The Glycolysis of Poly(ethylene terephthalate) Waste: Lewis Acidic Ionic Liquids as High Efficient Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Synthesis of Lewis Acidic Ionic Liquids
2.3. Glycolysis of PET Waste


3. Results and Discussion
3.1. Characterization of Ionic Liquids and Product
3.1.1. Determination of the Component of Ionic Liquids


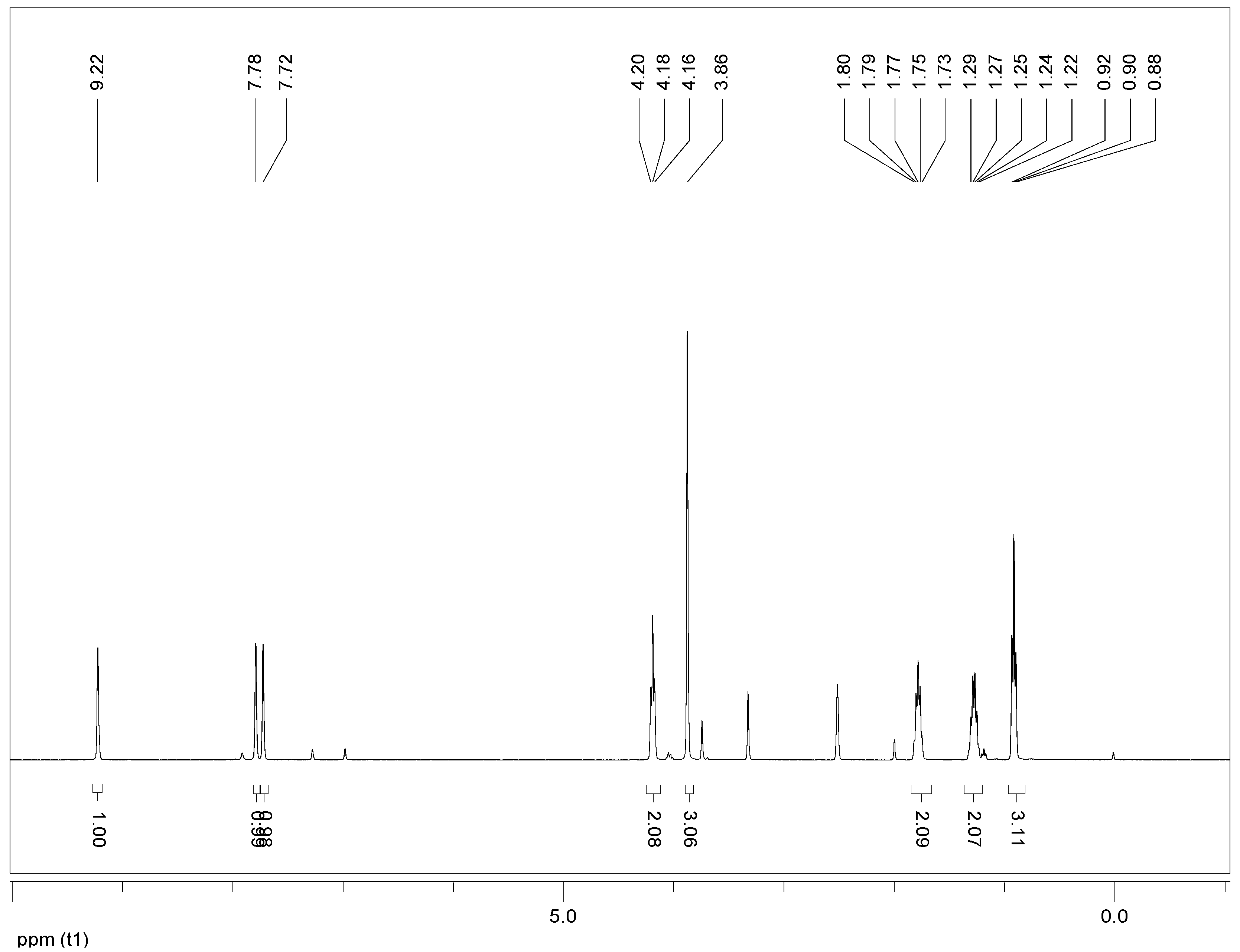
3.1.2. Qualitative Analysis of Glycolysis Product

3.2. The Effects of Catalysts on the Glycolysis of PET
| Entry | Catalyst | Dosage of catalyst (wt %) | Time (h) | PET conversion (%) | BHET yield b (%) |
|---|---|---|---|---|---|
| 1 | [Bmim]Cl | 5.00 | 2 | 3.3 | – |
| 2 | [Bmim]Cl | 5.00 | 4 | 35.4 | 8.8 |
| 3 | [Bmim]Cl | 5.00 | 6 | 57.2 | 14.0 |
| 4 | [Bmim]Cl | 5.00 | 8 | 100 | 57.1 |
| 5 | [Bmim]ZnCl3 | 5.00 | 2 | 100 | 82.6 |
| 6 | [Bmim]ZnCl3 | 2.50 | 2 | 100 | 83.0 |
| 7 | [Bmim]ZnCl3 | 1.25 | 2 | 100 | 84.9 |
| 8 | [Bmim]ZnCl3 | 0.63 | 2 | 100 | 83.4 |
| 9 | [Bmim]ZnCl3 | 0.31 | 2 | 100 | 79.5 |
| 10 | [Bmim]ZnCl3 | 0.16 | 2 | 52.2 | 36.0 |
| 11 | ZnCl2 | 2.18 c | 2 | 100 | 78.6 |
| 12 | [Bmim]MnCl3 | 5.00 | 2 | 100 | 83.3 |
| 13 | [Bmim]MnCl3 | 0.31 | 2 | 64.5 | 38.2 |
3.3. Effects of Reaction Parameters on the Glycolysis Reaction of PET
3.3.1. Effect of Catalyst Concentration
3.3.2. Effect of Reaction Time

3.3.3. Effects of Lewis Acidity of Ionic Liquids

3.3.4. Reusability Test of Lewis Acidic Ionic Liquid
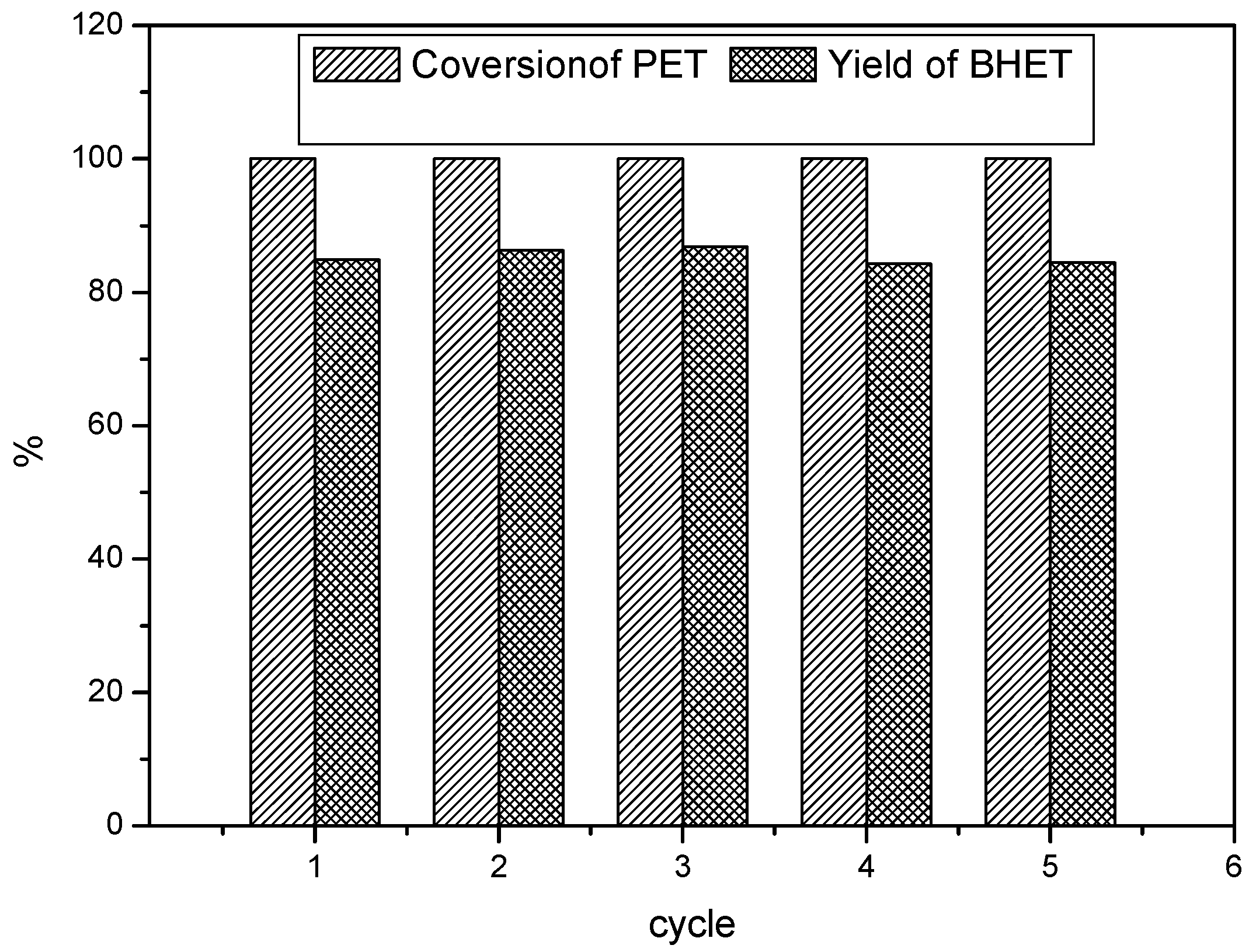
3.3.5. Possible Mechanism for the Glycolysis of PET in the Presence of [Bmim]ZnCl3

