One-Dimensional Coordination Polymers of Lanthanide Cations to Cucurbit[7]uril Built Using a Range of Tetrachloride Transition-Metal Dianion Structure Inducers
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
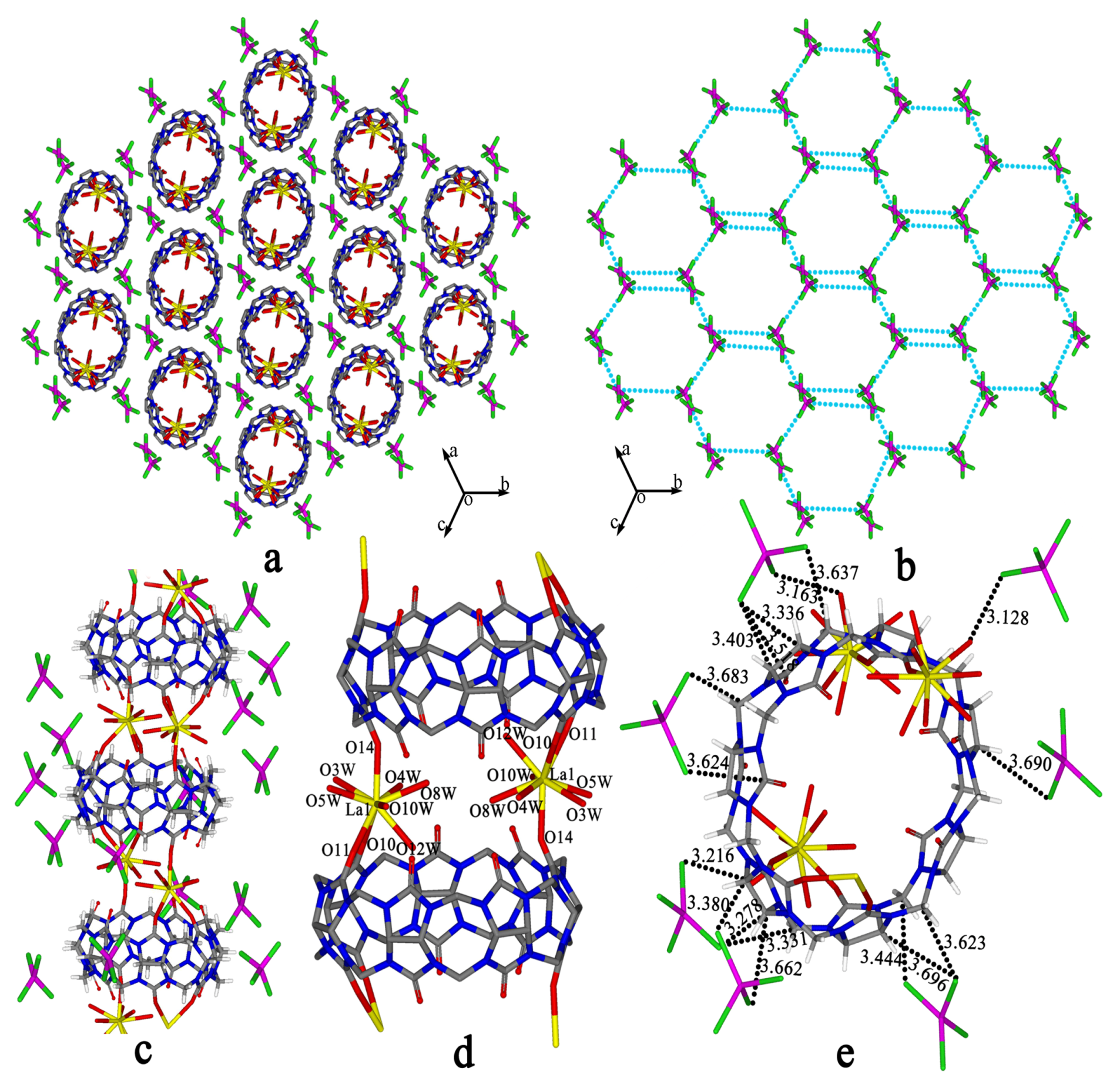
 Cl–Mtransition-metal–Cl are close to 109°28′, generally in the range 105°–112° [61,62]. In the present case, however, the bond angles
Cl–Mtransition-metal–Cl are close to 109°28′, generally in the range 105°–112° [61,62]. In the present case, however, the bond angles  Cl–Cu–Cl are in the range 101°–122°, so the [CuCl4]2− anions form a distorted tetrahedron. Similar results can be observed in the crystal structures of compounds with [CuCl4]2− anions, such as compounds 2, 5, 6 and 11, where the bond angles
Cl–Cu–Cl are in the range 101°–122°, so the [CuCl4]2− anions form a distorted tetrahedron. Similar results can be observed in the crystal structures of compounds with [CuCl4]2− anions, such as compounds 2, 5, 6 and 11, where the bond angles  Cl-Cu-Cl are in the range 101°–121°. The bond angles
Cl-Cu-Cl are in the range 101°–121°. The bond angles  Cl–Co–Cl in the [CoCl4]2− anions are similar to those of [CdCl4]2− and [ZnCl4]2− and are in the range 106°–112° for compounds with the [CoCl4]2− anions, such as compounds 3, 4, 7, 8, 9, 10 and 12.
