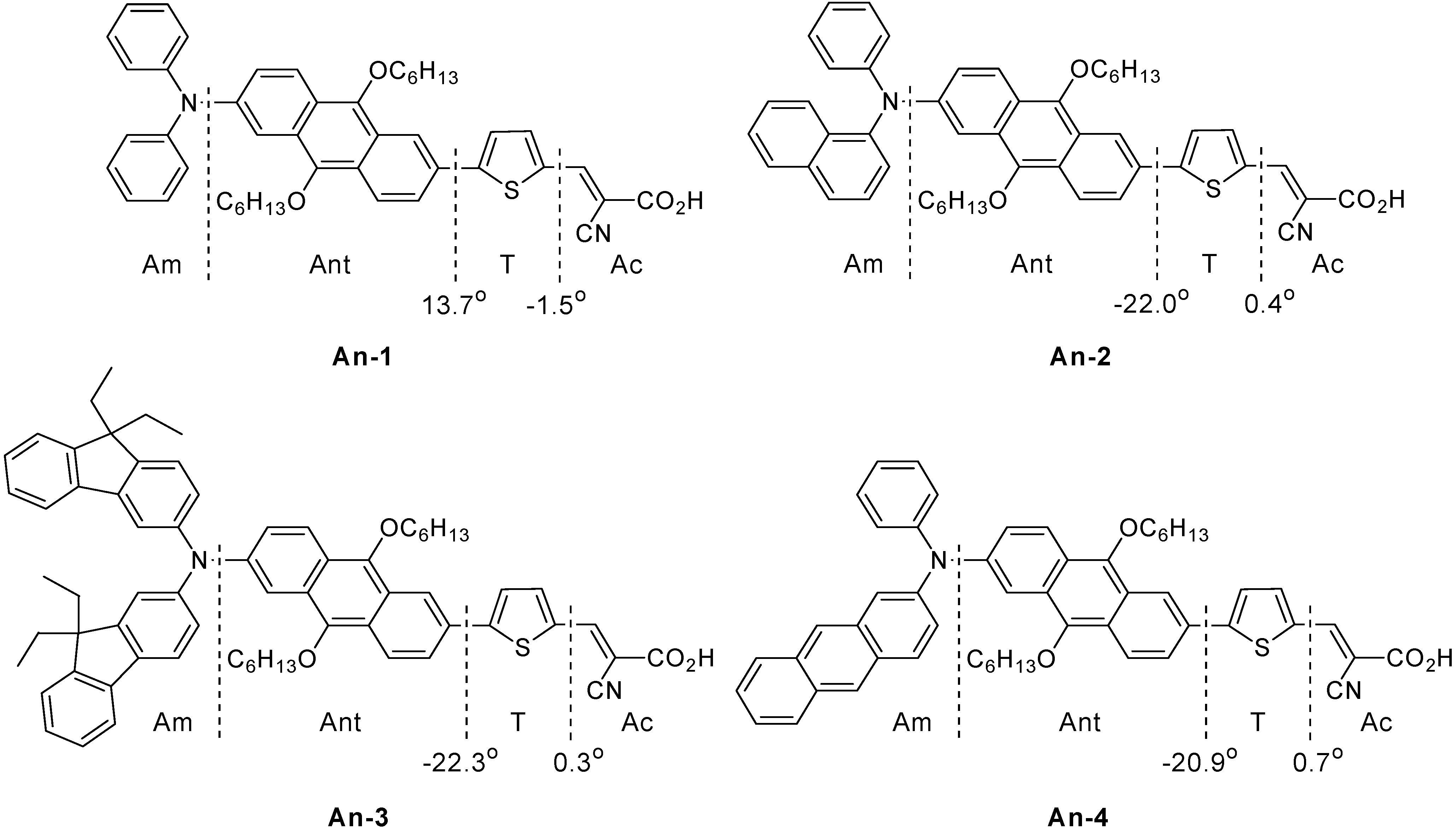
Novel Organic Sensitizers Containing 2,6-Difunctionalized Anthracene Unit for Dye Sensitized Solar Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Synthesis
2.3. Devices Fabrication
2.4. Quantum Chemistry Computation
3. Results and Discussion
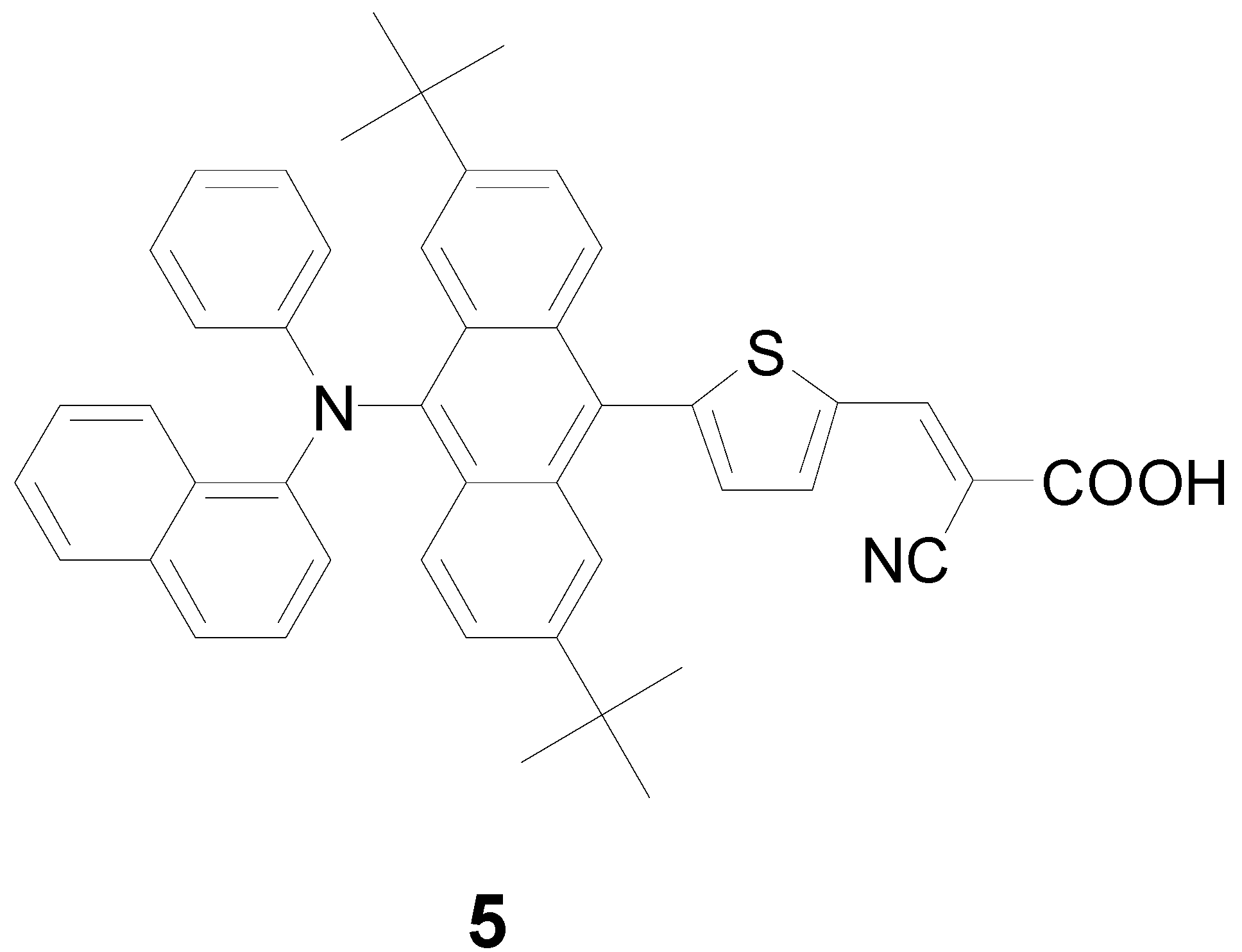
3.1. Synthesis

3.2. Optical Properties
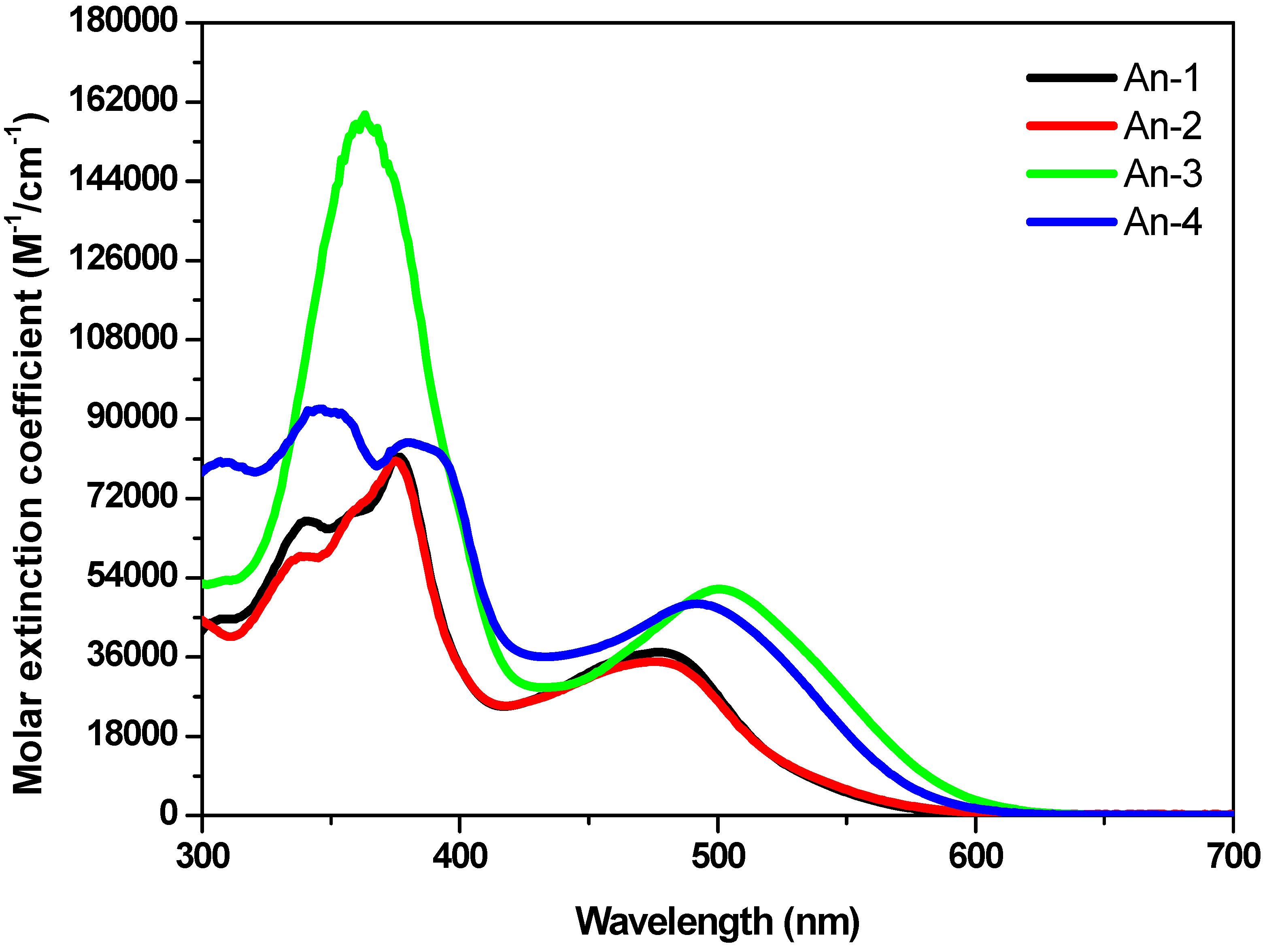
| Dye | λabs (ε × 10−4 M−1 cm−1)a nm | E1/2 (ox)b mV | Eoxc V | E0-0d eV | E0-0*e V |
|---|---|---|---|---|---|
| An-1 | 479 (3.70), 376 (8.14) | 348 | 1.05 | 2.29 | −1.24 |
| An-2 | 477 (3.48), 375 (8.05) | 360 | 1.06 | 2.29 | −1.23 |
| An-3 | 500 (5.13), 363 (15.91) | 312 | 1.01 | 2.12 | −1.11 |
| An-4 | 493 (4.81), 379 (8.47) | 356 | 1.06 | 2.14 | −1.08 |
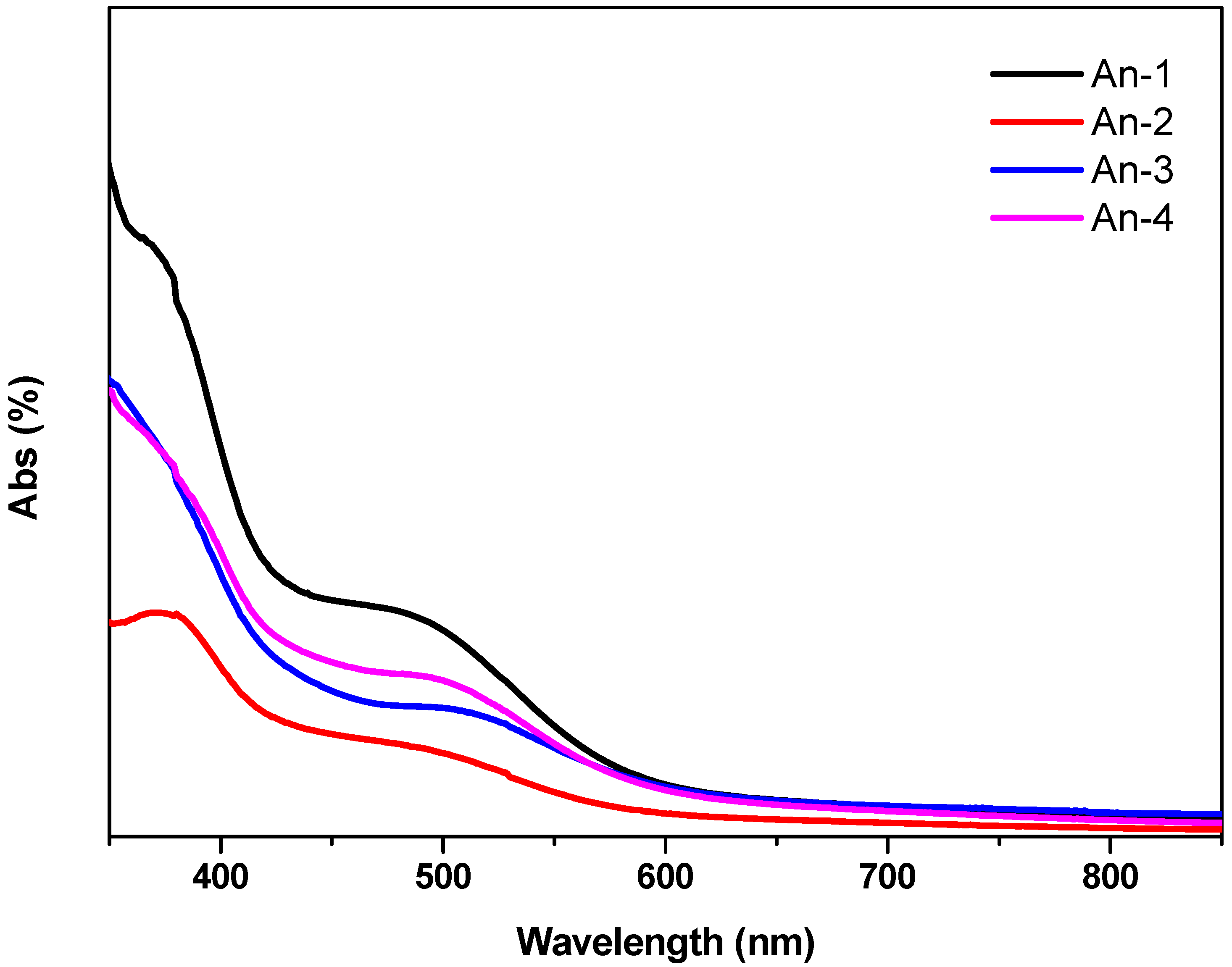
3.3. Optical Properties
3.4. Photovoltaic Device Performances
| Cell | VOC (V) | JSC (mA/cm2) | (%) | FF | Dye Loading (mol/cm2) |
|---|---|---|---|---|---|
| An-1 | 0.62 | 7.10 | 2.85 | 0.65 | 3.97 × 10−7 |
| An-2 | 0.64 | 6.06 | 2.61 | 0.67 | 3.17 × 10−7 |
| An-3 | 0.59 | 7.30 | 2.88 | 0.67 | 2.88 × 10−7 |
| An-4 | 0.55 | 4.52 | 1.62 | 0.65 | 3.14 × 10−7 |
| N719 | 0.77 | 13.87 | 7.11 | 0.67 | – |
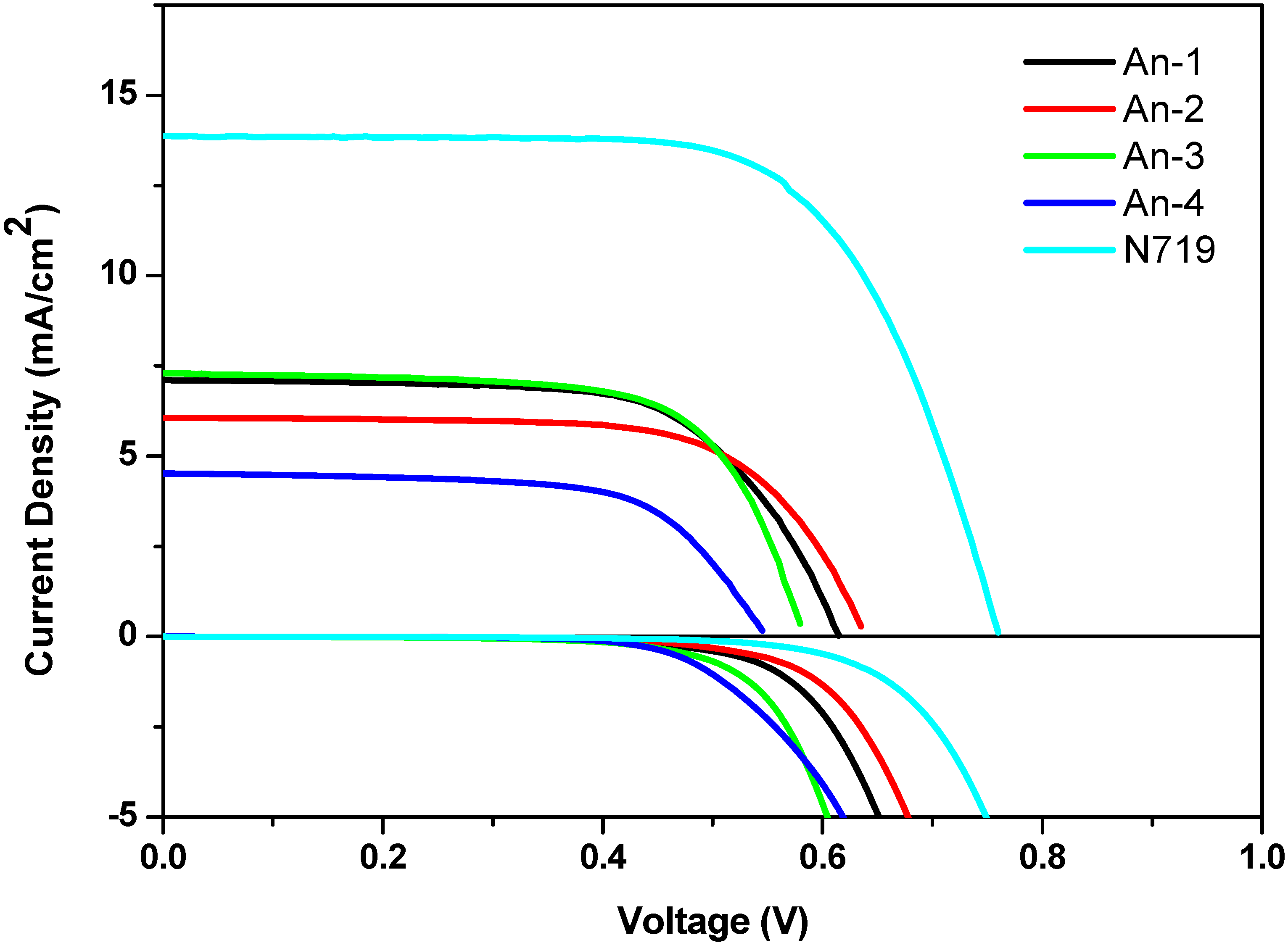
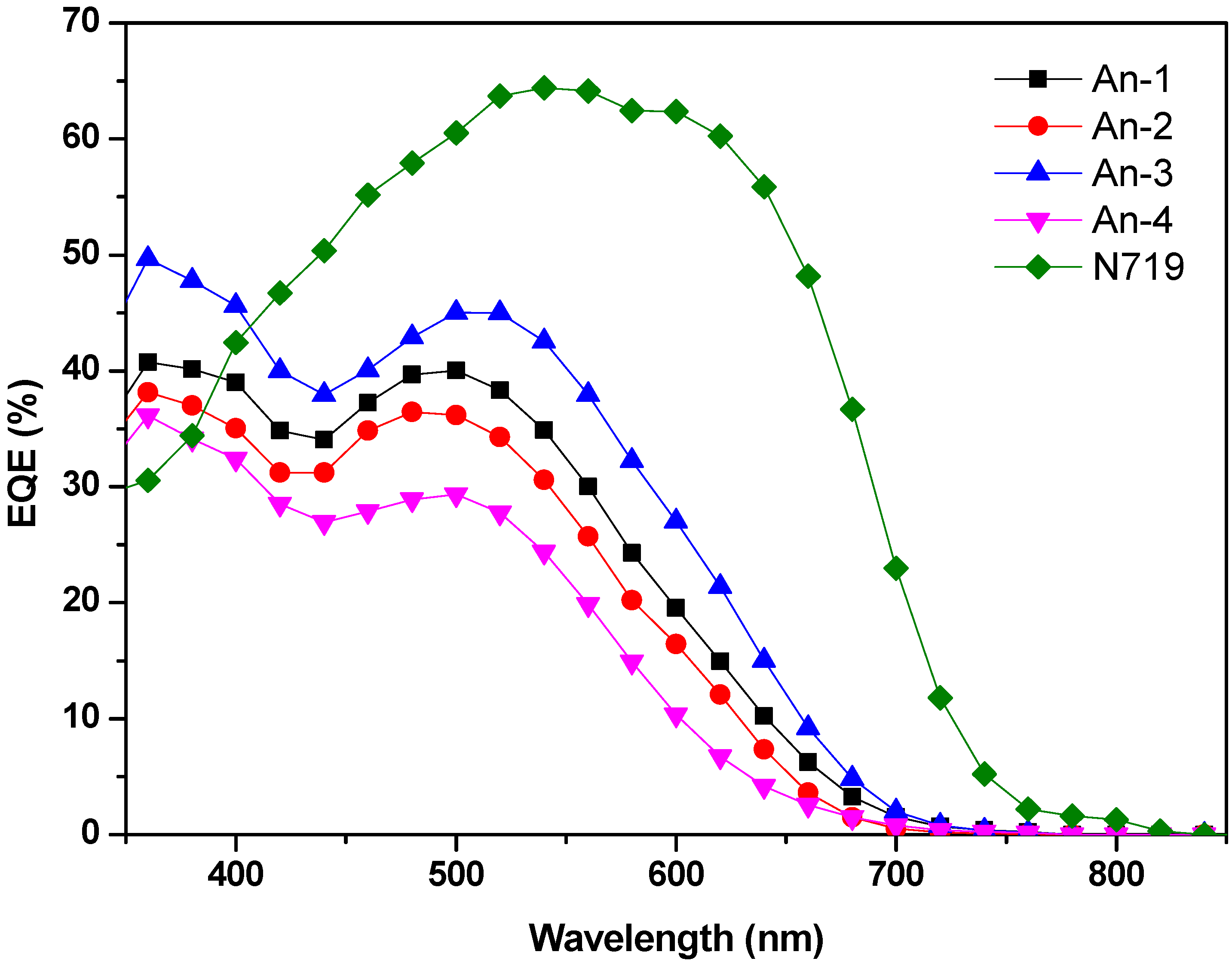
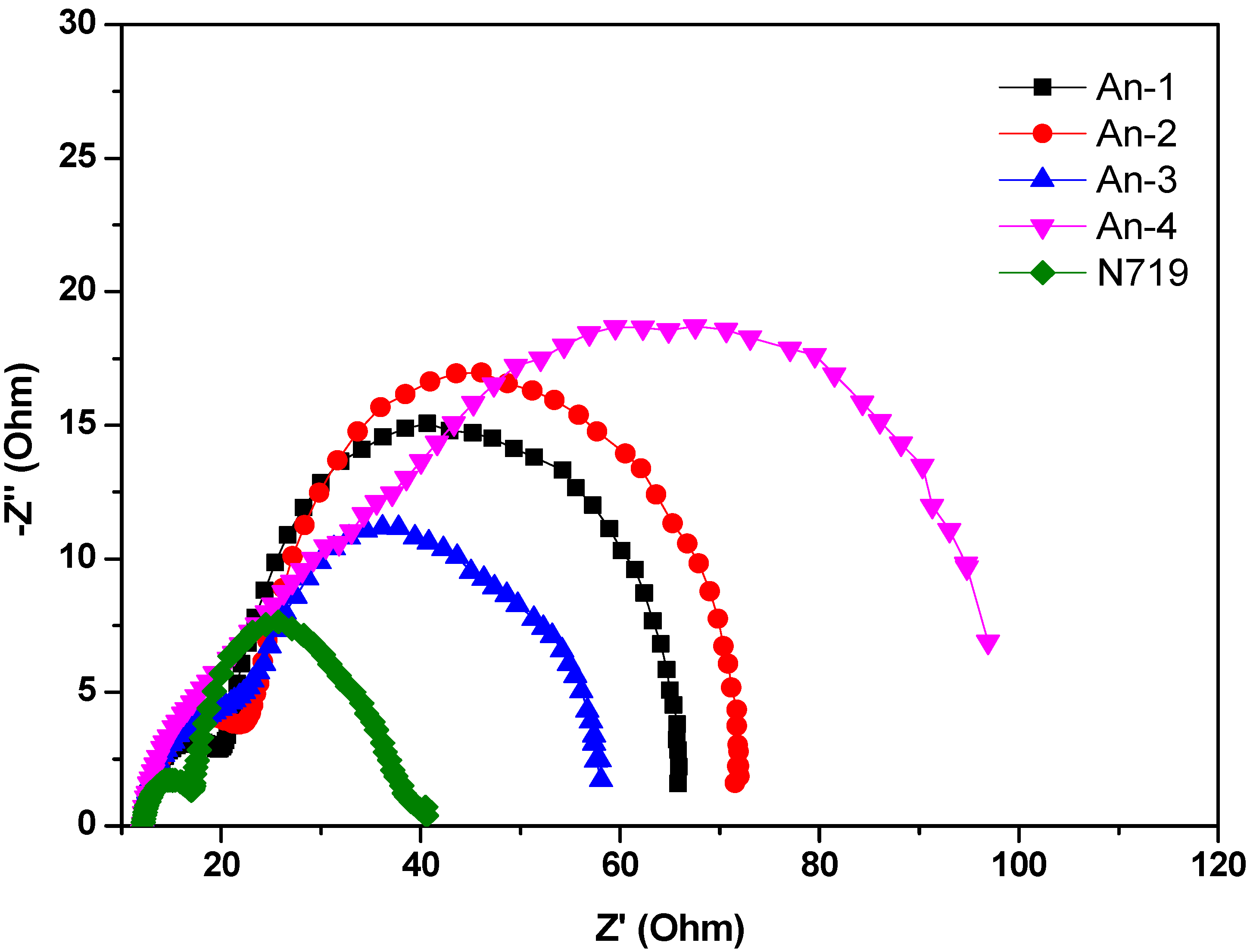
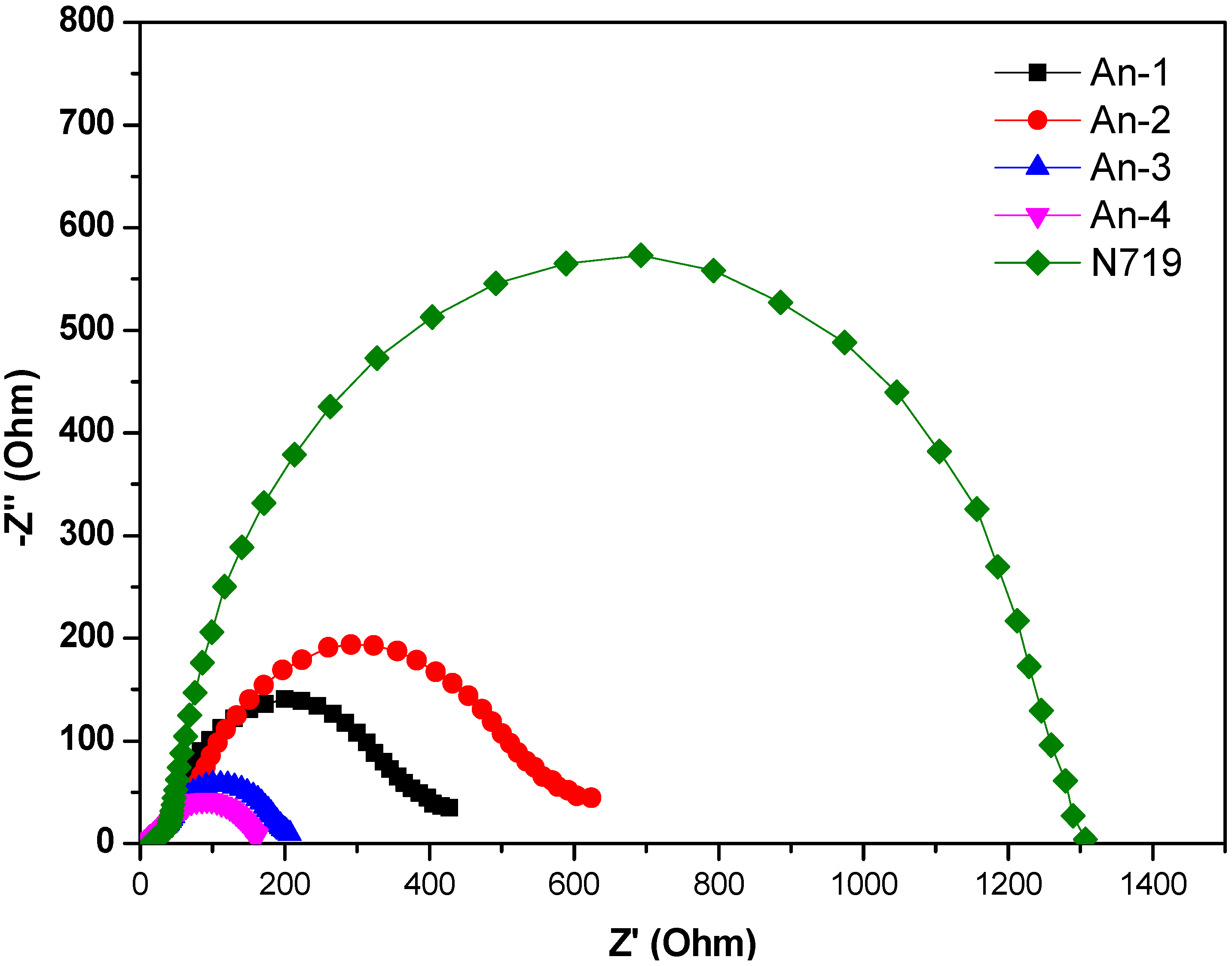
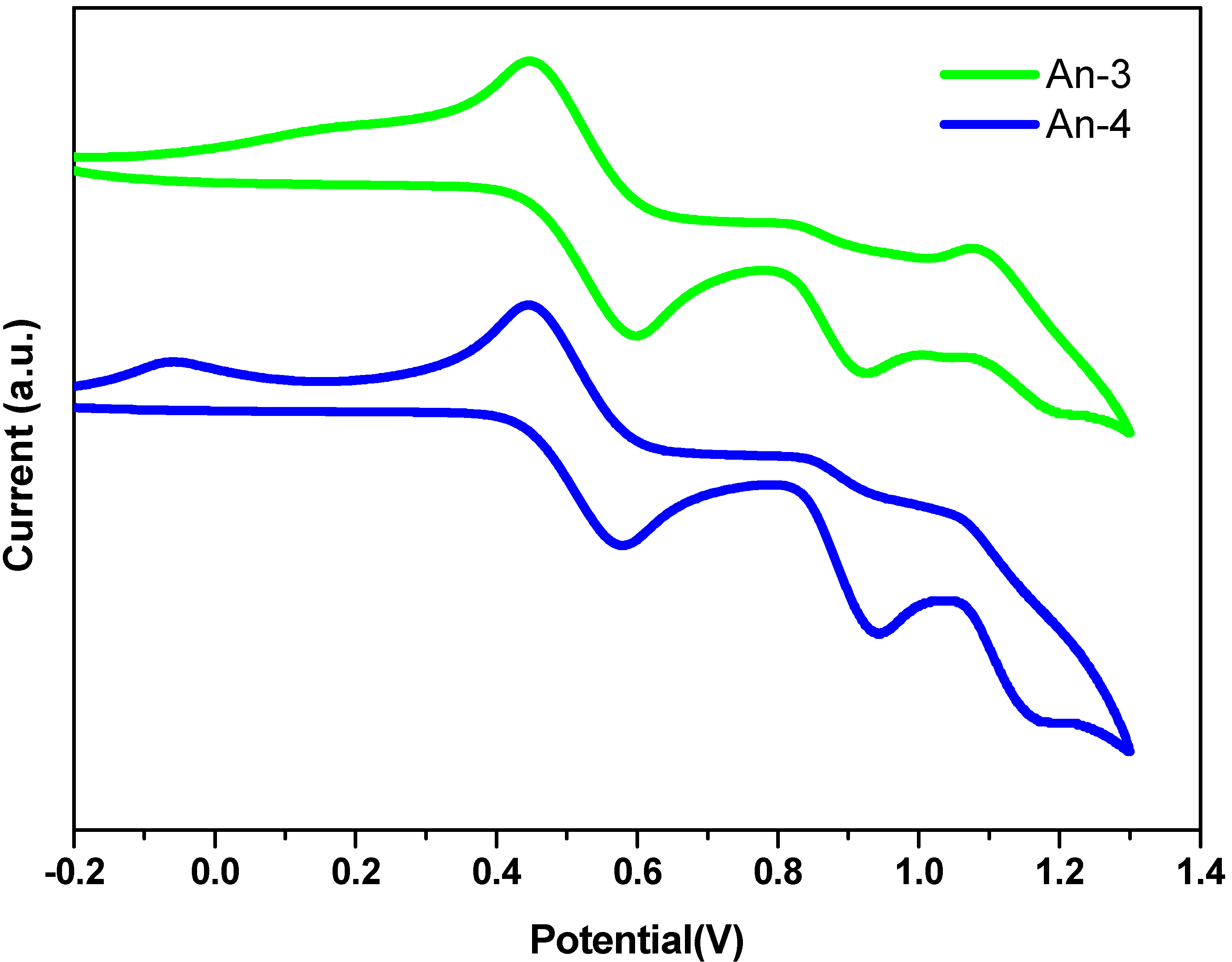
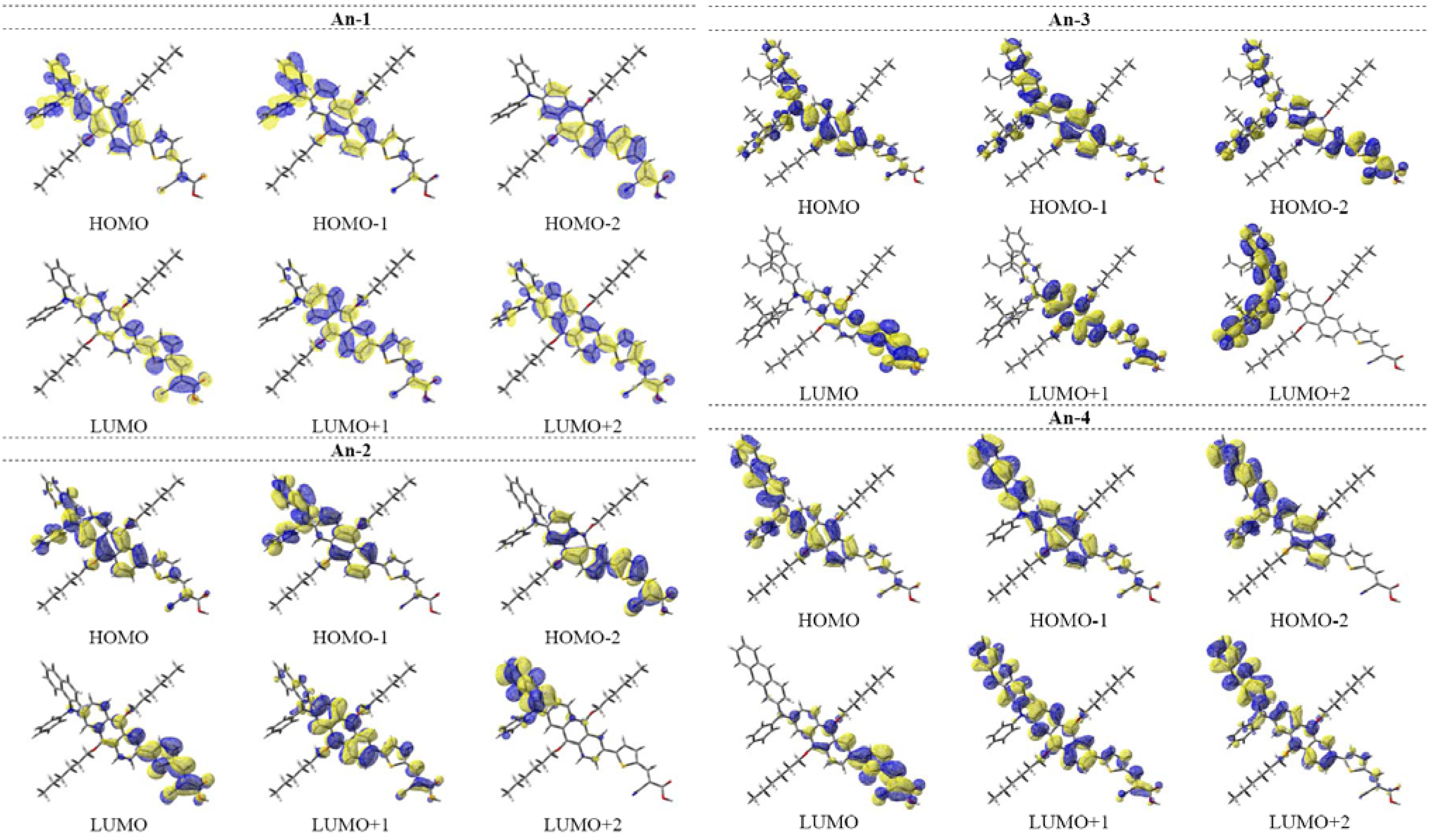
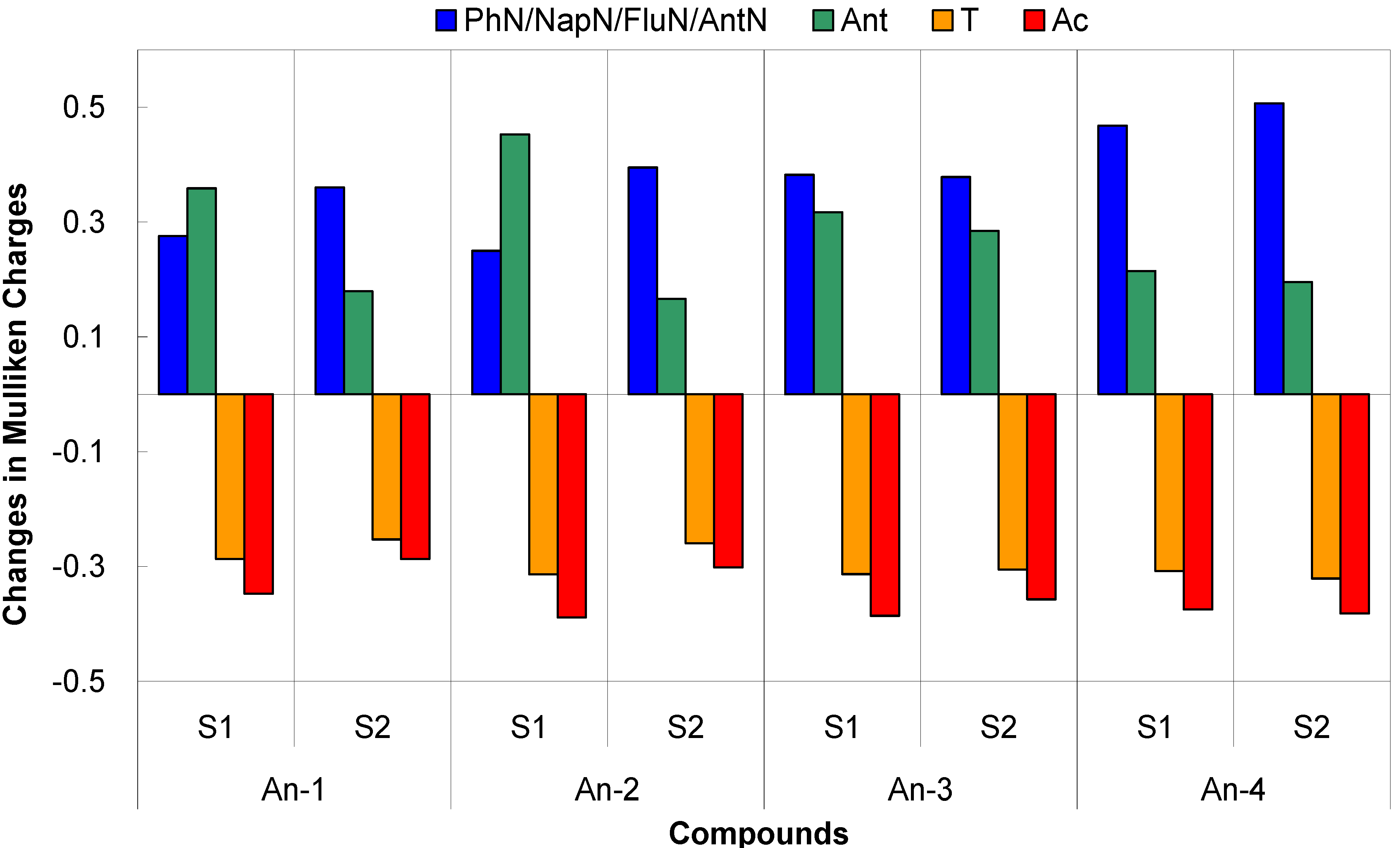
3.5. Theoretical Calculations

| dye | State | excitation a | λcal, eV | f b | ∆(Mulliken charge),c |e| | f × ∆q |
|---|---|---|---|---|---|---|
| An-1 | S1 | H → L (99%) | 2.07 | 0.57 | PhN: 0.28 | −0.20 |
| Ant: 0.36 | ||||||
| T: −0.29 | ||||||
| Ac: −0.35 | ||||||
