1. Introduction
Ring-opening polymerizations, which convert cyclic monomers into linear polymers, are a major type of polymerization. A recent authoritative treatise [
1] covered ring-opening polymerization of monocyclic monomers; this Review covers ring-opening polymerization of atom-bridged bicyclic monomers. “Atom-bridged” means all three chains connecting the bridgehead atoms contain at least one atom. Bicyclic compounds with bridgehead atoms directly attached to one another will not be considered here because they usually polymerize like monocyclics. Alkene metathesis of bicyclic monomers has been well-reviewed elsewhere and will not be included here.
The reactions are arranged below according to mechanism:
UNCOORDINATED ANIONIC POLYMERIZATIONS of lactams, ureas, and urethanes
COORDINATED ANIONIC POLYMERIZATIONS of lactones and carbonates
CATIONIC POLYMERIZATIONS of ethers, acetals, orthoesters and amines
Although these monomers may appear exotic, they are often synthesized rather easily. Hydrogenation of suitable benzene derivatives followed by cyclization has been used most often. This is easy for industrial research laboratories equipped with high pressure equipment but less so for academic laboratories, perhaps accounting for the limited data in this field.
All of the syntheses involve the possibility of forming linear polymer directly instead of bicyclic monomer. The synthesis conditions may have to be adjusted to favor the formation of bicyclic monomer. These include dilute solution, high temperature, and other factors.
Since the polymer structure is usually obvious from the monomer structure, the polymer structure is only given below for the first example and in some later cases where more than one polymer structure is possible.
2. Discussion
2.1. Lactams (1–15)
Lactams dominate the group of bicyclic monomers which undergo uncoordinated anionic polymerization (
Table 1).
Table 1.
Bicyclic lactam monomers
1–15 [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13].
Table 1.
Bicyclic lactam monomers 1–15 [2,3,4,5,6,7,8,9,10,11,12,13].
| Bicyclo[2.2.1]heptanes |
![Polymers 04 01674 i001]() | ![Polymers 04 01674 i002]()
![Polymers 04 01674 i003]() |
| Bicyclo[2.2.2]octanes |
![Polymers 04 01674 i004]() |
| Bicyclo[3.2.1]octanes | Bicyclo[4.1.1]octane | Bicyclo[3.2.2]nonane |
![Polymers 04 01674 i005]() | ![Polymers 04 01674 i006]() | ![Polymers 04 01674 i007]() | ![Polymers 04 01674 i008]() |
| Bicyclo[3.3.1]nonanes | Bicyclo[4.2.1]nonane | Bicyclo[4.3.1]decane |
![Polymers 04 01674 i009]() |
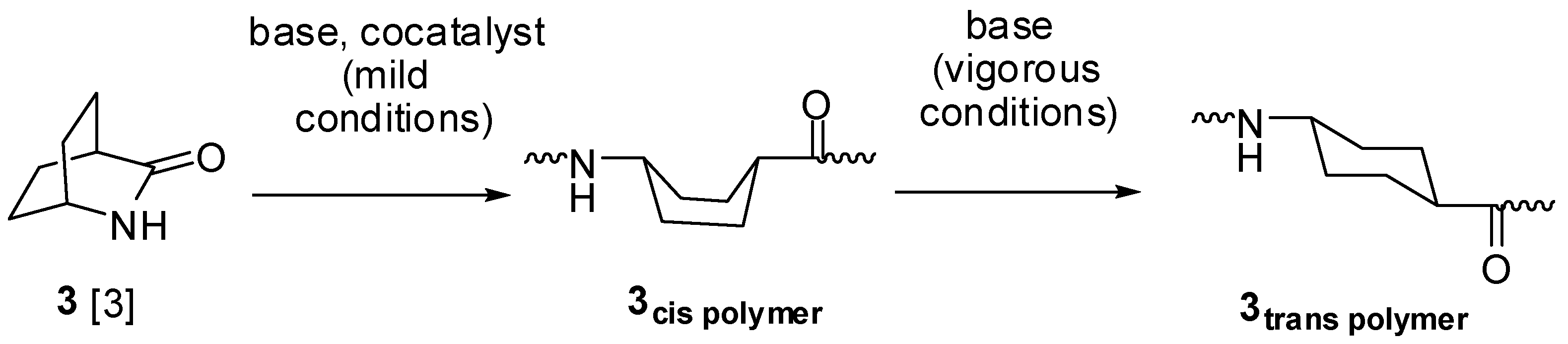
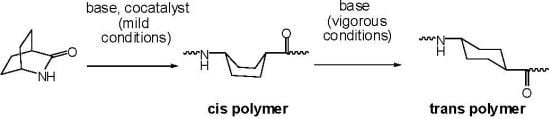
Lactam synthesis usually involves removal of a small molecule from an amino acid derivative, often by simply heating the amino acid under vacuum to remove water. Cyclization of an aminoacyl chloride hydrochloride with triethylamine has also been employed. The polymerizations are catalyzed by the lactam anion, assisted by cocatalyst N-acyllactam. Bicyclic amides 1–15 shown below (except the one in brackets) have been converted to polymers; the polymer is shown for monomer 1 only.
When the carbonyl group is attached to the ring in the initial polymer, it may be epimerized by the strongly basic lactam anion (
Scheme I).