Cl–Co–Cl in the [CoCl4]2− anions are similar to those of [CdCl4]2− and [ZnCl4]2− and are in the range 106°–112° for compounds with the [CoCl4]2− anions, such as compounds 3, 4, 7, 8, 9, 10 and 12.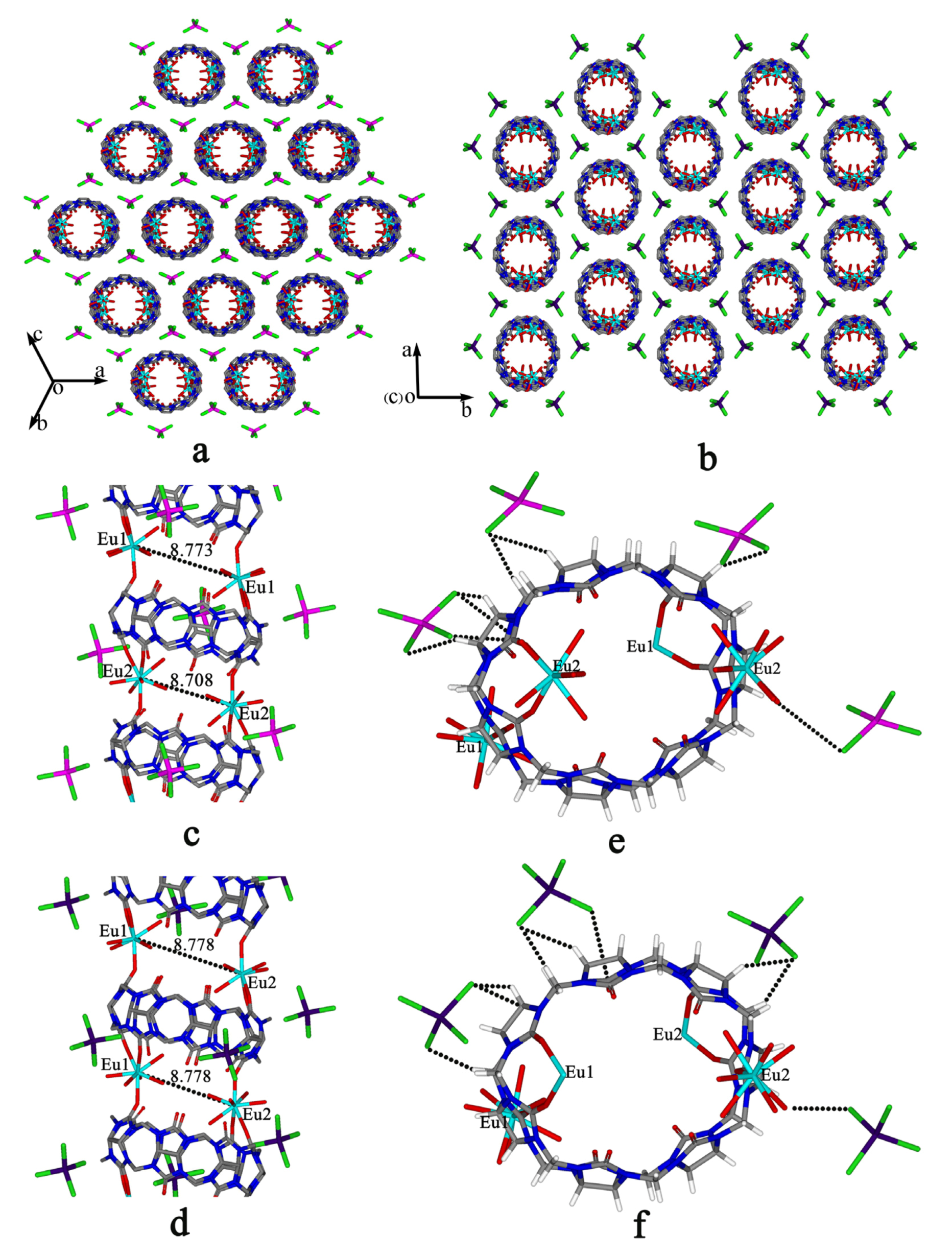

4. Conclusions
Acknowledgments
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Javier, G.M.; Kitagawa, S.; Ohrstrom, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef]
- Ye, B.H.; Tong, M.L.; Chen, X.M. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coordin. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Côté, A.P.; Shimizu, G.K.H. Coordination solids via assembly of adaptable components: Systematic structural variation in alkaline earth organosulfonate networks. Chem. Eur. J. 2003, 9, 5361–5370. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Bogliaa, A.; Leonea, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Rizzato, S. New polymeric networks from the self-assembly of silver(I) salts and the flexible ligand 1,3-bis(4-pyridyl)propane (bpp). A systematic investigation of the effects of the counterions and a survey of the coordination polymers based on bpp. CrystEngComm 2002, 4, 121–129. [Google Scholar] [CrossRef]
- Bureekaew, S.; Shimomura, S.; Kitagawa, S. Chemistry and application of flexible porous coordination polymers. Sci. Technol. Adv. Mater. 2008, 9, 014108–014120. [Google Scholar] [CrossRef]
- Fromm, K.M. Coordination polymer networks with s-block metal ions. Coord. Chem. Rev. 2008, 252, 856–885. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, G.Q.; Li, Y.Z.; Bai, J.F.; Pan, Y.; You, X.Z. Synthesis, structures andproperties of alkaline earth metal benzene-1,4-dioxylacetates with three-dimensional hybrid networks. Inorg. Chim. Acta 2006, 359, 3257–3263. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Day, A.I.; Arnold, A.P. Method for Synthesis Cucurbiturils. U.S. Patent 6,793,839 B1, 21 September 2004. [Google Scholar]
- Kim, J.; Jung, I.S.; Kim, S.Y.; Lee, E.; Kang, J.K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New cucurbituril homo-logues: Syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Day, A.I.; Blanch, R.J.; Arnold, A.P.; Lorenzo, S.; Lewis, G.R.; Dance, I. A cucurbituril-based gyroscane: A new supramolecular form. Angew. Chem. Int. Ed. 2002, 41, 275–277. [Google Scholar]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Masson, E.; Ling, X.X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X.Y. Cucurbituril chemistry: A tale of supramolecular success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar]
- Samsonenko, D.G.; Lipkowski, J.; Gerasko, O.A.; Virovets, A.V.; Sokolov, M.N.; Fedin, V.P.; Platas, J.G.; Rita, H.M.; Mederos, A. Cucurbituril as a new macrocyclic ligand for complexation of lanthanide cations in aqueous solutions. Eur. J. Inorg. Chem. 2002, 9, 2380–2388. [Google Scholar]