| S2 | H2 → L (5%) | 2.63 | 0.01 | PhN: 0.36 | 0.00 | |
| H1 → L (71%) | Ant: 0.18 | |||||
| H → L1 (21%) | T: −0.25 | |||||
| Ac: −0.29 | ||||||
| S3 | H1 → L (89%) | 2.79 | 0.06 | PhN: 0.38 | −0.01 | |
| Ant: −0.10 | ||||||
| T: −0.11 | ||||||
| Ac: −0.17 | ||||||
| S4 | H2 → L (90%) | 3.19 | 1.06 | PhN: 0.03 | −0.13 | |
| Ant: 0.06 | ||||||
| T: 0.03 | ||||||
| Ac: −0.12 | ||||||
| An-2 | S1 | H → L (99%) | 2.09 | 0.55 | NapN: 0.25 | −0.21 |
| Ant: 0.45 | ||||||
| T: −0.31 | ||||||
| Ac: −0.39 | ||||||
| S2 | H1 → L (72%) | 2.65 | 0.03 | NapN: 0.40 | −0.01 | |
| H → L1 (21%) | Ant: 0.17 | |||||
| T: −0.26 | ||||||
| Ac: −0.30 | ||||||
| S3 | H1 → L (24%) | 2.81 | 0.06 | NapN: 0.35 | −0.01 | |
| H → L1 (74%) | Ant: −0.07 | |||||
| T: −0.11 | ||||||
| Ac: −0.17 | ||||||
| S4 | H → L2 (91%) | 3.16 | 0.20 | NapN: −0.49 | 0.00 | |
| Ant: 0.46 | ||||||
| T: 0.03 | ||||||
| Ac: 0.01 | ||||||
| An-3 | S1 | H → L (99%) | 1.99 | 0.60 | FluN: 0.38 | −0.23 |
| Ant: 0.32 | ||||||
| T: −0.31 | ||||||
| Ac: −0.39 | ||||||
| S2 | H1 → L (85%) | 2.55 | 0.00 | FluN: 0.38 | 0.00 | |
| H → L1 (10%) | Ant: 0.28 | |||||
| T: −0.31 | ||||||
| Ac: −0.36 | ||||||
| S3 | H1 → L (12%) | 2.69 | 0.09 | FluN: 0.44 | −0.01 | |
| H → L1 (85%) | Ant: −0.23 | |||||
| T: −0.08 | ||||||
| Ac: −0.13 | ||||||
| S4 | H2 → L (53%) | 3.18 | 0.60 | FluN: −0.03 | −0.06 | |
| H → L2 (36%) | Ant: 0.15 | |||||
| T: −0.01 | ||||||
| Ac: −0.11 | ||||||
| An-4 | S1 | H → L (99%) | 1.98 | 0.55 | AntN: 0.47 | −0.21 |
| Ant: 0.21 | ||||||
| T: −0.31 | ||||||
| Ac: −0.37 | ||||||
| S2 | H1 → L (95%) | 2.44 | 0.06 | AntN: 0.51 | −0.02 | |
| Ant: 0.20 | ||||||
| T: −0.32 | ||||||
| Ac: −0.38 | ||||||
| S3 | H → L1 (93%) | 2.59 | 0.14 | AntN: 0.24 | −0.01 | |
| Ant: −0.11 | ||||||
| T: −0.04 | ||||||
| Ac: −0.08 | ||||||