Scheme I.
Ring-opening of bicyclic lactam
3 to give either cis or trans polymer [
3].
Scheme I.
Ring-opening of bicyclic lactam
3 to give either cis or trans polymer [
3].
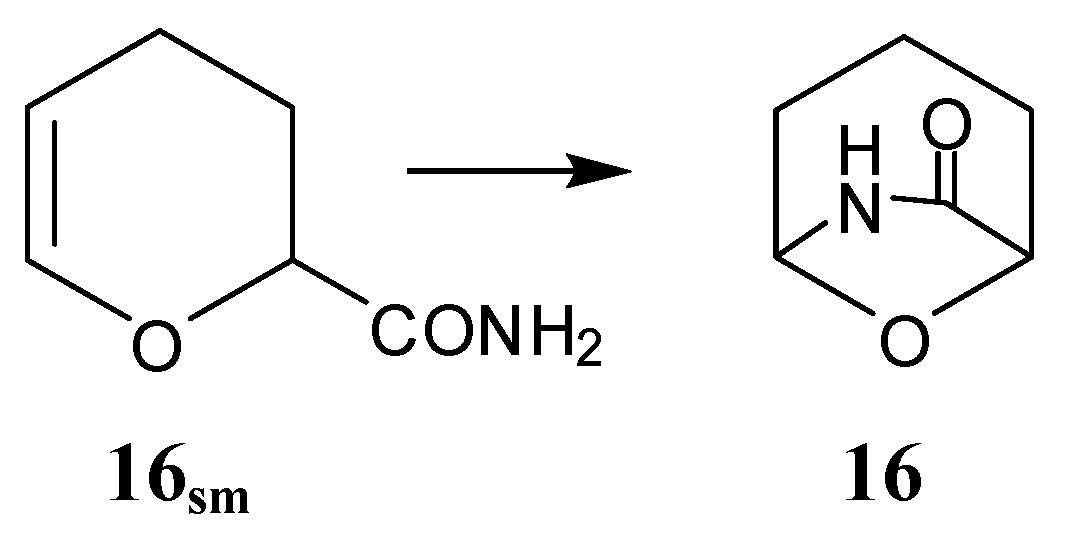
Oxabicyclic lactam
16 (
Scheme II) and derivatives were synthesized from starting material
16sm by internal addition of the carboxamide group to the dihydropyran group. Although monomer
16 is both a bicyclic lactam and a bicyclic ether, it has only been polymerized as the former by an anionic mechanism; cationic ether polymerization is excluded because of the basic amide group. This topic has been reviewed [
14,
15,
16].
Scheme II.
Synthesis of bicyclic lactam monomer 16.
Scheme II.
Synthesis of bicyclic lactam monomer 16.
An especially interesting class of lactam and related
N-containing monomers contains a bridgehead N next to a carbonyl group. These are greatly destabilized relative to normal amides because the usual N–CO resonance is absent according to Bredt’s Rule, which forbids the occurrence of double bonds at bridgeheads as pointed out by Lukes [
5]. Yakhontov also discussed this problem [
6]. Pracejus [
7] synthesized 1-aza-6,6-dimethylbicyclo[2.2.2]octan-2-one, while recent workers synthesized the parent structure as a salt by a nitrene insertion reaction; the free lactam could not be generated from the salt [
8]. Although polymerization studies were not reported, these anti-Bredt lactams undoubtedly polymerize, being destabilized by their boat form as well as Bredt’s Rule violation. The effect can be offset with an adjacent heteroatom, or in ureas and urethanes; the results of these studies are discussed below.
2.2. Ureas (17–23)
Bicyclic ureas (
Table 2) are prepared by carbonylation of the corresponding diamine [
3]. They polymerize anionically. The bicyclo[3.3.1]nonane-
N-bridgehead ureas
22 and
23 were stable because of electron donation to the carbonyl by the non-bridgehead N atom [
17,
18]. The molecules adopted a chair-boat conformation, as does bicyclo[3.3.1]non-1-ene.
Table 2.
Bicyclic urea monomers
17–29 [
3,
4,
11,
17,
18].
2.3. Urethanes(24–29)
Bicyclic urethanes (
Table 3) are synthesized from amino alcohols with carbonylating agents such as phosgene and diphenyl carbonate [
3]. Attempted cyclizations of 3-hydroxypyrrolidine and 4-hydroxypiperidine failed to yield bicyclic urethanes
24 and
29, respectively. However, the
N-bridgehead urethanes
25 and
27 were sufficiently stable to be isolated because of the resonance stabilization provided by electron donation from the oxygen adjacent to the carbonyl [
19,
20,
21]. This topic has been reviewed [
22].
Table 3.
Bicyclic urethane monomers
24–29 [
3,
19,
20,
21].
2.4. Lactones (30–37) and Carbonates(38–39)
Lactones (
Table 4) can undergo “uncoordinated” anionic polymerization, but “coordinated” anionic polymerization, in which the addition of a Lewis acid of a metal like aluminum, tantalum, or titanium provides simultaneous Lewis acid-base activation, is much more selective and does not lead to reshuffling of the polymer chains. The lactone monomers are synthesized by loss of small molecules from hydroxyacids or hydroxyesters. Also in this group are cyclic carbonates, prepared by carbonylation of alicyclic diols.