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hayden, K.W.; Jaimys, A.; Russell, J.C.; Elena, P.I. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Hideki, K.; Masa-aki, O.; Kazuo, S.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Chen, J.W.; Chen, L.W. The glycolysis of poly(ethylene terephthalate). J. Appl. Polym. Sci. 1999, 73, 35–40. [Google Scholar] [CrossRef]
- Xi, G.X.; Lu, M.X.; Sun, C. Study on depolymerization of waste polyethylene terephthalate into monomer of bis(2-hydroxyethyl terephthalate). Polym. Degrad. Stab. 2005, 87, 117–120. [Google Scholar] [CrossRef]
- Firas, A.; Dumitru, P. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Ghaemy, M.; Mossaddegh, K. Depolymerisation of poly(ethylene terephthalate) fibre wastes using ethylene glycol. Polym. Degrad. Stab. 2005, 90, 570–576. [Google Scholar] [CrossRef]
- Baliga, S.; Wong, W.T. Depolymerization of poly(ethylene terephthalate) recycled from post-consumer soft-drink bottles. J. Polym. Sci. A Polym. Chem. 1989, 27, 2071–2082. [Google Scholar] [CrossRef]
- Troev, K.; Granchrov, G.; Tsevi, R.; Gitsov, I. A novel catalyst for the glycolysis of poly(ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 1148–1152. [Google Scholar]
- Wang, H.; Yan, R.Y.; Li, Z.X.; Zhang, X.P.; Zhang, S.J. Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly(ethylene terephthalate). Catal. Commun. 2010, 11, 763–767. [Google Scholar] [CrossRef]