| S4 | H → L2 (92%) | 2.82 | 0.01 | AntN: −0.13 | 0.00 | |
| Ant: 0.16 | ||||||
| T: −0.01 | ||||||
| Ac: −0.02 |
4. Conclusions
Acknowledgments
References
- O’Reagen, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Y.; Yu, Q.; Cheng, Y.; Liu, S.; Shi, D.; Gao, F.; Wang, P. Dye-sensitized solar cells with a high absorptivity ruthenium sensitizer featuring a 2-(hexylthio)thiophene conjugated bipyridine. J. Phys. Chem. C 2009, 113, 6290–6297. [Google Scholar]
- Hagfeldt, A.; Boschloo, G.; Sun, L.C.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar]
- Mishra, A.; Fischer, M.K.R.; Bauerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Chen, B.S.; Chen, D.Y.; Chen, C.L.; Hsu, C.W.; Hsu, H.C.; Wu, K.L.; Liu, S.H.; Chou, P.T.; Chi, Y. Donor-acceptor dyes with fluorine substituted phenylene spacer for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 1937–1945. [Google Scholar]
- Zhou, D.; Cai, N.; Long, H.; Zhang, M.; Wang, Y.; Wang, P. An energetic and kinetic view on cyclopentadithiophene dye-sensitized solar cells: the influence of fluorine vs. ethyl substituent. J. Phys. Chem. C 2011, 115, 3163–3171. [Google Scholar]
- Zhu, W.; Wu, Y.; Wang, S.; Li, W.; Li, X.; Chen, J.; Wang, Z.S.; Tian, H. Organic D-A-π-A solar cell sensitizers with improved stability and spectral response. Adv. Funct. Mater. 2011, 21, 756–763. [Google Scholar]
- Ning, Z.; Fu, Y.; Tian, H. Improvement of dye-sensitized solar cells: What we know and what we need to know. Energy Environ. Sci. 2010, 3, 1170–1181. [Google Scholar]
- Ning, Z.; Zhang, Q.; Pei, H.; Luan, J.; Lu, C.; Cui, Y.; Tian, H. Photovoltage improvement for dye-sensitized solar cells via cone-shaped structural design. J. Phys. Chem. C 2009, 113, 10307–10313. [Google Scholar]