The polymer from monomer
31 retains the cis form when the polymerization is carried out under mild conditions [
3,
24]. Monomer
33 readily gave high polymer while monomer
32 gave lower molecular weight polymer. Monomer
36 showed a propensity for macrocyclic oligomerization. The bicyclic oxalactone polymers, also stemming from dihydropyrans, have been reviewed [
14,
15,
16].
2.5. Ethers (40–43)
Ethers (
Table 5)comprise the first group of oxygen-containing monomers we will consider which undergo cationic polymerization. Their polymerization involves oxonium ion intermediates. This topic has been reviewed [
31].
The stereochemistry of these polymerizations, involving nucleophilic displacement on the propagating oxonium ion, is trans. The isomeric 2-methyl-7-oxabicyclo[2.2.1]heptanes gave stereoregular polymers [
32,
33,
34].
2.6. Acetals (44–52)
Unsubstituted acetal monomers (
Table 6) were prepared by syntheses involving malonic ester alkylation followed by acid-catalyzed bicyclization with loss of methanol. Crucial to the success of these reactions was removal of the sensitive acetal by distillation under reduced pressure as it formed [
37,
38,
39,
40]. This topic has been reviewed [
41,
42,
43].
Table 6.
Bicyclic acetal monomers
44–49 [
37,
38,
39,
40,
44,
45].
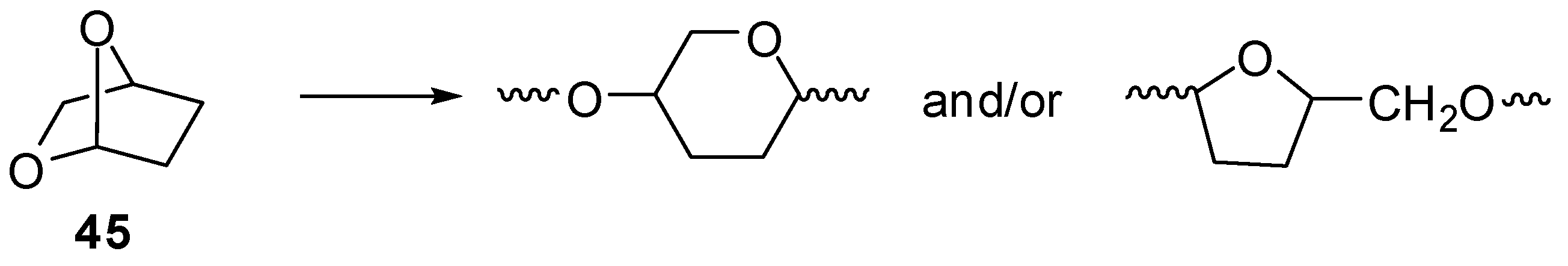
Unsymmetrical acetals like
45 can cleave in either or both of two ways (
Scheme III).
Scheme III.
Ring-opening of an unsymmetrical acetal.
Scheme III.
Ring-opening of an unsymmetrical acetal.
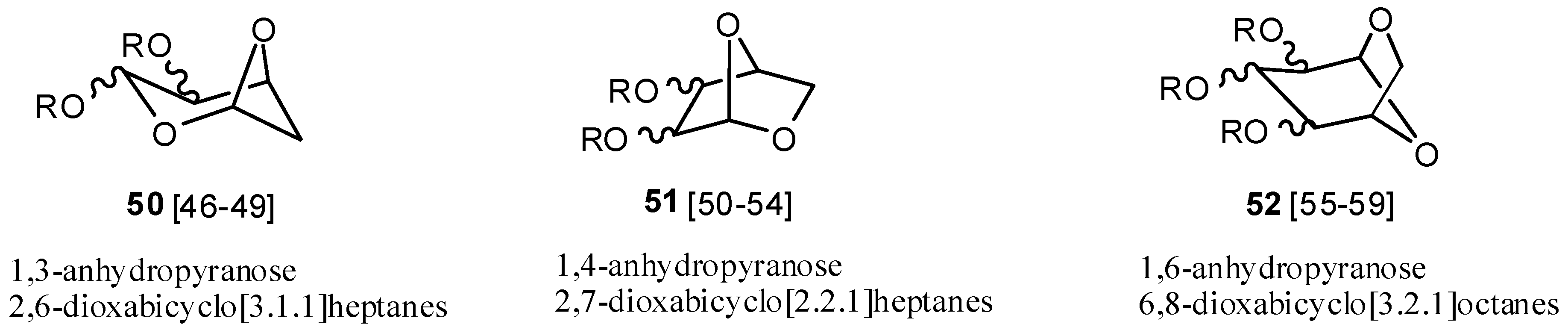
Although atom-bridged monomers have generally been unsubstituted, the acetals represent a marked exception. Numerous atom-bridged bicyclic acetals like
50–
52 derived from sugars (
Scheme IV) have been used to synthesize unnatural polysaccharides. Their hydroxyl groups are protected by benzylation or acylation before polymerization and can be removed after polymerization [
46,
47,
48,
49]. These substituents can produce stereoregularity in the oxacarbenium ion polymerization.
Scheme IV.
Bicyclic acetal monomers
50–52 from sugars [
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59].
