Progress in Imidazolium Ionic Liquids Assisted Fabrication of Carbon Nanotube and Graphene Polymer Composites
Abstract
:1. Introduction
2. Exfoliation of Carbon Nanotubes and Graphene Sheets in Imidazolium Ionic Liquids
2.1. Carbon Nanotubes in Imidazolium Ionic Liquids
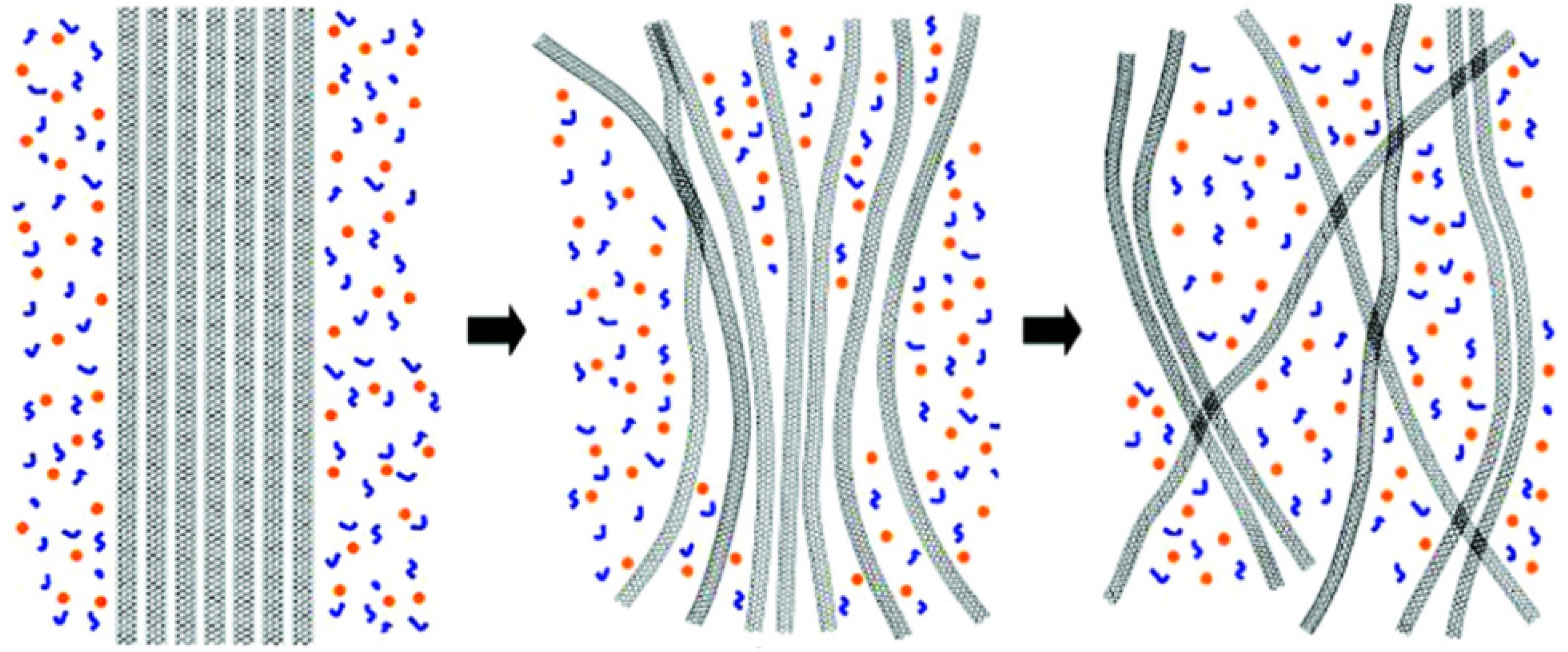
2.2. Graphene Sheets in Imidazolium Ionic Liquids
3. Imidazolium Ionic Liquids Assisted Fabrication of Polymer Composites
3.1. Fluoropolymer Composites
3.1.1. With Carbon Nanotubes
3.1.2. With Graphene
3.2. Hydrocarbon Polymer Composites
3.2.1. With Carbon Nanotubes
3.2.2. With Graphene
3.3. Polyacrylate Composites
3.3.1. With Carbon Nanotubes
3.3.2. With Graphene
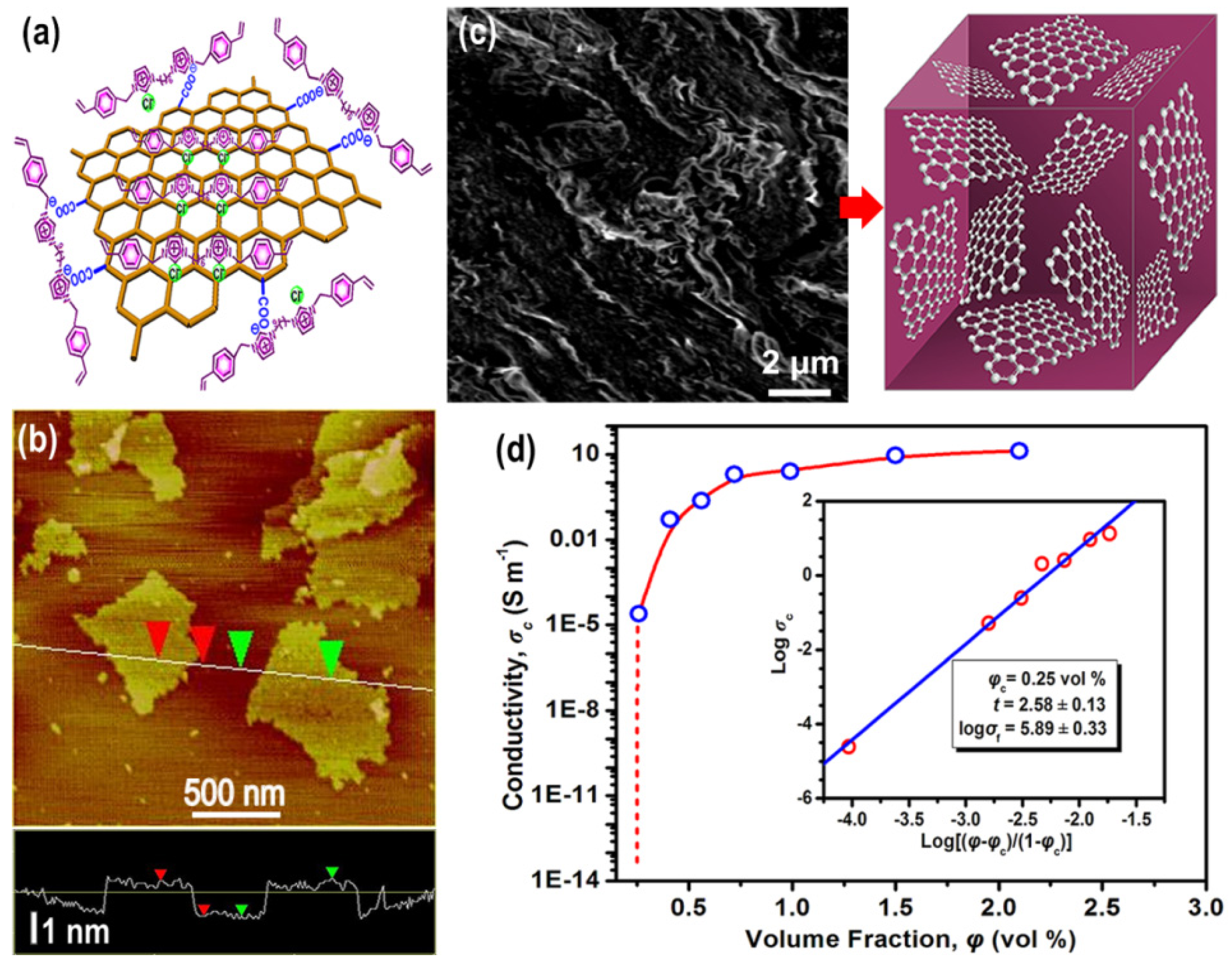
3.4. Cellulose Composites
3.4.1. With Carbon Nanotubes
3.4.2. With Graphene
4. Poly(ionic liquid) Composites
4.1. With Carbon Nanotubes
4.2. With Graphene
5. Conclusions
Acknowledgments
Conflict of interest
References
- Winey, K.I.; Vaia, R.A. Polymer nanocomposites. MRS Bull. 2007, 32, 314–322. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gun’ko, Y.K. Recent advances in research on carbon nanotube—Polymer composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Tour, J.M. Materials science: Nanotube composites. Nature 2007, 447, 1066–1068. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopez-Manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef]
- Novoselov, K.S. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; Devries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layerwalls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tomanek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef]
- Durkop, T.; Getty, S.A.; Cobas, E.; Fuhrer, M.S. Extraordinary mobility in semiconducting carbon nanotubes. Nano Lett. 2004, 4, 35–39. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.J.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308–1308. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.F.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Xie, X.L.; Mai, Y.-W.; Zhou, X.P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Cheng, Q.; Bao, J.; Park, J.; Liang, Z.; Zhang, C.; Wang, B. High mechanical performance composite conductor: Multi-walled carbon nanotube sheet/bismaleimide nanocomposites. Adv. Funct. Mater. 2009, 19, 3219–3225. [Google Scholar] [CrossRef]
- Yuan, W.; Che, J.F.; Chan-Park, M.B. A novel polyimide dispersing matrix for highly electrically conductive solution-cast carbon nanotube-based composite. Chem. Mater. 2011, 23, 4149–4157. [Google Scholar] [CrossRef]
- Lin, H.; Li, L.; Ren, J.; Cai, Z.; Qiu, L.; Yang, Z.; Peng, H. Conducting polymer composite film incorporated with aligned carbon nanotubes for transparent, flexible and efficient supercapacitor. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Kim, S.N.; Rusling, J.F.; Papadimitrakopoulos, F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv. Mater. 2007, 19, 3214–3228. [Google Scholar] [CrossRef]
- Lu, F.; Gu, L.; Meziani, M.J.; Wang, X.; Luo, P.G.; Veca, L.M.; Cao, L.; Sun, Y.-P. Advances in bioapplications of carbon nanotubes. Adv. Mater. 2009, 21, 139–152. [Google Scholar] [CrossRef]
- Lu, L.; Chen, W. Biocompatible composite actuator: A supramolecular structure consisting of the biopolymer chitosan, carbon nanotubes, and an ionic Liquid. Adv. Mater. 2010, 22, 3745–3748. [Google Scholar] [CrossRef]
- Koerner, H.; Price, G.; Pearce, N.A.; Alexander, M.; Vaia, R.A. Remotely actuated polymer nanocomposites—Stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 2004, 3, 115–120. [Google Scholar] [CrossRef]
- Ahir, S.V.; Terentjev, E.M. Photomechanical actuation in polymer-nanotube composites. Nat. Mater. 2005, 4, 491–495. [Google Scholar] [CrossRef]
- Hasan, T.; Sun, Z.; Wang, F.; Bonaccorso, F.; Tan, P.H.; Rozhin, A.G.; Ferrari, A.C. Nanotube-polymer composites for ultrafast photonics. Adv. Mater. 2009, 21, 3874–3899. [Google Scholar] [CrossRef]