- Samsonenko, D.G.; Gerasko, O.A.; Lipkowski, J.; Virovets, A.V.; Fedin, V.P. Synthesis and crystal structure of the nanosized supramolecular SmIII complex with macrocyclic cavitand cucurbituril {[Sm(H2O)4]2(C36H36N24O12)3}Br6·44H2O. Russ Chem. Bull. 2002, 51, 1915–1918. [Google Scholar] [CrossRef]
- Samsonenko, D.G.; Sokolov, M.N.; Gerasko, O.A.; Virovets, A.V.; Lipkowski, J.; Fenske, D.; Fedin, V.P. Syntheses and crystal structures of SmIII and ThIV complexes with macrocyclic cavitand cucurbituril. Russ Chem. Bull. 2003, 52, 2132–2139. [Google Scholar] [CrossRef]
- Gerasko, O.A.; Sokolov, M.N.; Fedin, V.P. Mono- and polynuclear aqua complexes and cucurbit[6]uril: Versatile building blocks for supramolecular chemistry. Pure Appl. Chem. 2004, 76, 1633–1646. [Google Scholar] [CrossRef]
- Yan, K.; Huang, Z.X.; Liu, S.M.; Feng, L.; Wu, C.T. Synthesis and crystal structure of new supramolecular adducts of [PtCl6]2− with cucurbit[7]uril: [(H3O)2(PtCl6)]3(C42H42N28O14)2·H2O. Wuhan Univ. J. Nat. Sci. 2004, 9, 99–101. [Google Scholar] [CrossRef]
- Mit’kina, T.V.; Gerasko, O.A.; Sokolov, M.N.; Naumov, D.Y.; Fedin, V.P. Syntheses and crystal structures of supramolecular compounds of tetranuclear ZrIV and HfIV aqua hydroxo complexes with macrocyclic cavitand cucurbituril. Russ. Chem. Bull. 2004, 53, 80–85. [Google Scholar] [CrossRef]
- Samsonenko, D.G.; Gerasko, O.А.; Virovets, А.V.; Fedin, V.P. Synthesis and crystal structure of a supramolecular adduct of trinuclear molybdenum oxocluster with macrocyclic cavitand cucurbit[5]uril containing the included ionic associate Na+ClNa+. Russ. Chem. Bull. 2005, 54, 1557–1562. [Google Scholar] [CrossRef]
- Tripolskaya, A.A.; Mainicheva, E.A.; Mit’kina, T.V.; Gerasko, O.A.; Naumov, D.Y.; Fedin, V.P. Sc(III), Eu(III), and Gd(III) complexes with macrocyclic cavitand cucurbit[6]uril: Synthesis and crystal structures. Russ. J. Coord. Chem. 2005, 31, 768–774. [Google Scholar] [CrossRef]
- Liu, J.X.; Long, L.S.; Huang, R.B.; Zheng, L.S. Molecular capsules based on cucurbit[5]uril encapsulating “naked” anion chlorine. Cryst. Growth Des. 2006, 6, 2611–2614. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Y.Z.; Shi, J.P.; Lu, G.Y. [μ-Cucurbit[6]uril(2-)]bis[pentaaquacalcium(II)] bis[tetrachloridozincate(II)]. Acta Crystallogr. 2007, E63, m1480–m1481. [Google Scholar]
- Kasuga, N.C.; Umeda, M.; Kidokoro, H.; Ueda, K.; Hattori, K.; Yamaguchi, K. Four novel solid-state supramolecular assemblies constructed from decavanadate salts and decamethylcucurbit[5]uril. Cryst. Growth Des. 2009, 9, 1494–1498. [Google Scholar] [CrossRef]