Scheme IV.
Bicyclic acetal monomers
50–52 from sugars [
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59].
2.7. Orthoesters (53–56)
Orthoesters (
Table 7) are the third member of this oxygen group. They are synthesized from condensation of triols with ethyl orthoformate, again removing the bicyclic monomer as it forms [
60,
61,
62,
63,
64,
65]. This topic has been reviewed [
41,
42,
43,
66].
For the bicyclic acetals and orthoesters, oxonium ion propagation is still predominant. However, for these monomers, cleavage of the oxonium ion to oxacarbenium ion can lead to loss of stereoregularity [
66]. Moreover, in the many sugar-derived monomers, neighboring group participation by adjacent benzoate or benzyloxy groups plays a role in determining polymer stereochemistry.
2.8. Amines (57–58)
Finally, bicyclic amines
57 and
58 (
Table 8) have also been polymerized cationically via S
N2 displacements on ammonium ion intermediates [
66]. 1,4-Diazabicyclo[2.2.2]octane (
57), widely used as a catalyst, is now seen to be a monomer itself.
Table 8.
Bicyclic amine monomers
57–58 [
68].
Table 8.
Bicyclic amine monomers 57–58 [68].
| Bicyclo[2.2.2]octane | Bicyclo[3.2.2]nonane |
![Polymers 04 01674 i051]() | ![Polymers 04 01674 i052]() |
2.9. Attempted Correlations with Monomer Properties
2.9.1. Infrared
Carbonyl groups in small rings absorb infrared radiation at much higher frequencies than carbonyls in open chain analogs. However, we determined that the carbonyl absorption did not correlate with polymerizability of monocyclic monomers [
69]. This also proved true for bicyclic monomers [
70]. The higher carbonyl absorption frequency in small rings is attributed to orbital hybridization effects.
2.9.2. Saponification Rates
We tried and failed to correlate saponification rates with polymerizability for monocyclic monomers [
71]. Our thought was that strain relief in polymerization might parallel strain relief in saponification reactions. However, no parallel was found [
70]. The same was true for bicyclic monomers [
72]. The reason is that formation of tetrahedral intermediates, not ring opening, is the rate-determining step.
2.9.3. Dipole Moments
We noticed that lactones possess much higher dipole moments than their open-chain analogs and wondered whether this factor might be related to polymerization. However, this proved not to be the case [
73]. For example, γ-butyrolactone and δ-valerolactone both have high dipole moments, yet only the latter polymerizes.
2.9.4. Polymer Properties and Uses
The physical properties of the polymers described herein have not been investigated to any great extent. Ring-opening polymerization of an atom-bridged-bicyclic monomer gives a linear polymer containing a ring in the chain. This often imparts desirable physical properties to the polymer, including increased melting point and glass transition temperature [
74,
75]. Polymerization of bicyclic ether
41 gives a crystalline polymer melting at 250 °C. while the polymer from tetrahydrofuran melts at 50 °C [
31]. Given that the polymerization mechanisms for the bicyclic and monocyclic monomers are the same, the synthesis of new copolymers can be anticipated.
The polymers from bicyclic acetals, after deprotection, have been intensively studied as synthetic polysaccharides [
76]. The polymeric orthoesters have been examined as bioerodible materials [
66].
3. Conclusions
The polymerizabilities of the atom-bridged bicyclic compounds in this review are listed in
Table 9. They almost all polymerize! The sole exceptions are bicyclo[3.3.1]nonanes; even here,
N-bridgehead anti-Bredt lactams do polymerize.
Table 9.
Polymerizability of bicyclic monomers in this review.
Table 9.
Polymerizability of bicyclic monomers in this review.
| Monomer Structure | Polymerizability |
|---|
| Bicyclohexane [2.1.1] | + |
| Bicycloheptane [2.2.1] | + |
| Bicyclooctanes [2.2.2] | + |
| [3.2.1] | + |
| [4.1.1] | + |
| Bicyclononanes [3.3.1] | ± |
| [3.2.2] | + |
| [4.2.1] | + |
| Bicyclodecane [4.3.1] | + |
This surprising generalization requires explanation. For the small ring sizes (three and four members), angle strain and eclipsing interactions are important. For the common ring sizes (five to seven members), eclipsing interactions dominate. Often the six-membered rings in bicyclic monomers are locked into either boat or chair forms (the boat forms of cyclohexane are destabilized by about 8 kcal/mole relative to the chair forms). For larger rings, transannular hydrogen crowding as well as entropic considerations come into play. The monomers described possess combined 5- and 6-membered rings. Although no significant angle strain occurs, ring fusion causes eclipsing H–H repulsions. The cyclohexane rings in many of the monomers are locked into boat forms with strain energies of ~8 kcal/mole. The non-polymerizability of bicyclo[3.3.1]nonanes possessing two stable chair forms supports this line of thought. Second, amide and ester bridges are forced into energetically unfavorable cis conformations. Third, entropic considerations favor polymerization; chain polymers have many more available conformations than the rigid bicyclic monomers. Fourth, the bridgehead N–C=O structure present in the anti-Bredt monomers described above is a powerful driving force for polymerization. Fifth, isomerization of an initially formed cis-disubstituted ring to a thermodynamically favored trans-disubstituted ring is another driving force in cases where the carbonyl group is attached to the ring and epimerization is permitted. Bicyclic monomers possess these attributes to varying degrees, but the sum is almost always sufficient to cause polymerization.