- Park, H.; Brown, P.R.; Bulović, V.; Kong, J. Graphene as transparent conducting electrodes in organic photovoltaics: Studies in graphene morphology, hole transporting layers, and counter electrodes. Nano Lett. 2011, 12, 133–140. [Google Scholar]
- Ren, S.Q.; Bernardi, M.; Lunt, R.R.; Bulovic, V.; Grossman, J.C.; Gradecak, S. Toward efficient carbon nanotube/P3HT solar cells: Active layer morphology, electrical, and optical propertie. Nano Lett. 2011, 11, 5316–5321. [Google Scholar] [CrossRef]
- Han, J.; Kim, H.; Kim, D.Y.; Jo, S.M.; Jang, S.Y. Water-soluble polyelectrolyte-grafted multiwalled carbon nanotube thin films for efficient counter electrode of dye-sensitized solar cells. ACS Nano 2010, 4, 3503–3509. [Google Scholar] [CrossRef]
- Hellstrom, S.L.; Lee, H.W.; Bao, Z.N. Polymer-assisted direct deposition of uniform carbon nanotube bundle networks for high performance transparent electrodes. ACS Nano 2009, 3, 1423–1430. [Google Scholar] [CrossRef]
- Huang, S.Q.; Li, L.; Yang, Z.B.; Zhang, L.L.; Saiyin, H.; Chen, T.; Peng, H.S. A new and general fabrication of an aligned carbon nanotube/polymer film for electrode applications. Adv. Mater. 2011, 23, 4707–4710. [Google Scholar] [CrossRef]
- Yang, Y.K.; Xie, X.L.; Mai, Y.-W. Functionalization of Carbon Nanotubes for Polymer Nanocomposites. In Functionalization of Carbon Nanotubes for Polymer Nanocomposites: Synthesis, Properties and Applications; McNally, T., Pötschke, P., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 55–91. [Google Scholar]
- Yang, Y.K.; Xie, X.L.; Cui, W. Functionalization of Carbon Nanotubes with Ionic Liquids. In Green Solvents II: Properties and Applications in Chemistry; Mohammad, A., Inamuddin, Eds.; Springer Dordrecht Heidelberg: New York, London, 2012; pp. 399–434. [Google Scholar]
- Yang, Y.K.; Qiu, S.Q.; Wang, X.B.; Xie, X.L. Functionalization and structure control of carbon nanotubes with polymers: Polymer-grafted carbon nanotubes. Prog. Chem. 2010, 22, 684–695. [Google Scholar]
- Kaiser, A.B.; Skakalova, V. Electronic conduction in polymers, carbon nanotubes and graphene. Chem. Soc. Rev. 2011, 40, 3786–3801. [Google Scholar] [CrossRef]
- Yang, Y.K.; Qiu, S.Q.; Xie, X.L.; Wang, X.B.; Li, R.K.Y. A facile, green, and tunable method to functionalize carbon nanotubes with water-soluble azo initiators by one-step free radical addition. Appl. Surf. Sci. 2010, 256, 3286–3292. [Google Scholar] [CrossRef]
- Yang, Y.K.; Yu, L.J.; Peng, R.G.; Huang, Y.L.; He, C.E.; Liu, H.Y.; Wang, X.B.; Xie, X.L.; Mai, Y.-W. Incorporation of liquid-like multiwalled carbon nanotubes into an epoxy matrix by solvent-free processing. Nanotechnology 2012, 23, e225701. [Google Scholar] [CrossRef]
- Yang, Y.K.; Xie, X.L.; Wu, J.G.; Mai, Y.W. Synthesis and self-assembly of polystyrene-grafted multiwalled carbon nanotubes with a hairy-rod nanostructure. J. Polym. Sci. Polym. Chem. 2006, 44, 3869–3881. [Google Scholar] [CrossRef]
- Yang, Y.K.; Mao, L.B.; Xie, X.L.; Mai, Y.W. Liquid crystallinity and novel assembly of amorphous polymer grafted carbon nanotubes. Solid State Phenom. 2007, 1411–1414. [Google Scholar] [CrossRef]
- Yang, Y.K.; Xie, X.L.; Wu, J.G.; Yang, Z.F.; Wang, X.T.; Mai, Y.W. Multiwalled carbon nanotubes functionalized by hyperbranched poly(urea-urethane)s by a one-pot polycondensation. Macromol. Rapid Comm. 2006, 27, 1695–1701. [Google Scholar] [CrossRef]
- Yang, Y.K.; Xie, X.L.; Yang, Z.F.; Wang, X.T.; Cui, W.; Yang, J.Y.; Mai, Y.W. Controlled synthesis and novel solution rheology of hyperbranched poly(urea-urethane)-functionalized multiwalled carbon nanotubes. Macromolecules 2007, 40, 5858–5867. [Google Scholar] [CrossRef]
- Yang, Y.K.; Tsui, C.P.; Tang, C.Y.; Qiu, S.Q.; Zhao, Q.; Cheng, X.J.; Sun, Z.G.; Li, R.K.Y.; Xie, X.L. Functionalization of carbon nanotubes with biodegradable supramolecular polypseudorotaxanes from grafted-poly(ε-caprolactone) and α-cyclodextrins. Eur. Polym. J. 2010, 46, 145–155. [Google Scholar] [CrossRef]