- Yen, Y.-S.; Chou, H.-H.; Chen, Y.-C.; Hsu, C.-Y.; Lin, J.T. Recent progress of organic materials for dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 8734–8747. [Google Scholar]
- Velusamy, M.; Justin Thomas, K.R.; Lin, J.T.; Hsu, Y.-C.; Ho, K.-C. Organic dyes incorporating low-band-gap chromophores for dye-sensitized solar cells. Org. Lett. 2005, 7, 1899–1902. [Google Scholar] [CrossRef]
- Justin Thomas, K.R.; Lin, J.T.; Hsu, Y.-C.; Ho, K.-C. Organic dyes containing thienylfluorene conjugation for solar cells. Chem. Commun. 2005, 4098–4100. [Google Scholar]
- Tsai, M.-S.; Hsu, Y.-C.; Lin, J.T.; Chen, H.-C.; Hsu, C.-P. Organic dyes containing 1H-phenanthro[9,10-d]imidazole conjugation for solar cells. J. Phys. Chem. C 2007, 111, 18785–18793. [Google Scholar]
- Justin Thomas, K.R.; Hsu, Y.-C.; Lin, J.T.; Lee, K.-M.; Ho, K.-C.; Lai, C.-H.; Cheng, Y.-M.; Chou, P.-T. 2,3-Disubstituted thiophene-based organic dyes for solar cells. Chem. Mater. 2008, 20, 1830–1840. [Google Scholar]
- Yen, Y.-S.; Hsu, Y.-C.; Lin, J.T.; Chang, C.-W.; Hsu, C.-P.; Yin, D.-J. Pyrrole-based organic dyes for dye-sensitized solar cells. J. Phys. Chem. C. 2008, 112, 12557–12567. [Google Scholar]
- Huang, S.-T.; Hsu, Y.-C.; Yen, Y.-S.; Chou, H.-H.; Lin, J.T.; Chang, C.-W.; Hsu, C.-P.; Tsai, C.; Yin, D.-J. Organic dyes containing a cyanovinyl entity in the spacer for solar cells applications. J. Phys. Chem. C 2008, 112, 19739–19747. [Google Scholar]
- Lin, J.T.; Chen, P.-C.; Yen, Y.-S.; Hsu, Y.-C.; Chou, H.-H.; Yeh, M.-C.P. Organic dyes containing furan moiety for high-performance dye-sensitized solar cells. Org. Lett. 2009, 11, 97–100. [Google Scholar]