Acknowledgments
The author greatly acknowledges financial support from NSF-DMR, PRF, DuPont Textile Fibers Department, Pioneering Research Laboratory, and scientific work performed by Robert B. Bates, Anne Padias, and many fine postdoctoral associates and graduate students.
References
- Dubois, P.; Coulembier, O.; Raquez, J.-M. Handbook of Ring-Opening Polymerization; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Cho, H.N.; Choi, K.Y.; Choi, S.K. Polymerization of 2-azabicyclo[2.2.1]heptane-3-one. J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 623–634. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Polymerization and ring strain in bridged bicyclic compounds. J. Am. Chem. Soc. 1958, 80, 6412–6420. [Google Scholar] [CrossRef]
- Al-Obeidi, F.A.; Micheli, B.J.M.; Barfield, M.; Padias, A.B.; Wei, Y.; Hall, H.K., Jr. Synthesis and NMR studies of activated derivatives of cis- and trans-5-amino-6-oxopiperidine-2-carboxylic acid and the corresponding bicyclic dilactam DBO: Potential building blocks for stereoregular polyamides and peptides. Macromolecules 1999, 32, 6507–6516. [Google Scholar] [CrossRef]
- Lukes, R. A new application of Bredt’s rule. Coll. Czech. Chem. Comm. 1938, 10, 148–152. [Google Scholar]
- Yakhontov, L.N.; Rubstov, M.V. Synthesis of 2-quinuclidone. Z. Obsh. Khimi 1957, 27, 72–77. [Google Scholar]
- Pracejus, H. 2,2-Dimethyl-6-quinuclidone, a resonance-free amide. Chem. Ber. 1959, 92, 988–989. [Google Scholar] [CrossRef]
- Claydon, J.; Moran, W.J. The twisted amide 2-quinuclidone. Angew. Chem. Int. Ed. 2006, 45, 7118–7120. [Google Scholar] [CrossRef]
- Okada, M.; Sumitomo, H.; Mori, H.; Hall, H.K., Jr.; Chan, J.H.; Bruck, M. Synthesis and ring-opening polymerization of novel bicyclic oxalactams: 2-Oxa-5-azabicyclo[2.2.2]octan-6-one. J. Polym. Sci. Polym. Chem. Ed. 1990, 8, 3251–3260. [Google Scholar]
- Okada, M.; Sumitomo, H.; Sassa, T.; Takai, M.; Hall, H.K., Jr.; Bruck, M. Synthesis and ring-opening polymerization of novel bicyclic oxalactams, 2-oxa-6-azabicyclo[2.2.2]octan-5-one. Macromolecules 1990, 23, 2427–2432. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Synthesis and polymerization of atom-bridged bicyclic lactams. J. Am. Chem. Soc. 1960, 82, 1209–1215. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Synthesis and polymerization of 3-azabicyclo[4.3.1]decan-4-one and 7,7-dimethyl-2-azabicyclo[4.1.1]octan-3-one. J. Org. Chem. 1963, 28, 3213–3214. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; Shaw, R.A.; Deutschmann, A., Jr. Anti-bredt molecules. 2. 1-azabicyclo[3.3.1]nonan-2-one, a new bicyclic lactam containing bridgehead nitrogen. J. Org. Chem. 1980, 45, 3722–3724. [Google Scholar] [CrossRef]
- Okada, M. Ring-opening polymerization of bicyclic and spiro compounds. Reactivities and polymerization mechanisms. Adv. Polym. Sci. 1992, 102, 1–46. [Google Scholar] [CrossRef]
- Sumitomo, H.; Okada, M. Ring-opening polymerization of bicyclic acetals, lactones, and lactams. Adv. Polym. Sci. 1978, 28, 47–82. [Google Scholar] [CrossRef]
- Okada, M.; Sumitomo, H.; Atsumi, M.; Hall, H.K., Jr. Ring-opening polymerization of bicyclic oxalactones and oxalactams. Makromol. Chem. Macromol. Symp. 1991, 42-43, 355–364. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; Johnson, R.C. 3-Isopropyl-1,3-diazabicyclo[3.3.1]nonan-2-one. A simple bicyclic urea with a bridgehead nitrogen atom. J. Org. Chem. 1972, 37, 697–699. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; Ekuchukwu, O.E.; Deutschmann, A., Jr.; Rose, C. Anti-bredt molecules. 6. Synthesis and polymerization of 1,3-diazabicyclo[3.3.1]nonan-2-one. Polym. Bull. 1980, 3, 375–382. [Google Scholar]