- Yang, Y.K.; Qiu, S.Q.; He, C.G.; He, W.J.; Yu, L.J.; Xie, X.L. Green chemical functionalization of multiwalled carbon nanotubes with poly(ε-caprolactone) in ionic liquids. Appl. Surf. Sci. 2010, 257, 1010–1014. [Google Scholar] [CrossRef]
- Yang, Y.K.; Wang, X.T.; Liu, L.; Xie, X.L.; Yang, Z.F.; Li, R.K.Y.; Mai, Y.W. Structure and photoresponsive behaviors of multiwalled carbon nanotubes grafted by polyurethanes containing azobenzene side chains. J. Phys. Chem. C 2007, 111, 11231–11239. [Google Scholar] [CrossRef]
- Du, F.P.; Wu, K.B.; Yang, Y.K.; Liu, L.; Gan, T.; Xie, X.L. Synthesis and electrochemical probing of water-soluble poly(sodium 4-styrenesulfonate-co-acrylic acid)-grafted multiwalled carbon nanotubes. Nanotechnology 2008, 19, e085716. [Google Scholar] [CrossRef]
- Zhang, R.H.; Yang, Y.K.; Xie, X.L.; Li, R.K.Y. Dispersion and crystallization studies of hyper-branched poly(urea-urethane)s-grafted carbon nanotubes filled polyamide-6 nanocomposites. Compos. A 2010, 41, 670–677. [Google Scholar] [CrossRef]
- Cui, W.; Du, F.P.; Zhao, J.C.; Zhang, W.; Yang, Y.K.; Xie, X.L.; Mai, Y.-W. Improving thermal conductivity while retaining high electrical resistivity of epoxy composites by incorporating silica-coated multi-walled carbon nanotubes. Carbon 2011, 49, 495–500. [Google Scholar] [CrossRef]
- Weingärtner, H. Understanding ionic liquids at the molecular level: Facts, problems, and controversies. Angew. Chem. Int. Ed. 2008, 47, 654–670. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2007, 108, 206–237. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1709–1726. [Google Scholar] [CrossRef]
- Antonietti, M.; Kuang, D.; Smarsly, B.; Zhou, Y. Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef]
- Price, B.K.; Hudson, J.L.; Tour, J.M. Green chemical functionalization of single-walled carbon nanotubes in ionic liquids. J. Am. Chem. Soc. 2005, 127, 14867–14870. [Google Scholar] [CrossRef]
- Zhou, X.S.; Wu, T.B.; Ding, K.L.; Hu, B.J.; Hou, M.Q.; Han, B.X. Dispersion of graphene sheets in ionic liquid bmim PF6 stabilized by an ionic liquid polymer. Chem. Commun. 2010, 46, 386–388. [Google Scholar]
- Yang, Y.K.; He, C.E.; Peng, R.G.; Baji, A.; Du, X.S.; Huang, Y.L.; Xie, X.L.; Mai, Y.-W. Non-covalently modified graphene sheets by imidazolium ionic liquids for multifunctional polymer nanocomposites. J. Mater. Chem. 2012, 22, 5666–5675. [Google Scholar]
- Fukushima, T.; Aida, T. Ionic liquids for soft functional materials with carbon nanotubes. Chem. Eur. J. 2007, 13, 5048–5058. [Google Scholar] [CrossRef]
- Ausman, K.D.; Piner, R.; Lourie, O.; Ruoff, R.S.; Korobov, M. Organic solvent dispersions of single-walled carbon nanotubes: Toward solutions of pristine nanotubes. J. Phys. Chem. B 2000, 104, 8911–8915. [Google Scholar] [CrossRef]
- Furtado, C.A.; Kim, U.J.; Gutierrez, H.R.; Pan, L.; Dickey, E.C.; Eklund, P.C. Debundling and dissolution of single-walled carbon nanotubes in amide solvents. J. Am. Chem. Soc. 2004, 126, 6095–6105. [Google Scholar] [CrossRef]
- Bahr, J.L.; Mickelson, E.T.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem. Commun. 2001, 2, 193–194. [Google Scholar]
- Giordani, S.; Bergin, S.D.; Nicolosi, V.; Lebedkin, S.; Kappes, M.M.; Blau, W.J.; Coleman, J.N. Debundling of single-walled nanotubes by dilution: Observation of large populations of individual nanotubes in amide solvent dispersions. J. Phys. Chem. B 2006, 110, 15708–15718. [Google Scholar]
- Landi, B.J.; Ruf, H.J.; Worman, J.J.; Raffaelle, R.P. Effects of alkyl amide solvents on the dispersion of single-wall carbon nanotubes. J. Phys. Chem. B 2004, 108, 17089–17095. [Google Scholar] [CrossRef]
- Cheng, Q.; Debnath, S.; Gregan, E.; Byrne, H.J. Effect of solvent solubility parameters on the dispersion of single-walled carbon nanotubes. J. Phys. Chem. C 2008, 112, 20154–20158. [Google Scholar] [CrossRef]