- Liu, J.X.; Dong, C.H.; Long, L.S.; Huang, R.B.; Zheng, L.S. From 1D zigzag chain to 1D tubular structure, weak field ligand-dependent assembly of cucurbit[6]uril-based tubular coordination polymer. Dalton Trans. 2009, 36, 7344–7346. [Google Scholar]
- Mainicheva, E.A.; Tripolskaya, A.A.; Gerasko, O.A.; Naumov, D.Y.; Fedin, V.P. Synthesis and crystal structures of PrIII and NdIII complexes with the macrocyclic cavitand cucurbit[6]uril. Russ. Chem. Bull. 2006, 55, 1566–1573. [Google Scholar] [CrossRef]
- Mainicheva, E.A.; Gerasko, O.A.; Sheludyakova, L.A.; Naumov, D.Y.; Karsanova, I.I.; Amirov, R.R.; Fedin, V.P. Use of the macrocyclic ligand cucurbit[6]uril for isolation of tetranuclear lanthanide aquahydroxo-carboxylate complexes from aqueous solutions. Russ. Chem. Bull. 2006, 55, 1956–1965. [Google Scholar] [CrossRef]
- Tripol’skaya, A.A.; Mainicheva, E.A.; Gerasko, O.A.; Naumov, D.Y.; Fedin, V.P. Synthesis and crystal structure of a supramolecular adduct of the aqua nitrato complex of gadolinium [Gd(NO3)(H2O)7]2+ with macrocyclic cavitand cucurbit[6]uril. J. Struct. Chem. 2007, 48, 547–551. [Google Scholar] [CrossRef]
- Gerasko, O.A.; Mainicheva, E.A.; Naumova, M.I.; Yurjeva, O.P.; Alberola, A.; Vicent, C.; Liusar, R.; Fedin, V.P. Tetranuclear lanthanide aqua hydroxo complexes with macrocyclic ligand cucurbit[6]uril. Eur. J. Inorg. Chem. 2008, 416–424. [Google Scholar]
- Thuéry, P. Uranylion complexes with cucurbit[n]urils (n = 6, 7, and 8): A new family of uranyl-organic frameworks. Cryst. Growth Des. 2008, 8, 4132–4143. [Google Scholar] [CrossRef]
- Gerasko, O.A.; Mainicheva, E.A.; Naumova, M.I.; Neumaier, M.; Kappes, M.M.; Lebedkin, S.; Fenske, D.; Fedin, V.P. Sandwich-type tetranuclear lanthanide complexes with cucurbit[6]uril: From molecular compounds to coordination polymers. Inorg. Chem. 2008, 47, 8869–8880. [Google Scholar] [CrossRef]
- Thuéry, P. Lanthanide complexes with cucurbit[n]urils (n = 5, 6, 7) and perrhenateligands: New examples of encapsulation of perrhenateanions. Inorg. Chem. 2009, 48, 4497–4513. [Google Scholar] [CrossRef]
- Thuéry, P. Uranyl-lanthanide heterometallic complexes with cucurbit[6]uril and perrhenate ligands. Inorg. Chem. 2009, 48, 825–827. [Google Scholar] [CrossRef]
- Thuéry, P. Uranyl ion complexes of cucurbit[7]uril with zero-, one- and two-dimensionality. CrystEngComm 2009, 11, 1150–1156. [Google Scholar] [CrossRef]
- Thuéry, P. Uranylion complexes with cucurbit[5]uril: From molecular capsules to uranyl-organic frameworks. Cryst. Growth Des. 2009, 9, 1208–1215. [Google Scholar]