- Hall, H.K., Jr.; El-Shekeil, A. Anti-bredt molecules. 3. Synthesis of two bicyclic urethanes possessing bridgehead nitrogen. J. Org. Chem. 1980, 45, 5325–5328. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; El-Shekeil, A. Anti-bredt molecules. 5. Synthesis and polymerization of 1-aza-7-oxabicyclo[3.2.1]octan-7-one. Polym. Bull. 1980, 3, 233–239. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; El-Shekeil, A. Anti-bredt molecules. 4. Polymerization of 1-aza-3-oxabicyclo[3.3.1]nonan-2-one. Polym. Bull. 1980, 2, 829–836. [Google Scholar]
- Hall, H.K., Jr.; El-Shekeil, A. Anti-bredt bridgehead nitrogen compounds in ring-opening polymerization. Chem. Rev. 1983, 83, 549–555. [Google Scholar]
- Hall, H.K., Jr.; Blanchard, E.P., Jr.; Martin, E.L. Synthesis and polymerization of 2-oxabicyclo[2.1.1] hexan-3-ones (cyclobutane1,3-lactones). Macromolecules 1971, 4, 142–146. [Google Scholar] [CrossRef]
- Ceccarelli, C.; Andruzzi, F.; Paci, M. NMR spectroscopy of polyesters from 2-oxabicyclo- [2.2.2]octan-3-one. Polymer 1979, 20, 605–610. [Google Scholar] [CrossRef]
- Okada, M.; Sumitomo, H.; Yamada, S.; Atsumi, M.; Hall, H.K., Jr.; Chan, R.J.H.; Ortega, R.B. Synthesis and ring-opening polymerization of bicyclic lactones containing a tetrahydropyran ring. 2,5-Dioxabicyclo[2.2.2]octan-3-one. Macromolecules 1986, 19, 953–959. [Google Scholar] [CrossRef]
- Okada, M.; Sumitomo, H.; Atsumi, M.; Hall, H.K., Jr.; Ortega, R.B. Synthesis and ring-opening polymerization of bicyclic lactones containing a tetrahydropyran ring. 2,6-Dioxabicyclo[2.2.2]octan-3-one. Macromolecules 1986, 19, 503–509. [Google Scholar] [CrossRef]
- Okada, M.; Sumitomo, H.; Atsumi, M.; Hall, H.K., Jr. Synthesis of polyesters having pendant ester groups by ring-opening polymerization of 4-methoxycarbonyl-2,6-dioxabicyclo[2.2.2]octan-3-one. Macromolecules 1987, 20, 1199–1205. [Google Scholar] [CrossRef]
- Sandin, R.B.; Rebel, W.J.; Levine, S. Ring closure of 2,5-dibromoadipic acids. J. Org. Chem. 1966, 31, 3879–3880. [Google Scholar] [CrossRef]
- Drumright, R.E.; Harmann, M.; Wolf, R. Copolymers of Cyclic Esters and Carbonates and Methods for Making Same. PCT. U.S. Patent 2002018443, 7 June 2002. [Google Scholar]
- Moore, J.A.; Kelly, J.E. Synthesis and polymerization of 2-oxo-3,8-dioxabicyclo[3.2.1]-octane. J. Polym. Sci. Polym. Lett. Ed. 1975, 13, 333–336. [Google Scholar] [CrossRef]
- Wittbecker, E.L.; Hall, H.K., Jr.; Campbell, T.W. Synthesis and polymerization of bridged bicyclic ethers. J. Am. Chem. Soc. 1960, 82, 1218–1222. [Google Scholar]
- Saegusa, T.; Motoi, M.; Matsumoto, S.; Fujei, H. Stereochemistry of the ring-opening polymerization of 2-methyl-7-oxabicyclo[2.2.1]heptane. Macromolecules 1972, 5, 233–236. [Google Scholar] [CrossRef]
- Baccaredda, M.; Giusti, P.; Andruzzi, F.; Cerrai, D.; DiMaina, N. PF5-catalyzed polymer of exo-2-methyl-7-oxabicyclo[2.2.1]heptane. J. Polym. Sci. Polym. Symp. 1970, 31, 159–176. [Google Scholar]
- Kops, J.; Spanggard, D. Polymerization of 2-methyl-7-oxabicyclo[2.2.1]heptane. J. Macromol. Sci. Chem. 1973, 7, 1455–1469. [Google Scholar] [CrossRef]
- Saegusa, T.; Hadaka, T.; Fujii, H. Polymer of 2-oxabicyclo[2.2.2]octane. Polym. J. 1971, 2, 670–671. [Google Scholar]
- Andruzzi, F.; Ceccarelli, G.; Paci, M. NMR spectra of poly-3-oxabicyclo[3.2.2]nonane. Polymer 1980, 21, 1180–1184. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; de Blauwe, F. 2,6- And 2,7-dioxabicyclo[2.2.1]heptanes. J. Am. Chem. Soc. 1975, 97, 655–656. [Google Scholar]
- Hall, H.K., Jr.; Carr, L.J.; Kellman, R.; de Blauwe, F. New ring system. 2,6-Dioxabicyclo[2.2.2]octane, a highly reactive bicyclic acetal. J. Am. Chem. Soc. 1974, 96, 7265–7269. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; de Blauwe, F.; Carr, L.J.; Rao, V.S.; Reddy, G.S. Synthesis, hydrolytic reactivity, and polymerization of 2,6- and 2,7-dioxabicyclo[2.2.1]heptanes. J. Polym. Sci. Symp. 1976, 56, 101–115. [Google Scholar]
- Hall, H.K., Jr.; Steuck, M.J. Polymerization of 6,8-dioxabicyclo[3.2.1]octane and 3,6,8-trioxabicyclo[3.2.1]octane. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 1035–1042. [Google Scholar] [CrossRef]