- Kim, D.S.; Nepal, D.; Geckeler, K.E. Individualization of single-walled carbon nanotubes: Is the solvent important? Small 2005, 1, 1117–1124. [Google Scholar] [CrossRef]
- Cheng, Q.; Debnath, S.; O’Neill, L.; Hedderman, T.G.; Gregan, E.; Byrne, H.J. Systematic study of the dispersion of SWNTs in organic solvents. J. Phys. Chem. C 2010, 114, 4857–4863. [Google Scholar]
- Coleman, J.N. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 2009, 19, 3680–3695. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Bellayer, S.; Gilman, J.W.; Eidelman, N.; Bourbigot, S.; Flambard, X.; Fox, D.M.; de Long, H.C.; Trulove, P.C. Preparation of homogeneously dispersed multiwalled carbon nanotube/polystyrene nanocomposites via melt extrusion using trialkyl imidazolium compatibilizer. Adv. Funct. Mater. 2005, 15, 910–916. [Google Scholar] [CrossRef]
- Shim, Y.; Kim, H.J. Solvation of carbon nanotubes in a room-temperature ionic liquid. ACS Nano 2009, 3, 1693–1702. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chu, H.B.; Li, Y. Why single-walled carbon nanotubes can be dispersed in imidazolium-based ionic liquids. ACS Nano 2008, 2, 2540–2546. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Demurtas, D.; Renzetti, A.; Siani, G.; de Maria, P.; Meneghetti, M.; Prato, M.; Fontana, A. Disaggregation of single-walled carbon nanotubes (SWNTs) promoted by the ionic liquid-based surfactant 1-hexadecyl-3-vinyl-imidazolium bromide in aqueous solution. Soft Matter 2009, 5, 62–66. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shen, Y.F.; Li, J.H.; Niu, L.; Dong, S.J.; Ivaska, A. Electrochemical functionalization of single-walled carbon nanotubes in large quantities at a room-temperature ionic liquid supported three-dimensional network electrode. Langmuir 2005, 21, 4797–4800. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, S. Electrochromic device based on carbon nanotubes functionalized poly (methyl pyrrole) synthesized in hydrophobic ionic liquid medium. Electrochem. Commun. 2008, 10, 895–898. [Google Scholar] [CrossRef]
- Wei, D.; Kvarnstrom, C.; Lindfors, T.; Ivaska, A. Electrochemical functionalization of single walled carbon nanotubes with polyaniline in ionic liquids. Electrochem. Commun. 2007, 9, 206–210. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar]
- Compton, O.C.; Jain, B.; Dikin, D.A.; Abouimrane, A.; Amine, K.; Nguyen, S.T. Chemically active reduced graphene oxide with tunable C/O ratios. ACS Nano 2011, 5, 4380–4391. [Google Scholar] [CrossRef]
- Liang, Y.T.; Vijayan, B.K.; Gray, K.A.; Hersam, M.C. Minimizing graphene defects enhances titania nanocomposite-based photocatalytic reduction of Co(II) for improved solar fuel production. Nano Lett. 2011, 11, 2865–2870. [Google Scholar] [CrossRef]
- Viet, H.P.; Tran, V.C.; Hur, S.H.; Oh, E.; Kim, E.J.; Shin, E.W.; Chung, J.S. Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J. Mater. Chem. 2011, 21, 3371–3377. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef]
- Chen, W.F.; Yan, L.F.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen, D.; Ruoff, R.S. Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano 2010, 4, 1227–1233. [Google Scholar] [CrossRef]
- Keeley, G.P.; O’Neill, A.; McEvoy, N.; Peltekis, N.; Coleman, J.N.; Duesberg, G.S. Electrochemical ascorbic acid sensor based on DMF-exfoliated graphene. J. Mater. Chem. 2010, 20, 7864–7869. [Google Scholar] [CrossRef]
- Zhao, W.F.; Fang, M.; Wu, F.R.; Wu, H.; Wang, L.W.; Chen, G.H. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Choi, E.K.; Jeon, I.Y.; Bae, S.Y.; Lee, H.J.; Shin, H.S.; Dai, L.M.; Baek, J.B. High-yield exfoliation of three-dimensional graphite into two-dimensional graphene-like sheets. Chem. Commun. 2010, 46, 6320–6322. [Google Scholar]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.Y.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.Z.; Tour, J.M.; Barron, A.R. High-yield organic dispersions of unfunctionalized graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Rangappa, D.; Sone, K.; Wang, M.; Gautam, U.K.; Golberg, D.; Itoh, H.; Ichihara, M.; Honma, I. Rapid and direct conversion of graphite crystals into high-yielding, good-quality graphene by supercritical fluid exfoliation. Chem. Eur. J. 2010, 16, 6488–6494. [Google Scholar]