- Thuéry, P.; Masci, B. Uranylion complexation by cucurbiturils in the presence of perrhenic, phosphoric, or polycarboxylic acids. Novel mixed-ligand uranyl-organic frameworks. Cryst. Growth Des. 2010, 10, 716–722. [Google Scholar] [CrossRef]
- Thuéry, P. Second-sphere tethering of rare-earth ions to cucurbit[6]uril by iminodiacetic acid involving carboxylic group encapsulation. Inorg. Chem. 2010, 49, 9078–9085. [Google Scholar] [CrossRef]
- Thuéry, P. Uranylion complexation by aliphatic dicarboxylic acids in the presence of cucurbiturils as additional ligands or structure-directing agents. Cryst. Growth Des. 2011, 11, 2606–2620. [Google Scholar] [CrossRef]
- Thuéry, P. l-Cysteine as a chiral linker in lanthanide–cucurbit[6]uril one-dimensional assemblies. Inorg. Chem. 2011, 50, 10558–10560. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Sokolov, M.N.; Sykes, A.G. Behavioral patterns of heterometallic cuboidal derivatives of [M3Q4(H2O)9]4+ (M = Mo, W; Q = S, Se). Acc. Chem. Res. 2001, 34, 223–230. [Google Scholar]
- Hernandez, M.R.; Sokolov, M.; Esparza, P.; Vicent, C.; Llusar, R. Aqueous solution chemistry of [Mo3CuSe4]n+ (n = 4, 5) and [W3CuQ4]5+ (Q = S, Se) clusters. Dalton Trans. 2004, 6, 847–851. [Google Scholar]
- Fedin, V.P. New lines of research in chemistry of chalcogenide complexes: From clusters to supramolecular compounds. J. Coord. Chem. 2004, 30, 151–152. [Google Scholar] [CrossRef]
- Molina, R.H.; Sokolov, M.N.; Clausen, M.; Clegg, W. Synthesis and structure of nickel-containing cuboidal clusters derived from [W3Se4(H2O)9]4+. Site-differentiated substitution at the nickel site in the series [W3NiQ4(H2O)10]4+ (Q = S, Se). Inorg. Chem. 2006, 45, 10567–10567. [Google Scholar] [CrossRef]
- Chubarova, E.V.; Sokolov, M.N.; Samsonenko, D.G.; Vicent, C.; Fedin, V.P. upramolecular compounds of chloroaqua complexes [Mo3Q4(H2O)9−xClx](4−x)+ (Q=S, Se; x=2, 3, 5) with cucurbit[n]urils. J. Struct. Chem. 2006, 47, 939–945. [Google Scholar] [CrossRef]
- Molina, R.H.; Kalinina, I.; Sokolov, M.; Clausen, M.; Platas, J.G.; Vicent, C.; Llusar, R. Synthesis, structure and reactivity of cuboidal-type cluster aqua complexes with W3PdS44+ core. Dalton Trans. 2007, 550–557. [Google Scholar]
- Abramov, P.A.; Sokolov, M.N.; Virovets, A.V.; Peresypkina, E.V.; Fedin, V.P. Synthesis and crystal structure of cucurbit[6]uril adduct of hydrogen-bonded cluster complex [Mo3(μ3-Se)(μ2-O)3(H2O)6Cl3]+. J. Clust. Sci. 2007, 18, 597–605. [Google Scholar]
- Hernandez-Molina, R.; Kalinina, I.V.; Sokolov, M.N.; Peris, G.; Llusar, R. Studies on iron-containing chalcogenide clusters with core M3FeQ4 (M = Mo, W; Q = S, Se). Synth. React. Inorg. M. 2007, 37, 765–770. [Google Scholar]