- Padias, A.B.; Szymanski, R.; Hall, H.K., Jr. Synthesis and polymerization of atom-bridged bicyclic acetals and orthoesters; A dioxacarbenium ion mechanism for orthoester polymerization. In Ring Opening Polymerization; McGrath, J.E., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; pp. 313–333, Chapter 286. [Google Scholar]
- Padias, A.B.; Szymanski, R.; Hall, H.K., Jr. Synthesis and polymerization of atom-bridged bicyclic acetals and orthoesters: A new mechanism. ACS Polym. Prepr. 1984, 25, 258–259. [Google Scholar]
- Yokoyama, Y.; Hall, H.K., Jr. Ring-opening polymerization of atom-bridged and bond-bridged bicyclic ethers, acetals and orthoester. Adv. Polym. Sci. 1982, 42, 107–138. [Google Scholar] [CrossRef]
- Reich, W.; Schwalm, R.; Haussling, L.; Nuyken, O.; Raether, R.B. Preparation Method for 2,7-Dioxabicyclo[3.2.1]octane. Ger. Patent DE19710992A1, 30 October 1971. [Google Scholar]
- Reich, W.; Schwalm, R.; Haussling, L.; Nuyken, O.; Raether, R.B. Polymers From 2,7-Dioxa-bicyclo[3.2.1]octane. Ger. Patent DE19614635A1, 16 October 1961. [Google Scholar]
- Good, F.J., Jr.; Schuerch, C. Synthesis of (1→3)-α-D-glucopyranan by stereoregular cationic polymerization of substituted 2,6-dioxabicyclo[3.1.1]heptanes: 1,3-anhydrotri-(p-substituted-benzyl)-β-D-glucopyranoses. Macromolecules 1985, 18, 595–599. [Google Scholar] [CrossRef]
- Kong, F.; Schuerch, C. Synthesis of (1→3)-α-D-mannopyranan by stereoregular cationic polymerization of substituted 2,6-dioxabicyclo[3.1.1]heptanes. Macromolecules 1984, 17, 983–989. [Google Scholar] [CrossRef]
- Good, F.; Schuerch, C. Improved synthesis of substituted 2,6-dioxabicyclo[3.1.1]heptanes: 1,3-anhydro-2,4,6-tri-O-benzyl-2,4,6-tri-O-p-bromobenzyl- and -2,4,6-tri-O-p-methylbenzyl-β-D-glucopyranose. Carbohydr. Res. 1984, 125, 165–171. [Google Scholar] [CrossRef]
- Kong, F.; Schuerch, C. Improved synthesis of substituted 2,6-dioxabicyclo[3.1.1]heptanes: 1,3-anhydro-2,4,6-tri-O-benzyl- and 1,3-anhydro-2,4,6-tri-O-p-bromobenzyl-β-D-mannopyranose. Carbohydr. Res. 1983, 112, 141–147. [Google Scholar] [CrossRef]
- Kops, J.; Schuerch, C. Polymerization of 1,4-Anhydro Sugar Derivative. J. Polym. Sci. C 1965, 11, 119–138. [Google Scholar] [CrossRef]
- Hagino, A.; Yoshida, S.; Shinpuku, T.; Matsuzaki, K.; Uryu, T. Selective ring-opening polymerization of 1,4-anhydro-α-D-lyxopyranose derivatives and synthesis of stereoregular (1→5)-α-D-lyxofuranan. Macromolecules 1986, 19, 1–7. [Google Scholar] [CrossRef]
- Ogawa, M.; Hatanaka, K.; Uryu, T. Synthesis of a novel cellulose-type hexopyranan 6-deoxy-(1→4)-α-L-talopyranan by selective ring opening polymerization of 1,4-anhydro sugar derivatives. Macromolecules 1991, 24, 987–992. [Google Scholar] [CrossRef]
- Uryu, T.; Yamanouchi, J.; Hayashi, S.; Tamaki, H.; Matsuzaki, K. Selective ring-opening polymerization of 1,4-anhydro-2,3-di-O-benzyl-α-D-xylopyranose and synthesis of stereoregular (1→5)-alpha-D-xylofuranan. Macromolecules 1983, 16, 320–326. [Google Scholar] [CrossRef]
- Uryu, T.; Kitano, K.; Ito, K.; Yamanouchi, J.; Matsuzaki, K. Selective ring-opening polymerization of 1,4-anhydro-α-D-ribopyranose derivatives and synthesis of stereoregular (1→4)-beta-D-ribopyranan. Macromolecules 1981, 14, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ichikawa, H.; Sumitomo, H.; Schuerch, C. Sterically controlled ring-opening polymerization of a 1,6-anhydro-beta-D-galactopyranose derivative by neighboring group participation. (1→6)-beta-D-Galactopyranan. Macromolecules 1988, 21, 542–543. [Google Scholar] [CrossRef]
- Uryu, T.; Katsuhiro, I.; Kobazashi, K.-I.; Matsuzahi, K. Spectroscopic studies on key-opening polymerization of 1,6-anhydro-2,3,4-tri-O-benzyl-β-D-glucopyranose. Macromol. Chem. Phys. 1979, 180, 1509–1519. [Google Scholar] [CrossRef]
