Enzymatic Synthesis and Characterization of Thermosensitive Polyester with Pendent Ketoprofen
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
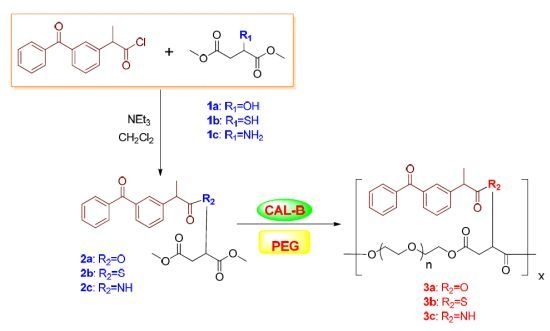
2.3. Synthesis of 2-Ketoprofen Malic Acid (Thiomalic Acid or Aspartic Acid) Dimethyl Ester
2.4. Copolymerization of 2-Ketoprofen Malic Acid (Thiomalic Acid or Aspartic Acid) Dimethyl Ester with Peg
2.5. Temperature Responsive Behavior
2.6. In Vitro Drug Release
3. Results and Discussion
3.1. Enzyme-Catalyzed Polycondensation for the Preparation of Polyester

| Entry | Substrate | Time (h) | Mw (g/mol) b | Mw/Mn | Yield (%) c |
|---|---|---|---|---|---|
| 1 | 2a | 24 | 6,250 | 1.9 | 85.1 |
| 2 | 2a | 48 | 8,970 | 4.8 | 80.8 |
| 3 | 2a | 96 | 6,560 | 2.4 | 79.6 |
| 4 | 2b | 24 | 8,570 | 2.6 | 86.7 |
| 5 | 2b | 48 | 11,230 | 3.1 | 87.0 |
| 6 | 2b | 96 | 13,760 | 3.1 | 89.9 |
| 7 | 2c | 24 | 2,560 | 2.9 | 91.7 |
| 8 | 2c | 48 | 2,430 | 3.0 | 91.0 |
| 9 | 2c | 96 | 4,270 | 1.6 | 92.8 |
3.2. Temperature Responsivity of Polyester in Aqueous Solution