- Cui, X.; Zhang, C.Z.; Hao, R.; Hou, Y.L. Liquid-phase exfoliation, functionalization and applications of graphene. Nanoscale 2011, 3, 2118–2126. [Google Scholar] [CrossRef]
- Liu, N.; Luo, F.; Wu, H.X.; Liu, Y.H.; Zhang, C.; Chen, J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Lu, J.; Yang, J.X.; Wang, J.Z.; Lim, A.L.; Wang, S.; Loh, K.P. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef]
- Wang, X.Q.; Fulvio, P.F.; Baker, G.A.; Veith, G.M.; Unocic, R.R.; Mahurin, S.M.; Chi, M.F.; Dai, S. Direct exfoliation of natural graphite into micrometre size few layers graphene sheets using ionic liquids. Chem. Commun. 2010, 46, 4487–4489. [Google Scholar]
- Nuvoli, D.; Valentini, L.; Alzari, V.; Scognamillo, S.; Bon, S.B.; Piccinini, M.; Illescas, J.; Mariani, A. High concentration few-layer graphene sheets obtained by liquid phase exfoliation of graphite in ionic liquid. J. Mater. Chem. 2011, 21, 3428–3431. [Google Scholar] [CrossRef]
- Shang, N.G.; Papakonstantinou, P.; Sharma, S.; Lubarsky, G.; Li, M.; McNeill, D.W.; Quinn, A.J.; Zhou, W.; Blackley, R. Controllable selective exfoliation of high-quality graphene nanosheets and nanodots by ionic liquid assisted grinding. Chem. Commun. 2012, 48, 1877–1879. [Google Scholar] [CrossRef]
- Safavi, A.; Tohidi, M.; Mahyari, F.A.; Shahbaazi, H. One-pot synthesis of large scale graphene nanosheets from graphite-liquid crystal composite via thermal treatment. J. Mater. Chem. 2012, 22, 3825–3831. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, T.; Song, H.K.; Cho, J.; Kim, B.S. Ionic liquid modified graphene nanosheets anchoring manganese oxide nanoparticles as efficient electrocatalysts for Zn-air batteries. Energy Environ. Sci. 2011, 4, 4148–4154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.Q.; Ning, W.; Zhang, J.M.; Qiao, X.; Zhang, J.; He, J.S.; Liu, C.Y. Stable dispersions of reduced graphene oxide in ionic liquids. J. Mater. Chem. 2010, 20, 5401–5403. [Google Scholar] [CrossRef]
- Ji, Q.M.; Honma, I.; Paek, S.M.; Akada, M.; Hill, J.P.; Vinu, A.; Ariga, K. Layer-by-layer films of graphene and ionic liquids for highly selective gas sensing. Angew. Chem. Int. Ed. 2010, 49, 9737–9739. [Google Scholar] [CrossRef]
- Guo, C.X.; Lu, Z.S.; Lei, Y.; Li, C.M. Ionic liquid-graphene composite for ultratrace explosive trinitrotoluene detection. Electrochem. Commun. 2010, 12, 1237–1240. [Google Scholar] [CrossRef]
- Guo, S.J.; Wen, D.; Zhai, Y.M.; Dong, S.J.; Wang, E.K. Ionic liquid-graphene hybrid nanosheets as an enhanced material for electrochemical determination of trinitrotoluene. Biosens. Bioelectron. 2011, 26, 3475–3481. [Google Scholar] [CrossRef]
- Ng, S.R.; Guo, C.X.; Li, C.M. Highly sensitive nitric oxide sensing using three-dimensional graphene/ionic liquid nanocomposite. Electroanalysis 2011, 23, 442–448. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhu, J.J. Sonoelectrochemical fabrication of Pd-graphene nanocomposite and its application in the determination of chlorophenols. Electrochim. Acta 2011, 56, 6008–6013. [Google Scholar] [CrossRef]
- Yang, M.H.; Choi, B.G.; Park, H.; Park, T.J.; Hong, W.H.; Lee, S.Y. Directed self-assembly of gold nanoparticles on graphene-ionic liquid hybrid for enhancing electrocatalytic activity. Electroanalysis 2011, 23, 850–857. [Google Scholar] [CrossRef]
- Peng, J.Y.; Hou, C.T.; Liu, X.X.; Li, H.B.; Hu, X.Y. Electrochemical behavior of azithromycin at graphene and ionic liquid composite film modified electrode. Talanta 2011, 86, 227–232. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wang, Z.L.; Cao, Y.Y.; Jing, Y.F.; Liu, Y.L. High sensitive simultaneous determination of hydroquinone and catechol based on graphene/BMIMPF6 nanocomposite modified electrode. Sens. Actuators B 2011, 157, 540–546. [Google Scholar] [CrossRef]