- Uryu, T.; Tachikawa, H.; Ohaku, K.-I.; Terui, K.; Matsuzahi, K. Synthesis of 2,3,4-tri-O-benzyl-[1→6]-α-D-glucopyranan. Macromol. Chem. Phys. 1977, 178, 1929–1940. [Google Scholar] [CrossRef]
- Uryu, T.; Ito, K.; Kobayashi, K.I.; Matsuzaki, K. Ring opening polymerization of 1,6-anhydro-2,3,4-tri-O-benzyl-β-D-glucopyranose. Makromol. Chem. 1979, 180, 1509–1519. [Google Scholar] [CrossRef]
- Uryu, T.; Tachikawa, H.; Ohaku, K.I.; Terui, K.; Matsuzaki, K. Polymerization of 1,6-anhydro-2,3,4-tri-O-benzyl-β-D-glucopyranose. Makromol. Chem. 1977, 178, 1929–1940. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; de Blauwe, F.; Pyriadi, T. 2,6,7-Trioxabicyclo[2.2.1]heptane. J. Am. Chem. Soc. 1975, 97, 3854. [Google Scholar]
- Hall, H.K., Jr.; Yokoyama, Y. Polymerization of 2,6,7-trioxabicyclo[2.2.1]heptane with either contraction or expansion. Polym. Bull. 1980, 2, 281–287. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Padias, A.B.; de Blauwe, F.; Hall, H.K., Jr. Synthesis and polymerization of 2,6,7-trioxabicyclo[2.2.1]heptane and 1-methyl-2,6,7-trioxabicyclo[2.2.1]heptane. Macromolecules 1980, 13, 252–261. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Padias, A.B.; Bratoeff, E.A.; Hall, H.K., Jr. Synthesis and polymerization of 2,6,7-trioxabicyclo[2.2.2]octane and its derivatives. Macromolecules 1982, 15, 11–17. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Hall, H.K., Jr. Polymerization of 2,7,8-trioxabicyclo[3.2.1]octane and 2,8,9-trioxabicyclo[3.3.1]nonane. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 3133–3147. [Google Scholar] [CrossRef]
- Crank, G.; Eastwood, F.W. Derivatives of Orthoesters. 1. Bicyclic Orthoesters. Aust. J. Chem. 1964, 17, 1385–1391. [Google Scholar] [CrossRef]
- Heller, J.; Barr, J.; Ng, S.Y.; Abdellanci, K.S.; Gurry, R. Poly(orthoesters). Adv. Drug Deliv. Rev. 2002, 547, 1015–1039. [Google Scholar]
- Burt, R.A.; Chiang, Y.; Hall, H.K., Jr.; Kresge, A.J. The hydrolysis of bicyclic orthoesters in the 2,6,7-trioxabicyclo[2.2.1]heptane series. Confirmation of the absence of strain-relief rate acceleration. J. Am. Chem. Soc. 1982, 104, 3687–3690. [Google Scholar]
- Hall, H.K., Jr. Polymerization of two atom-bridged bicyclic amines. J. Org. Chem. 1963, 28, 223–224. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; Zbinden, R. Infrared spectra and strain in cyclic carbonyl compounds. J. Am. Chem. Soc. 1958, 80, 6428–6432. [Google Scholar] [CrossRef]
- Zbinden, R.; Hall, H.K., Jr. Infrared carbonyl and carbon-hydrogen frequencies in bridged bicyclic ketones. J. Am. Chem. Soc. 1960, 82, 1215–1218. [Google Scholar] [CrossRef]
- Hall, H.K., Jr.; Brandt, M.K.; Mason, R.M. Hydrolysis rates and mechanisms of cyclic monomers. J. Am. Chem. Soc. 1958, 80, 6420–6427. [Google Scholar]
- Hall, H.K., Jr. Mechanisms of hydrolysis of several atom-bridged bicyclic anhydrides, N-methylimides and lactone. J. Org. Chem. 1963, 28, 2027–2029. [Google Scholar] [CrossRef]
- Lee, C.M.; Kumler, W.D. Dipole moments and structure of cyclic compounds: Lactones, lactams, anhydrides, carbonates, carbamates, ureides, and imides. J. Org. Chem. 1963, 28, 1438–1439. [Google Scholar] [CrossRef]
- Berti, C.; Binassi, E.; Celli, A.; Colonna, M.; Fiorini, M.; Marchese, P.; Marianucci, E.; Gazzano, M.; Di Credico, F.; Brunelle, D. Poly(1,4-cyclohexylenedimethylene 1,4-cyclohexanedicarboxylate): Influence of stereochemistry of 1,4-cyclohexylene units on the thermal properties. J. Polym. Sci. B Polym. Phys. 2008, 46, 619–630. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, S.R. Synthesis and properties of cyclic diester-based aliphatic copolyesters. J. Polym. Sci. A Polym. Chem. 2010, 48, 2162–2169. [Google Scholar] [CrossRef]
- Schuerch, C. Biomedical applications of synthetic polysaccharides. In Polymer and Fiber Science: Recent Advances; Fornes, R.E., Gilbert, R.D., Mark, H.F., Eds.; Wiley-VCH: New York, NY, USA, 1992; pp. 9–16. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).