- Algarra, A.G.; Sokolov, M.N.; Gonzalez-Platas, J.; Fernandez-Trujillo, M.J.; Basallote, M.G.; Hernandez-Molina, R. Synthesis, reactivity, and kinetics of substitution in W3PdSe4 cuboidal clusters. Are examination of the kinetics of substitution of the related W3S4 cluster with thiocyanate. Inorg. Chem. 2009, 48, 3639–3649. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Ooi, B.; Harris, P.; Vicent, C.; Sokolov, M.N. Synthesis and characterization of mixed chalcogen triangular complexes with new Mo3(µ3-S)(µ2-Se2)34+ and M3(µ3-S)(µ2-Se)34+ (M = Mo, W) cluster cores. Inorg. Chem. 2009, 48, 3832–3839. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.Y.; Whang, D.; Kim, K. Shape-induced, hexagonal, open frameworks: Rubidium ion complexed cucurbituril. Angew. Chem. Int. Ed. 1999, 38, 641–643. [Google Scholar]
- Heo, J.; Kim, J.; Whang, D.; Kim, K. Columnar one-dimensional coordination polymer formed with a metal ion and a host-guest complex as building blocks: potassium ion complexed cucurbituril. Inorg. Chim. Acta 2000, 297, 307–312. [Google Scholar] [CrossRef]
- Zhang, F.; Yajima, T.; Li, Y.Z.; Xu, G.Z.; Chen, H.L.; Liu, Q.T.; Yamauchi, O. Iodine-assisted assembly of helical coordination polymers of cucurbituril and asymmetric copper(ii) complexes. Angew. Chem. Int. Ed. 2005, 44, 3402–3407. [Google Scholar] [CrossRef]
- Chen, K.; Liang, L.L.; Zhang, Y.Q.; Zhu, Q.J.; Xue, S.F.; Tao, Z. Novel supramolecular assemblies based on coordination of samarium cation to cucurbit[5]uril. Inorg. Chem. 2011, 50, 7754–7760. [Google Scholar] [CrossRef]
- Chen, K.; Feng, X.; Liang, L.L.; Zhang, Y.Q.; Zhu, Q.J.; Xue, S.F.; Tao, Z. Coordination and supramolecular self-assemblies of alkali and alkaline earth metal ions to cucurbit[5]uril in the presence of nitrophenol. Cryst. Growth Des. 2011, 11, 5712–5722. [Google Scholar] [CrossRef]
- Chen, W.J.; Yu, D.H.; Xiao, X.; Zhang, Y.Q.; Zhu, Q.J.; Xue, S.F.; Tao, Z.; Wei, G. Difference of coordination between alkali- and alkaline-earth-metal ions to a symmetrical α,α′,δ,δ′-tetramethylcucurbit[6]uril. Inorg. Chem. 2011, 50, 6956–6964. [Google Scholar] [CrossRef]
- Feng, X.; Du, H.; Chen, K.; Xiao, X.; Luo, S.X.; Xue, S.F.; Zhang, Y.Q.; Zhu, Q.J.; Tao, Z.; Zhang, X.Y.; Wei, G. Design and synthesis of self-assembly supramolecular entities based on noncovalent interaction of cucurbit[5]uril, metal ions, and hydroxybenzene or its derivatives. Cryst. Growth Des. 2010, 10, 2901–2907. [Google Scholar] [CrossRef]
- Chen, K.; Liang, L.L.; Cong, H.; Xiao, X.; Zhang, Y.Q.; Xue, S.F.; Zhu, Q.J.; Tao, Z. p-Hydroxybenzoic acid-induced formation of a novel framework based on direct coordination of caesium ions to cucurbit[8]uril. CrystEngComm 2012, 14, 3862–3864. [Google Scholar] [CrossRef]