- Kim, T.; Kang, H.C.; Tung, T.T.; Lee, J.D.; Kim, H.; Yang, W.S.; Yoon, H.G.; Suh, K.S. Ionic liquid-assisted microwave reduction of graphite oxide for supercapacitors. RSC Adv. 2012, 2, 8808–8812. [Google Scholar]
- Marquardt, D.; Vollmer, C.; Thomann, R.; Steurer, P.; Mulhaupt, R.; Redel, E.; Janiak, C. The use of microwave irradiation for the easy synthesis of graphene-supported transition metal nanoparticles in ionic liquids. Carbon 2011, 49, 1326–1332. [Google Scholar] [CrossRef]
- Shen, J.F.; Shi, M.; Yan, B.; Ma, H.W.; Li, N.; Ye, M.X. Ionic liquid-assisted one-step hydrothermal synthesis of TiO2-reduced graphene oxide composites. Nano Res. 2011, 4, 795–806. [Google Scholar] [CrossRef]
- Shen, J.F.; Shi, M.; Yan, B.; Ma, H.W.; Li, N.; Ye, M.X. One-pot hydrothermal synthesis of Ag-reduced graphene oxide composite with ionic liquid. J. Mater. Chem. 2011, 21, 7795–7801. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Ma, H.L.; Zhang, Q.; Xu, W.; Peng, J.; Li, J.; Yu, Z.Z.; Zhai, M. Ionic-liquid-assisted facile synthesis of silver nanoparticle-reduced graphene oxide hybrids by gamma irradiation. Carbon 2013, 55, 245–252. [Google Scholar] [CrossRef]
- Fu, C.P.; Kuang, Y.F.; Huang, Z.Y.; Wang, X.; Du, N.N.; Chen, J.H.; Zhou, H.H. Electrochemical co-reduction synthesis of graphene/Au nanocomposites in ionic liquid and their electrochemical activity. Chem. Phys. Lett. 2010, 499, 250–253. [Google Scholar] [CrossRef]
- Yang, H.F.; Shan, C.S.; Li, F.H.; Han, D.X.; Zhang, Q.X.; Niu, L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009, 26, 3880–3882. [Google Scholar]
- Shan, C.S.; Yang, H.F.; Han, D.X.; Zhang, Q.X.; Ivaska, A.; Niu, L. Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosens. Bioelectron. 2010, 25, 1504–1508. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Guo, S.J.; Zhai, Y.M.; Dong, S.J. Layer-by-layer self-assembly for constructing a graphene/platinum nanoparticle three-dimensional hybrid nanostructure using ionic liquid as a linker. Langmuir 2010, 26, 7614–7618. [Google Scholar] [CrossRef]
- Landi, B.J.; Raffaelle, R.P.; Heben, M.J.; Alleman, J.L.; van de Rveer, W.; Gennett, T. Single wall carbon nanotube-Nafion composite actuators. Nano Lett. 2002, 2, 1329–1332. [Google Scholar] [CrossRef]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully plastic actuator through layer-by-layer casting with ionic-liquid-based bucky gel. Angew. Chem. Int. Ed. 2005, 44, 2410–2413. [Google Scholar] [CrossRef]
- Mukai, K.; Asaka, K.; Kiyohara, K.; Sugino, T.; Takeuchi, I.; Fukushima, T.; Aida, T. High performance fully plastic actuator based on ionic-liquid-based bucky gel. Electrochim. Acta 2008, 53, 5555–5562. [Google Scholar] [CrossRef]
- Takeuchi, I.; Asaka, K.; Kiyohara, K.; Sugino, T.; Terasawa, N.; Mukai, K.; Fukushima, T.; Aida, T. Electromechanical behavior of fully plastic actuators based on bucky gel containing various internal ionic liquids. Electrochim. Acta 2009, 54, 1762–1768. [Google Scholar] [CrossRef]
- Sugino, T.; Kiyohara, K.; Takeuchi, I.; Mukai, K.; Asaka, K. Actuator properties of the complexes composed by carbon nanotube and ionic liquid: The effects of additives. Sens. Actuators B 2009, 141, 179–186. [Google Scholar] [CrossRef]
- Takeuchi, I.; Asaka, K.; Kiyohara, K.; Sugino, T.; Terasawa, N.; Mukai, K.; Shiraishi, S. Electromechanical behavior of a fully plastic actuator based on dispersed nano-carbon/ionic-liquid-gel electrodes. Carbon 2009, 47, 1373–1380. [Google Scholar] [CrossRef]
- Sekitani, T.; Noguchi, Y.; Hata, K.; Fukushima, T.; Aida, T.; Someya, T. A rubberlike stretchable active matrix using elastic conductors. Science 2008, 321, 1468–1472. [Google Scholar] [CrossRef]