- Yoshioka, T.; Handa, T.; Grause, G.; Lei, Z.G.; Inomata, H.; Mizoguchi, T. Effects of metal oxides on the pyroilsis of poly(ethylene terephthalate). J. Anal. Appl. Pyrolusis 2005, 73, 139–144. [Google Scholar] [CrossRef]
- Liu, F.S.; Cui, X.; Yu, S.T.; Li, Z.; Ge, X.P. Hydrolysis reaction of poly(ethylene terephthalate) using ionic liquids as solvent and catalyst. J. Appl. Polym. Sci. 2009, 114, 3561–3565. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.X.; Liu, Y.Q.; Zhang, X.; Zhang, S.J. Degradation of poly(ethylene terephthalate) using ionic liquids. Green Chem. 2009, 11, 1568–1575. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.Q.; Li, Z.X.; Zhang, Z.P.; Zhang, S.J.; Zhang, Y.Q. Glycolysis of poly(ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. 2009, 45, 1535–1544. [Google Scholar] [CrossRef]
- Yue, Q.F.; Wang, C.X.; Zhang, L.N.; Ni, Y.; Jin, Y.X. Glycolysis of poly(ethylene terephthalate) (PET) using basic ionic liquids as catalysts. Polym. Degrad. Stab. 2011, 96, 399–403. [Google Scholar] [CrossRef]
- Li, F.W.; Xiao, L.F.; Xia, C.G.; Hu, B. Chemical fixation of CO2 with highly efficient ZnCl2/[BMIm]Br catalyst system. Tetrahedron Lett. 2004, 45, 8307–8310. [Google Scholar]
- Qiu, R.H.; Qiu, Y.M.; Yin, S.F.; Xu, X.H.; Luo, S.L.; Au, C.T.; Wong, W.Y.; Shimada, S. Highly efficient and selective synthesis of (E)-β-unsaturated ketones by crossed condensation of ketones and aldehydes catalyzed by an air-stable cationic organobismuth perfluorooctanesulfonate. Adv. Synth. Catal. 2010, 352, 153–162. [Google Scholar] [CrossRef]