- Chen, K.; Cong, H.; Xiao, X.; Zhang, Y.Q.; Xue, S.F.; Tao, Z.; Zhu, Q.J.; Wei, G. Hydroquinone-induced framework based on direct coordination of rubidium ions to cucurbit[7]uril. CrystEngComm 2011, 13, 5105–5110. [Google Scholar] [CrossRef]
- Feng, X.; Chen, K.; Zhang, Y.Q.; Xue, S.F.; Zhu, Q.J.; Tao, Z.; Day, A.I. Stable cucurbit[5]uril MOF structures as “beaded” rings built on a p-hydroxybenzoic acid template—A small molecule absorption material. CrystEngComm 2011, 13, 5049–5051. [Google Scholar] [CrossRef]
- Liang, L.L.; Ni, X.L.; Zhao, Y.; Chen, K.; Xiao, X.; Zhang, Y.Q.; Redshaw, C.; Zhu, Q.J.; Xue, S.F.; Tao, Z. Construction of cucurbit[7]uril-based tubular nanochannels incorporating associated [CdCl4]2− and lanthanide ions. Inorg. Chem. 2013, 52, 1909–1915. [Google Scholar] [CrossRef]
- Liang, L.L.; Zhao, Y.; Zhang, Y.Q.; Tao, Z.; Zhu, Q.J.; Xue, S.F.; Liu, J.X. Coordination nanotubes self-assembled from cucurbit[7]uril and lanthanide cations. CrystEngComm 2013, 15, 3943–3950. [Google Scholar] [CrossRef]
- Liang, L.L.; Chen, K.; Ji, N.N.; Cheng, X.J.; Zhao, Y.; Xiao, X.; Zhang, Y.Q.; Zhu, Q.J.; Xue, S.F.; Tao, Z. Tetrachloride transition-metal dianion-induced coordination and supramolecular self-assembly of strontium dications to cucurbit[8]uril. CrystEngComm 2013, 15, 2416–2421. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97 Program for the Solution and Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- CCDC CIF Depository Request Form. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 9 May 2013).
- Fang, X.K.; Kogerler, P.; Isaacs, L.; Uchida, S.; Mizuno, N. Cucurbit[n]uril–Polyoxoanion Hybrids. J. Am. Chem. Soc. 2009, 131, 432–433. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, L.-L.; Zhao, Y.; Chen, K.; Xiao, X.; Clegg, J.K.; Zhang, Y.-Q.; Tao, Z.; Xue, S.-F.; Zhu, Q.-J.; Wei, G. One-Dimensional Coordination Polymers of Lanthanide Cations to Cucurbit[7]uril Built Using a Range of Tetrachloride Transition-Metal Dianion Structure Inducers. Polymers 2013, 5, 418-430. https://doi.org/10.3390/polym5020418
Liang L-L, Zhao Y, Chen K, Xiao X, Clegg JK, Zhang Y-Q, Tao Z, Xue S-F, Zhu Q-J, Wei G. One-Dimensional Coordination Polymers of Lanthanide Cations to Cucurbit[7]uril Built Using a Range of Tetrachloride Transition-Metal Dianion Structure Inducers. Polymers. 2013; 5(2):418-430. https://doi.org/10.3390/polym5020418
Chicago/Turabian StyleLiang, Li-Li, Yi Zhao, Kai Chen, Xin Xiao, Jack K. Clegg, Yun-Qian Zhang, Zhu Tao, Sai-Feng Xue, Qian-Jiang Zhu, and Gang Wei. 2013. "One-Dimensional Coordination Polymers of Lanthanide Cations to Cucurbit[7]uril Built Using a Range of Tetrachloride Transition-Metal Dianion Structure Inducers" Polymers 5, no. 2: 418-430. https://doi.org/10.3390/polym5020418



