Organic Semiconductor/Insulator Polymer Blends for High-Performance Organic Transistors
Abstract
:1. Introduction
2. Fundamental Aspects of Phase Separation

3. Organic Semiconductor/Insulating Polymer Blends

3.1. Vertical Phase-Separation



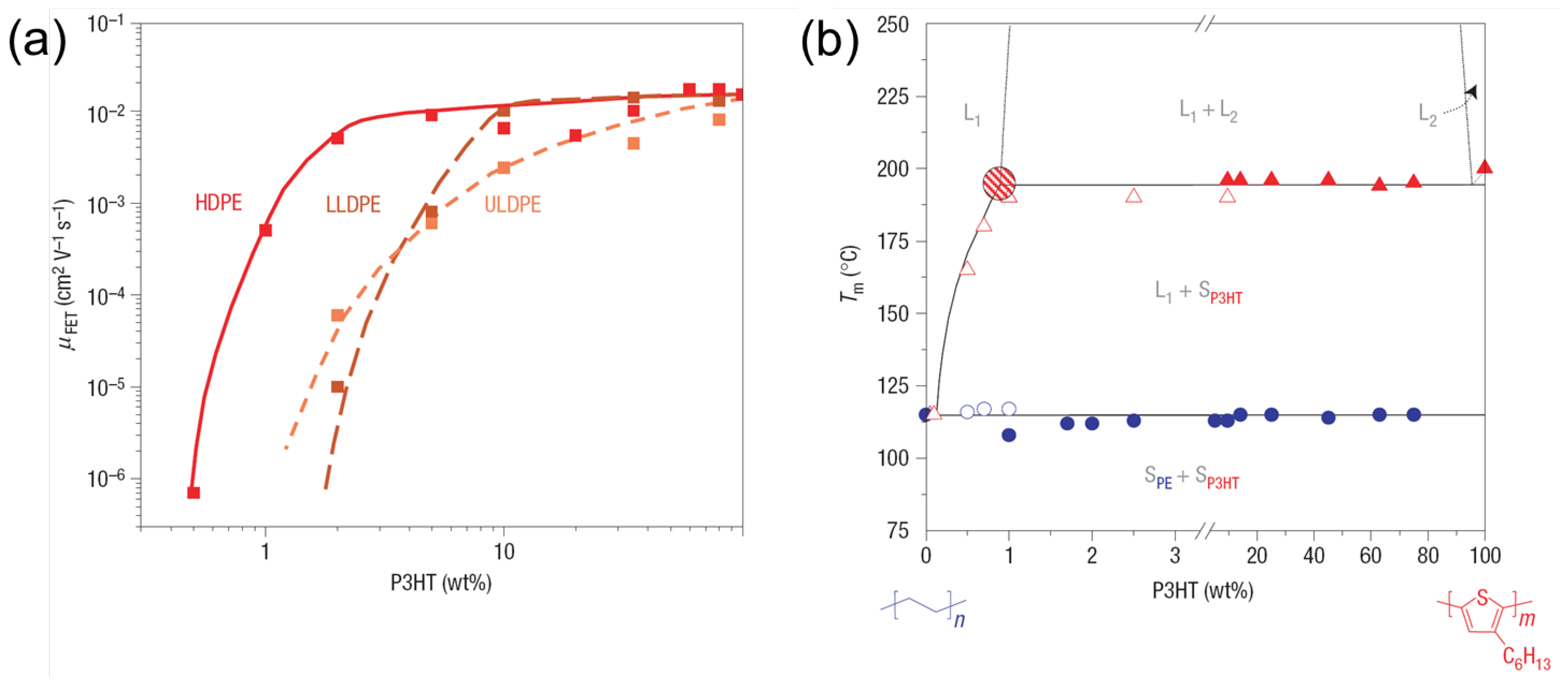
3.2. Processing Additive

3.3. Embedded Semiconductor Nanowires

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sirringhaus, H. Reliability of organic field-effect transistors. Adv. Mater. 2009, 21, 3859–3873. [Google Scholar] [CrossRef]
- Braga, D.; Horowitz, G. High-performance organic field-effect transistors. Adv. Mater. 2009, 21, 1473–1486. [Google Scholar] [CrossRef]
- Kang, B.; Lee, W.H.; Cho, K. Recent advances in organic transistor printing processes. ACS Appl. Mater. Interfaces 2013, 5, 2302–2315. [Google Scholar] [CrossRef]
- Smith, J.; Hamilton, R.; McCulloch, I.; Stingelin-Stutzmann, N.; Heeney, M.; Bradley, D.D.C.; Anthopoulos, T.D. Solution-processed organic transistors based on semiconducting blends. J. Mater. Chem. 2010, 20, 2562–2574. [Google Scholar] [CrossRef]
- Lee, W.H.; Cho, J.H.; Cho, K. Control of mesoscale and nanoscale ordering of organic semiconductors at the gate dielectric/semiconductor interface for organic transistors. J. Mater. Chem. 2010, 20, 2549–2561. [Google Scholar] [CrossRef]
- Allard, S.; Forster, M.; Souharce, B.; Thiem, H.; Scherf, U. Organic semiconductors for solution-processable field-effect transistors (ofets). Angew. Chem. Int. Ed. 2008, 47, 4070–4098. [Google Scholar] [CrossRef]
- Podzorov, V. Organic single crystals: Addressing the fundamentals of organic electronics. MRS Bull. 2013, 38, 15–27. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Turbiez, M.; McCulloch, I. Recent advances in the development of semiconducting dpp-containing polymers for transistor applications. Adv. Mater. 2013, 25, 1859–1880. [Google Scholar] [CrossRef]
- Noh, Y.Y.; Zhao, N.; Caironi, M.; Sirringhaus, H. Downscaling of self-aligned, all-printed polymer thin-film transistors. Nat. Nanotechnol. 2007, 2, 784–789. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Z.H.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dotz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef]
- Klauk, H. Organic thin-film transistors. Chem. Soc. Rev. 2010, 39, 2643–2666. [Google Scholar] [CrossRef]