- Sekitani, T.; Nakajima, H.; Maeda, H.; Fukushima, T.; Aida, T.; Hata, K.; Someya, T. Stretchable active-matrix organic light-emitting diode display using printable elastic conductors. Nat. Mater. 2009, 8, 494–499. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y.G. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef]
- Mandal, A.; Nandi, A.K. Ionic liquid integrated multiwalled carbon nanotube in a poly(vinylidene fluoride) matrix: Formation of a piezoelectric β-polymorph with significant reinforcement and conductivity improvement. ACS Appl. Mater. Interfaces 2013, 5, 747–760. [Google Scholar] [CrossRef]
- Pandey, G.P.; Rastogi, A.C. Graphene-based all-solid-state supercapacitor with ionic liquid gel polymer electrolyte. MRS Proc. 2012, 1440. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Bahr, J.L.; Arepalli, S.; Tour, J.M.; Krishnamoorti, R. Dispersion of functionalized carbon nanotubes in polystyrene. Macromolecules 2002, 35, 8825–8830. [Google Scholar] [CrossRef]
- Wei, D.; Baral, J.K.; Osterbacka, R.; Ivaska, A. Memory effect in an ionic liquid matrix containing single-walled carbon nanotubes and polystyrene. Nanotechnology 2008, 19, e055203. [Google Scholar] [CrossRef]
- Carrion, F.J.; Espejo, C.; Sanes, J.; Bermúdez, M.D. Single-walled carbon nanotubes modified by ionic liquid as antiwear additives of thermoplastics. Compos. Sci. Technol. 2010, 70, 2160–2167. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Cao, X.; You, J.; Dong, W. Multifunctional role of an ionic liquid in melt-blended poly(methyl methacrylate)/multi-walled carbon nanotube nanocomposites. Nanotechnology 2012, 23, e255702. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Zhu, S.D.; Wu, Y.X.; Chen, Q.M.; Yu, Z.N.; Wang, C.W.; Jin, S.W.; Ding, Y.G.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Li, L.; Meng, L.; Zhang, X.; Fu, C.; Lu, Q. The ionic liquid-associated synthesis of a cellulose/SWCNT complex and its remarkable biocompatibility. J. Mater. Chem. 2009, 19, 3612–3617. [Google Scholar] [CrossRef]
- Wan, J.; Yan, X.; Ding, J.J.; Ren, R. A simple method for preparing biocompatible composite of cellulose and carbon nanotubes for the cell sensor. Sens. Actuators B 2010, 146, 221–225. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.G.; Zhang, Z.N.; Wu, J.; Zhang, J.; He, H.S. Regenerated-cellulose/multiwalled-carbon-nanotube composite fibers with enhanced mechanical properties prepared with the ionic liquid 1-allyl-3-methylimidazolium chloride. Adv. Mater. 2007, 19, 698–704. [Google Scholar] [CrossRef]
- Miyauchi, M.; Miao, J.; Simmons, T.J.; Lee, J.-W.; Doherty, T.V.; Dordick, J.S.; Linhardt, R.J. Conductive cable fibers with insulating surface prepared by coaxial electrospinning of multiwalled nanotubes and cellulose. Biomacromolecules 2010, 11, 2440–2445. [Google Scholar] [CrossRef]
- Peng, H.; Meng, L.; Niu, L.; Lu, Q. Simultaneous reduction and surface functionalization of graphene oxide by natural cellulose with the assistance of the ionic liquid. J. Phys. Chem. C 2012, 116, 16294–16299. [Google Scholar] [CrossRef]
- Mahmoudian, S.; Wahit, M.U.; Imran, M.; Ismail, A.F.; Balakrishnan, H. A facile approach to prepare regenerated cellulose/graphene nanoplatelets nanocomposite using room-temperature ionic liquid. J. Nanosci. Nanotechnol. 2012, 12, 5233–5239. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid) latexes prepared by dispersion polymerization of ionic liquid monomers. Macromolecules 2011, 44, 744–750. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M. The design of polymeric ionic liquids for the preparation of functional materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Yamamoto, Y.; Aimiya, T.; Notazawa, S.; Takigawa, T.; Inabe, T.; Aida, T. Dramatic effect of dispersed carbon nanotubes on the mechanical and electroconductive properties of polymers derived from ionic liquids. Small 2006, 2, 554–560. [Google Scholar] [CrossRef]
- Hong, S.H.; Tung, T.T.; Huyen Trang, L.K.; Kim, T.Y.; Suh, K.S. Preparation of single-walled carbon nanotube (SWNT) gel composites using poly(ionic liquids). Colloid Polym. Sci. 2010, 288, 1013–1018. [Google Scholar] [CrossRef]