- Velusamy, M.; Hsu, Y.-C.; Lin, J.T.; Chang, C.-W.; Hsu, C.-P. 1-Alkyl-1H-imidazole-based dipolar organic compounds for dye-sensitized solar cells. Chem. Asian J. 2010, 5, 87–96. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsu, Y.-C.; Chou, H.-H.; Justin Thomas, K.R.; Lin, J.T.; Hsu, C.-P. Dipolar compounds containing fluorene and a heteroaromatic ring as the conjugating bridge for high-performance dye-sensitized solar cells. Chem. Eur. J. 2010, 16, 3184–3193. [Google Scholar]
- Silvestri, V.; Marrocchi, A.; Seri, M.; Kim, C.; Mark, T.J.; Facchetti, A.; Taticchi, A. Solution-processable low-molecular weight extended arylacetylenes: versatile p-type semiconductors for field-effect transistors and bulk heterojunction solar cells. J. Am. Chem. Soc. 2010, 132, 6108–6123. [Google Scholar]
- Chung, D.S.; Park, J.W.; Park, J.H.; Moon, D.; Kim, G.H.; Lee, D.H.; Shim, H.K.; Kwon, S.K.; Park, C.E. High mobility organic single crystal transistors based on soluble triisopropylsilylethynyl anthracene derivatives. J. Mater. Chem. 2010, 20, 524–530. [Google Scholar]
- Jung, K.H.; Bae, S.Y.; Kim, K.H.; Cho, M.J.; Lee, K.; Kim, Z.H.; Choi, D.H.; Chung, D.S.; Park, C.E. High-mobility anthracene-based X-shaped conjugated molecules for thin film transistors. Chem. Commun. 2009, 5290–5292. [Google Scholar]
- Xia, Z.Y.; Zhang, Z.Y.; Su, J.H.; Zhang, Q.; Fung, K.M.; Lam, M.K.; Li, K.F.; Wong, W.Y.; Cheah, K.W.; Tian, H.; Chen, C.H. Robust and highly efficient blue light-emitting hosts based on indene-substituted anthracene. J. Mater. Chem. 2010, 20, 3768–3774. [Google Scholar]
- Reddy, M.A.; Thomas, A.; Srinivas, K.; Rao, V.J.; Bhanuprakash, K.; Sridhar, B.; Kumar, A.; Kamalasanan, M.N.; Srivastava, R. Synthesis and characterization of 9,10-bis(2-phenyl-1,3,4-oxadiazole) derivatives of anthracene: Efficient n-type emitter for organic light-emitting diodes. J. Mater. Chem. 2009, 19, 6172–6184. [Google Scholar] [CrossRef]