- Gelinck, G.; Heremans, P.; Nomoto, K.; Anthopoulos, T.D. Organic transistors in optical displays and microelectronic applications. Adv. Mater. 2010, 22, 3778–3798. [Google Scholar]
- Hambsch, M.; Reuter, K.; Stanel, M.; Schmidt, G.; Kempa, H.; Fugmann, U.; Hahn, U.; Hubler, A.C. Uniformity of fully gravure printed organic field-effect transistors. Mater. Sci. Eng. B 2010, 170, 93–98. [Google Scholar] [CrossRef]
- Sheats, J.R. Manufacturing and commercialization issues in organic electronics. J. Mater. Res. 2004, 19, 1974–1989. [Google Scholar]
- Kang, I.; Yun, H.J.; Chung, D.S.; Kwon, S.K.; Kim, Y.H. Record high hole mobility in polymer semiconductors via side-chain engineering. J. Am. Chem. Soc. 2013, 135, 14896–14899. [Google Scholar]
- Li, J.; Zhao, Y.; Tan, H.S.; Guo, Y.L.; Di, C.A.; Yu, G.; Liu, Y.Q.; Lin, M.; Lim, S.H.; Zhou, Y.H.; et al. A stable solution-processed polymer semiconductor with record high-mobility for printed transistors. Sci. Rep. 2012, 2, 754:1–754:9. [Google Scholar]
- Anthopoulos, T.D.; Tanase, C.; Setayesh, S.; Meijer, E.J.; Hummelen, J.C.; Blom, P.W.M.; de Leeuw, D.M. Ambipolar organic field-effect transistors based on a solution-processed methanofullerene. Adv. Mater. 2004, 16, 2174–2179. [Google Scholar] [CrossRef]
- Meijer, E.J.; de Leeuw, D.M.; Setayesh, S.; van Veenendaal, E.; Huisman, B.H.; Blom, P.W.M.; Hummelen, J.C.; Scherf, U.; Kadam, J.; Klapwijk, T.M. Solution-processed ambipolar organic field-effect transistors and inverters. Nat. Mater. 2003, 2, 834–834. [Google Scholar] [CrossRef]
- You, J.B.; Dou, L.T.; Hong, Z.R.; Li, G.; Yang, Y. Recent trends in polymer tandem solar cells research. Prog. Polym. Sci. 2013, 38, 1909–1928. [Google Scholar] [CrossRef]
- Nelson, J. Polymer: Fullerene bulk heterojunction solar cells. Mater. Today 2011, 14, 462–470. [Google Scholar] [CrossRef]
- Arias, A.C. Vertically segregated polymer blends: Their use in organic electronics. Polym. Rev. 2006, 46, 103–125. [Google Scholar]
- Moons, E. Conjugated polymer blends: Linking film morphology to performance of light emitting diodes and photodiodes. J. Phys. Condens. Mat. 2002, 14, 12235–12260. [Google Scholar] [CrossRef]
