearing a brush with longer dextran chains when compared with NPs bearing loops of the same dextran or a shorter brush. ApoA-1 was found to be the main protein adsorbed on both types of NP bearing the longer dextran, whereas a fragment of fibrinogen was found as the main species adsorbed on both types of NPs bearing the shorter dextran. More details can be found in Labarre
et al. [
32]. ApoA-1 was also found as second or third protein adsorbed on the surface of all NPs, in accordance to the results obtained by Cornelius
et al. [
33]. Relevance of such adsorptions to
in vivo fate of injected NPs is an open question.
Figure 3.
Nanoparticles formed by aqueous emulsion polymerization of alkylcyanoacrylates in the presence of polysaccharides at pH1 with or without Ce(IV) ions.
Figure 3.
Nanoparticles formed by aqueous emulsion polymerization of alkylcyanoacrylates in the presence of polysaccharides at pH1 with or without Ce(IV) ions.
Table 1.
Complement activation measured in human serum in the presence of nanoparticles (NPs) obtained by aqueous emulsion polymerization of isobutylcyanoacrylate (IBCA) on either dextran or chitosan of different molecular weights in the presence or absence of Ce(IV) ions. Configuration: B = brush, L = loops. Complement activation was assessed by evaluating conversion of C3 into C3b by crossed immuno-electrophoresis of C3 after 1 h incubation at 37 °C of equivalent surface areas (2000 cm
2) of NPs in human serum diluted in veronal buffered saline supplemented with divalent ions. More data can be found in Bertholon
et al. [
31].
Table 1.
Complement activation measured in human serum in the presence of nanoparticles (NPs) obtained by aqueous emulsion polymerization of isobutylcyanoacrylate (IBCA) on either dextran or chitosan of different molecular weights in the presence or absence of Ce(IV) ions. Configuration: B = brush, L = loops. Complement activation was assessed by evaluating conversion of C3 into C3b by crossed immuno-electrophoresis of C3 after 1 h incubation at 37 °C of equivalent surface areas (2000 cm2) of NPs in human serum diluted in veronal buffered saline supplemented with divalent ions. More data can be found in Bertholon et al. [31].
|
| Dextran (Da) | 104 B | 104 L | 105 B | 105 L |
| C3b/C3 + C3b | 0.47 | 0.40 | 0.25 | 1.0 |
| Chitosan (Da) | 104 B | 104 L | 7 × 104 B | 7 × 104 L |
| C3b/C3 + C3b | 0.47 | 0.47 | 0.10 | 0.55 |
Table 2.
Evaluation of proteins adsorption from citrated human plasma. Sample volumes of Dextran-PIBCA NPs with equivalent surface area (3000 cm2) were incubated for 5 min with citrated plasma at 37 °C. Proteins and protein fragments were desorbed by SDS and analyzed by 2-D PAGE. Dex71-B and Dex71-L = NP obtained from dextran of MW 71 × 103 Da respectively with a brush and with loops on the surface. Dex15-B and Dex15-L = NP obtained from dextran of MW 15 × 103 Da.
Table 2.
Evaluation of proteins adsorption from citrated human plasma. Sample volumes of Dextran-PIBCA NPs with equivalent surface area (3000 cm2) were incubated for 5 min with citrated plasma at 37 °C. Proteins and protein fragments were desorbed by SDS and analyzed by 2-D PAGE. Dex71-B and Dex71-L = NP obtained from dextran of MW 71 × 103 Da respectively with a brush and with loops on the surface. Dex15-B and Dex15-L = NP obtained from dextran of MW 15 × 103 Da.
| Sample | Total amount desorbed (cpm) | Predominant protein or fragment |
|---|
| First | Second | Third |
|---|
| Name | Amount | Name | Amount | Name | Amount |
|---|
| Dex71-B | 11 | ApoA-1 | 4.1 | ApoC-II | 3.0 | Fg-α-chain | 1.5 |
| Dex15-B | 22 | Fg-α-chain | 7.8 | ApoA-1 | 6.0 | Fg-γ-chain | 3.3 |
| Dex71-L | 33 | ApoA-1 | 9.4 | Fg-β-chain | 7.0 | Albumin | 5.0 |
| Dex15-L | 96 | Fg-α-chain | 27.6 | Fg-β-chain | 15.3 | ApoA-1 | 14.4 |
2.5. Examples of Uses of the Brush-Bearing NPs for Carrying Particular Drugs
Loading a drug into NPs and releasing it is not always easy, especially if the drug is a rather large molecule. In addition, some fragile drugs such as hemoglobin or nucleotides have to be protected from enzymatic degradation. The shell of core-shell NPs can provide accommodation for these drugs, providing that the drug could be loaded and retained in an appropriate shell. The drug is not entrapped in the core of the NP, but is protected against large proteins and enzymes while remaining accessible to small molecules.
Hemoglobin could be loaded in the polysaccharidic brush made of heparin or carboxylated dextran covering NPs without changing the low or very low complement activating capacity of the NPs. The loaded hemoglobin was accessible to gases and kept its activity [
34].
A siRNA specific for an experimental mice tumor could be loaded in the chitosan brush of NPs. After intravenous injection to nude mice bearing such a tumor, the specific siRNA could be delivered to the tumor if the NPs were small enough. Tumoral growth was inhibited only in the presence of the NPs delivering specific siRNA and not in the presence of the NPs delivering a non-specific one [
35].
3. Conclusions
Nanoparticles can be used as drug carriers to protect sensitive drugs from degradation, or to protect tissues and organs from toxic drugs side effects. Classical NPs are “foreign bodies”, quickly taken up by macrophages. They can carry drugs to liver and spleen.
Long-circulating and/or low complement activating NPs can be obtained from amphiphilic PEO/PEG copolymers with the hydrophilic part forming brushes or loops on the surface of the core-shell NPs.
Core-shell NPs can also be obtained from polysaccharidic graft or block amphiphilic copolymers. Complement activation by the NPs and proteins adsorption depend on the chains’ structure, nature and the molecular weight of polysaccharidic shell. Low complement activating NPs can be obtained if polysaccharide is present as a long enough brush on the surface.
Fragile molecules such as hemoglobin or siRNA can be loaded and protected by the appropriate brush shells. The low complement activation and/or long-circulating properties are retained, making the delivery of siRNA to the tumor possible by intravenous route or prolonged presence of active hemoglobin in the circulation.