3.3. In Vitro Hydrolysis of Polyester

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dimitrov, I.; Trzebicka, B.; Muller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Prog. Polym. Sci. 2007, 32, 1275–1343. [Google Scholar] [CrossRef]
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Alarcon, C.H.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Deng, X.M.; Hao, J.Y. Thermogelling hydrogels of poly(ε-caprolactone-co-d,l-lactide)–poly(ethylene glycol)–poly(ε-caprolactone-co-d,l-lactide) and poly(ε-caprolactone-co-l-lactide)–poly(ethylene glycol)–poly(ε-caprolactone-co-l-lactide) aqueous solutions. J. Polym. Sci. A Polym. Chem. 2007, 45, 4091–4099. [Google Scholar] [CrossRef]
- Bae, S.J.; Suh, J.M.; Sohn, Y.S.; Bae, Y.H.; Kim, S.W.; Jeong, B. Thermogelling poly(caprolactone-b-ethylene glycol-b-caprolactone) aqueous solutions. Macromolecules 2005, 38, 5260–5365. [Google Scholar] [CrossRef]
- Kumar, A.; Kulshrestha, A.S.; Gao, W.; Gross, R.A. Versatile route to polyol polyesters by lipase catalysis. Macromolecules 2003, 36, 8219–8221. [Google Scholar] [CrossRef]
- Kato, M.; Toshima, K.; Matsumura, S. Direct enzymatic synthesis of a polyester with free pendant mercapto groups. Biomacromolecules 2009, 10, 366–373. [Google Scholar] [CrossRef]
- Dai, S.Y.; Xue, L.; Zinn, M.; Li, Z. Enzyme-catalyzed polycondensation of polyester macrodiols with divinyl adipate: A green method for the preparation of thermoplastic block copolyesters. Biomacromolecules 2009, 10, 3176–3181. [Google Scholar] [CrossRef]
- Xue, L.; Dai, S.Y.; Li, Z. Synthesis and characterization of three-arm poly(ε-caprolactone)-based poly(ester urethanes) with shape-memory effect at body temperature. Macromlecules 2009, 42, 964–972. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Z.Z.; Decatur, J.; Xie, W.C.; Gross, R.A. Chain growth and branch structure formation during lipase-catalyzed synthesis of aliphatic polycarbonate polyols. Macromlecules 2011, 44, 1471–1479. [Google Scholar] [CrossRef]
- Puskas, J.E.; Kwang, S.S.; Sen, M.Y. Green polymer chemistry: Precision synthesis of novel multifunctional poly(ethylene glycol)s using enzymatic catalysis. Eur. Polym. J. 2011, 47, 524–534. [Google Scholar] [CrossRef]
- Kadokawa, J.I.; Kobayashi, S. Polymer synthesis by enzymatic catalysis. Curr. Opin. Chem. Biol. 2010, 14, 145–153. [Google Scholar] [CrossRef]
- Kadokawa, J.I. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef]
- Gross, R.A.; Ganesh, M.; Lu, W.H. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef]
- Gross, R.A.; Kumar, A.; Kalra, B. Polymer synthesis by in vitro enzyme catalysis. Chem. Rev. 2001, 101, 2097–2124. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhou, Y.J.; Wang, Z.; Wang, N.; Li, K.; Yu, X.Q. Enzyme-catalyzed synthesis of a novel thermosensitive polyester with pendant ketoprofen. Macromol. Biosci. 2011, 11, 595–599. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, C.; Li, K.; Wang, N.; Li, K.; Feng, X.W.; He, T.; Yu, X.Q. Two-step enzymatic selective synthesis of water-soluble ketoprofen–saccharide conjugates in organic media. Bioorg. Med. Chem. 2009, 17, 1905–1910. [Google Scholar] [CrossRef]
- Babazadeh, M. Design, synthesis and in vitro evaluation of vinyl ether type polymeric prodrugs of ibuprofen, ketoprofen and naproxen. Int. J. Pharm. 2008, 356, 167–173. [Google Scholar] [CrossRef]
- Tyagi, R.; Kumar, R.; Pandey, M.K.; Kumar, J.; Parmar, V.S.; Watterson, A.C. Amino acid and poly(ethylene glycol) based self-organizing polymeric systems: Chemo-enzymatic synthesis and characterization. J. Macromol. Sci. A Pure Appl. Chem. 2008, 45, 957–962. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Pasut, G.; Guiotto, A.; Veronese, F.M. Protein, peptide and non-peptide drug PEGylation for therapeutic application. Expert Opin. Ther. Pat. 2004, 14, 859–894. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961. [Google Scholar] [CrossRef]
- Choi, H.K.; Chun, M.K.; Lee, S.H.; Jang, M.H.; Kim, H.D.; Jung, C.S.; Oh, S.Y. In vitro and in vivo study of poly(ethylene glycol) conjugated ketoprofen to extend the duration of action. Int. J. Pharm. 2007, 341, 50–57. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Gunnarsson, A.; Jonsson, P.; Marie, R.; Tegenfeldt, J.O.; Hook, F. Single-molecule detection and mismatch discrimination of unlabeled DNA targets. Nano Lett. 2008, 8, 183–188. [Google Scholar] [CrossRef]
- Hwang, M.J.; Suh, J.M.; Bae, Y.H.; Kim, S.W.; Jeong, B. Caprolactonic poloxamer analog: PEG-PCL-PEG. Biomacromolecules 2005, 6, 885–890. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, S.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Nagahama, K.; Hashizume, M.; Yamamoto, H.; Ouchi, T.; Ohya, Y. Hydrophobicallymodified biodegradable poly(ethylene glycol) copolymers that formtemperature-responsive nanogels. Langmuir 2009, 25, 9734–9740. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, W.-X.; Wang, H.-Y.; Wang, N.; Zhang, W.-W.; Lai, H.; Yu, X.-Q. Enzymatic Synthesis and Characterization of Thermosensitive Polyester with Pendent Ketoprofen. Polymers 2013, 5, 1158-1168. https://doi.org/10.3390/polym5031158
Wu W-X, Wang H-Y, Wang N, Zhang W-W, Lai H, Yu X-Q. Enzymatic Synthesis and Characterization of Thermosensitive Polyester with Pendent Ketoprofen. Polymers. 2013; 5(3):1158-1168. https://doi.org/10.3390/polym5031158
Chicago/Turabian StyleWu, Wan-Xia, Hai-Yang Wang, Na Wang, Wei-Wei Zhang, Han Lai, and Xiao-Qi Yu. 2013. "Enzymatic Synthesis and Characterization of Thermosensitive Polyester with Pendent Ketoprofen" Polymers 5, no. 3: 1158-1168. https://doi.org/10.3390/polym5031158