- Xiao, C.H.; Chu, X.C.; Wu, B.H.; Pang, H.L.; Zhang, X.H.; Chen, J.H. Polymerized ionic liquid-wrapped carbon nanotubes: The promising composites for direct electrochemistry and biosensing of redox protein. Talanta 2010, 80, 1719–1724. [Google Scholar] [CrossRef]
- Marcilla, R.; Curri, M.L.; Cozzoli, P.D.; Martínez, M.T.; Loinaz, I.; Grande, H.; Pomposo, J.A.; Mecerreyes, D. Nano-objects on a round trip from water to organics in a polymeric ionic liquid vehicle. Small 2006, 2, 507–512. [Google Scholar] [CrossRef]
- Meyer, F.; Raquez, J.-M.; Coulembier, O.; de Winter, J.; Gerbaux, P.; Dubois, P. Imidazolium end-functionalized poly(l-lactide) for efficient carbon nanotube dispersion. Chem. Commun. 2010, 46, 5527–5529. [Google Scholar] [CrossRef]
- Wu, B.H.; Hu, D.; Kuang, Y.J.; Liu, B.; Zhang, X.H.; Chen, J.H. Functionalization of carbon nanotubes by an ionic-liquid polymer: Dispersion of Pt and PtRu nanoparticles on carbon nanotubes and their electrocatalytic oxidation of methanol. Angew. Chem. Int. Ed. 2009, 48, 4751–4754. [Google Scholar] [CrossRef]
- Jia, F.; Shan, C.; Li, F.; Niu, L. Carbon nanotube/gold nanoparticles/polyethylenimine-functionalized ionic liquid thin film composites for glucose biosensing. Biosens. Bioelectron. 2008, 24, 945–950. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal nanoparticles and related materials supported on carbon nanotubes: Methods and applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef]
- Kim, T.; Lee, H.; Kim, J.; Suh, K.S. Synthesis of phase transferable graphene sheets using ionic liquid polymers. ACS Nano 2010, 4, 1612–1618. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, H.W.; Stoller, M.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S.; Suh, K.S. High-performance supercapacitors based on poly(ionic liquid)-modified graphene electrodes. ACS Nano 2011, 5, 436–442. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.Y.; Zhang, L.; Lu, J.; Verproot, F.; Liu, Y.; Xing, Z.Q.; Li, J.H.; Song, X.M. Fabrication of polymeric ionic liquid/graphene nanocomposite for glucose oxidase immobilization and direct electrochemistry. Biosens. Bioelectron. 2011, 26, 2632–2637. [Google Scholar] [CrossRef]
- Wang, Q.; Yun, Y. Nonenzymatic sensor for hydrogen peroxide based on the electrodeposition of silver nanoparticles on poly(ionic liquid)-stabilized graphene sheets. Microchim. Acta 2013, 180, 261–268. [Google Scholar] [CrossRef]
- Zhou, X.S.; Wu, T.B.; Hu, B.J.; Yang, G.Y.; Han, B.X. Synthesis of graphene/polyaniline composite nanosheets mediated by polymerized ionic liquid. Chem. Commun. 2010, 46, 3663–3665. [Google Scholar] [CrossRef]
- Tung, T.T.; Kim, T.Y.; Shim, J.P.; Yang, W.S.; Kim, H.; Suh, K.S. Poly(ionic liquid)-stabilized graphene sheets and their hybrid with poly(3,4-ethylenedioxythiophene). Org. Electron. 2011, 12, 2215–2224. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Bobenrieth, A.; Winter, J.D.; Gerbaux, P.; Raquez, J.-M.; Dubois, P. A supramolecular approach toward organo-dispersible graphene and its straightforward polymer nanocomposites. J. Mater. Chem. 2012, 22, 18124–18126. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, R.; Wang, Y.; Tang, W.; Yang, Y.; Xie, X. Progress in Imidazolium Ionic Liquids Assisted Fabrication of Carbon Nanotube and Graphene Polymer Composites. Polymers 2013, 5, 847-872. https://doi.org/10.3390/polym5020847
Peng R, Wang Y, Tang W, Yang Y, Xie X. Progress in Imidazolium Ionic Liquids Assisted Fabrication of Carbon Nanotube and Graphene Polymer Composites. Polymers. 2013; 5(2):847-872. https://doi.org/10.3390/polym5020847
Chicago/Turabian StylePeng, Rengui, Yuanzhen Wang, Wei Tang, Yingkui Yang, and Xiaolin Xie. 2013. "Progress in Imidazolium Ionic Liquids Assisted Fabrication of Carbon Nanotube and Graphene Polymer Composites" Polymers 5, no. 2: 847-872. https://doi.org/10.3390/polym5020847