- Tao, S.; Zhou, Y.; Lee, C.S.; Lee, S.T.; Huang, D.; Zhang, X. Highly efficient nondoped blue organic light-emitting diodes based on anthracene-triphenylamine derivatives. J. Phys. Chem. C 2008, 112, 14603–14606. [Google Scholar] [CrossRef]
- Wang, L.; Wong, W.-Y.; Lin, M.-F.; Wong, W.-K.; Cheah, K.-W.; Tam, H.-L.; Chen, C.H. Novel host materials for single-component white organic light-emitting diodes based on 9-naphthylanthracene derivatives. J. Mater. Chem. 2008, 18, 4529–4536. [Google Scholar] [CrossRef]
- Xia, Z.-Y.; Su, J.-H.; Wong, W.-Y.; Wang, L.; Cheah, K.-W.; Tian, H.; Chen, C.H. High performance organic light-emitting diodes based on tetra(methoxy)-containing anthracene derivatives as a hole transport and electron-blocking layer. J. Mater. Chem. 2010, 20, 8382–8388. [Google Scholar]
- Wang, L.; Wu, Z.-Y.; Wong, W.-Y.; Cheah, K.-W.; Huang, H.; Chen, C.H. New blue host materials based on anthracene-containing dibenzothiophene. Org. Electron. 2011, 12, 595–601. [Google Scholar]
- Marrocchi, A.; Silvestri, F.; Seri, M.; Facchetti, A.; Taticchi, A.; Marks, T.J. Conjugated anthracene derivatives as donor materials for bulk heterojunction solar cells: Olefinic versus acetylenic spacers. Chem. Commun. 2009, 1380–1382. [Google Scholar]
- Teng, C.; Yang, X.; Yang, C.; Li, S.; Cheng, M.; Hagfeldt, A.; Sun, L. Molecular design of anthracene-bridged metal-free organic dyes for efficient dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 9101–9110. [Google Scholar] [CrossRef]
- Srinivas, K.; Yesudas, K.; Bhanuprakash, K.; Rao, V.J.; Giribabu, L. A combined experimental and computational investigation of anthracene based sensitizers for DSSC: comparison of cyanoacrylic and malonic acid electron withdrawing groups binding onto the TiO2 anatase (101) surface. J. Phys. Chem. C 2009, 113, 20117–20126. [Google Scholar]