- Pierre, B.; Ana-Paula, D.; Nicholas, P.; Kuppuswamy, K.; Michael, G. Hydrophobic highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef]
- Sharma, R.K.; Rawat, D.; Pant, P. Synthesis, characterization and catalytic behaviour of silica supported zinc salicylaldimine complex. J. Macromol. Sci. A Pure Appl. Chem. 2008, 45, 394–399. [Google Scholar] [CrossRef]
- Pingale, N.D.; Palekar, V.S.; Shukla, S.R. Glycolysis of postconsumer polyethylene terephthalate waste. J. Appl. Polym. Sci. 2010, 115, 249–254. [Google Scholar] [CrossRef]
- Bao, Q.; Qiao, K.; Tomida, D.; Yokoyama, C. 1-Methylimidazolium chlorosulfate ([HMIm]SO3Cl): A novel ionic liquid with dual Brønsted-Lewis acidity. Chem. Lett. 2010, 39, 728–729. [Google Scholar] [CrossRef]
- Yang, Y.L.; Kou, Y. Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 226–227. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yue, Q.F.; Xiao, L.F.; Zhang, M.L.; Bai, X.F. The Glycolysis of Poly(ethylene terephthalate) Waste: Lewis Acidic Ionic Liquids as High Efficient Catalysts. Polymers 2013, 5, 1258-1271. https://doi.org/10.3390/polym5041258
Yue QF, Xiao LF, Zhang ML, Bai XF. The Glycolysis of Poly(ethylene terephthalate) Waste: Lewis Acidic Ionic Liquids as High Efficient Catalysts. Polymers. 2013; 5(4):1258-1271. https://doi.org/10.3390/polym5041258
Chicago/Turabian StyleYue, Qun Feng, Lin Fei Xiao, Mi Lin Zhang, and Xue Feng Bai. 2013. "The Glycolysis of Poly(ethylene terephthalate) Waste: Lewis Acidic Ionic Liquids as High Efficient Catalysts" Polymers 5, no. 4: 1258-1271. https://doi.org/10.3390/polym5041258