- Thomas, K.R.J.; Singh, P.; Baheti, A.; Hsu, Y.-C.; Ho, K.-C.; Lin, J.T. Electro-optical properties of new anthracene based organic dyes for dye-sensitized solar cells. Dye. Pigment. 2011, 91, 33–43. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, Y.-H.; Chou, H.-H.; Chaurasia, S.; Wen, Y.S.; Lin, J.T.; Yao, C.-F. Naphthyl and thienyl units as bridges for metal-free dye-sensitized solar cells. Chem. Asian J. 2012, 7, 1074–1084. [Google Scholar] [CrossRef]
- Q-CHEM, version 4.0, Q-Chem Inc.: Pittsburgh, PA, USA, 2011.
- Vaswani, H.M.; Hsu, C.P.; Head-Gordon, M.; Fleming, G.R. Quantum chemical evidence for an intramolecular charge-transfer state in the carotenoid peridinin of peridinin−chlorophyll−protein. J. Phys. Chem. B 2003, 107, 7940–7946. [Google Scholar]
- Kurashige, Y.; Nakajima, T.; Kurashige, S.; Hirao, K.; Nishikitani, Y. Theoretical investigation of the excited states of coumarin dyes for dye-sensitized solar cells. J. Phys. Chem. A 2007, 111, 5544–5548. [Google Scholar] [CrossRef]
- Dreuw, A.; Head-Gordon, M. Failure of time-dependent density functional theory for long-range charge-transfer excited states: The zincbacteriochlorin–bacteriochlorin and bacteriochlorophyll–spheroidene complexes. J. Am. Chem. Soc. 2004, 126, 4007–4016. [Google Scholar]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar]
- Hartwig, J.F.; Kawatsura, M.; Hauck, S.I.; Shaughnessy, L.M.; Alcazar-Roman, J. Room-temperature palladium-catalyzed amination of aryl bromides and chlorides and extended scope of aromatic C−N bond formation with a commercial ligand. J. Org. Chem. 1999, 64, 5575–5580. [Google Scholar]
- Driver, M.S.; Hartwig, J.F. A second-generation catalyst for aryl halide amination: Mixed secondary amines from aryl halides and primary amines catalyzed by (DPPF)PdCl2. J. Am. Chem. Soc. 1996, 118, 7217–7218. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.; Baik, C.; Song, K.; Kang, S.O.; Ko, J. Novel conjugated organic dyes containing bis-dimethylfluorenyl amino phenyl thiophene for efficient solar cell. Tetrahedron 2007, 63, 11436–11443. [Google Scholar]
- Hara, K.; Sato, T.; Katoh, R.; Furube, A.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Molecular design of coumarin dyes for efficient dye-sensitized solar cells. J. Phys. Chem. B 2003, 107, 597–606. [Google Scholar]
- Hara, K.; Tchibana, Y.; Ohga, Y.; Shinpo, A.; Suga, S.; Sayama, K.; Sugihara, H.; Arakawa, H. Dye-sensitized nanocrystalline TiO2 solar cells based on novel coumarin dyes. Sol. Energy Mater. Sol. Cells 2003, 77, 89–103. [Google Scholar] [CrossRef]
- Li, S.-L.; Jiang, K.-J.; Shao, K.-F.; Yang, L.-M. Novel organic dyes for efficient dye-sensitized solar cells. Chem. Commun. 2006, 2792–2794. [Google Scholar]
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yen, Y.-S.; Chen, Y.-C.; Chou, H.-H.; Huang, S.-T.; Lin, J.T. Novel Organic Sensitizers Containing 2,6-Difunctionalized Anthracene Unit for Dye Sensitized Solar Cells. Polymers 2012, 4, 1443-1461. https://doi.org/10.3390/polym4031443
Yen Y-S, Chen Y-C, Chou H-H, Huang S-T, Lin JT. Novel Organic Sensitizers Containing 2,6-Difunctionalized Anthracene Unit for Dye Sensitized Solar Cells. Polymers. 2012; 4(3):1443-1461. https://doi.org/10.3390/polym4031443
Chicago/Turabian StyleYen, Yung-Sheng, Yung-Chung Chen, Hsien-Hsin Chou, Shih-Tang Huang, and Jiann T. Lin. 2012. "Novel Organic Sensitizers Containing 2,6-Difunctionalized Anthracene Unit for Dye Sensitized Solar Cells" Polymers 4, no. 3: 1443-1461. https://doi.org/10.3390/polym4031443