- Chua, L.L.; Ho, P.K.H.; Sirringhaus, H.; Friend, R.H. Observation of field-effect transistor behavior at self-organized interfaces. Adv. Mater. 2004, 16, 1609–1615. [Google Scholar] [CrossRef]
- Heriot, S.Y.; Jones, R.A.L. An interfacial instability in a transient wetting layer leads to lateral phase separation in thin spin-cast polymer-blend films. Nat. Mater. 2005, 4, 782–786. [Google Scholar] [CrossRef]
- Salleo, A. Charge transport in polymeric transistors. Mater. Today 2007, 10, 38–45. [Google Scholar] [CrossRef]
- Park, Y.D.; Lim, J.A.; Lee, H.S.; Cho, K. Interface engineering in organic transistors. Mater. Today 2007, 10, 46–54. [Google Scholar] [CrossRef]
- Chung, D.S.; Lee, D.H.; Park, J.W.; Jang, J.; Nam, S.; Kim, Y.H.; Kwon, S.K.; Park, C.E. Phase-separated polydimethylsiloxane as a dielectric surface treatment layer for organic field effect transistors. Org. Electron. 2009, 10, 1041–1047. [Google Scholar] [CrossRef]
- Arias, A.C.; Endicott, F.; Street, R.A. Surface-induced self-encapsulation of polymer thin-film transistors. Adv. Mater. 2006, 18, 2900–2904. [Google Scholar] [CrossRef]
- Qiu, L.; Lim, J.A.; Wang, X.; Lee, W.H.; Hwang, M.; Cho, K. Versatile use of vertical-phase-separation-induced bilayer structures in organic thin-film transistors. Adv. Mater. 2008, 20, 1141–1145. [Google Scholar] [CrossRef]
- Wang, X.H.; Lee, W.H.; Zhang, G.B.; Wang, X.H.; Kang, B.; Lu, H.B.; Qiu, L.Z.; Cho, K. Self-stratified semiconductor/dielectric polymer blends: Vertical phase separation for facile fabrication of organic transistors. J. Mater. Chem. C 2013, 1, 3989–3998. [Google Scholar] [CrossRef]
- Lim, J.A.; Lee, H.S.; Lee, W.H.; Cho, K. Control of the morphology and structural development of solution-processed functionalized acenes for high-performance organic transistors. Adv. Funct. Mater. 2009, 19, 1515–1525. [Google Scholar] [CrossRef]
- Payne, M.M.; Parkin, S.R.; Anthony, J.E.; Kuo, C.C.; Jackson, T.N. Organic field-effect transistors from solution-deposited functionalized acenes with mobilities as high as 1 cm2/V·s. J. Am. Chem. Soc. 2005, 127, 4986–4987. [Google Scholar] [CrossRef]
- Llorente, G.R.; Dufourg-Madec, M.B.; Crouch, D.J.; Pritchard, R.G.; Ogier, S.; Yeates, S.G. High performance, acene-based organic thin film transistors. Chem. Commun. 2009, 3059–3061. [Google Scholar]
- Park, S.K.; Jackson, T.N.; Anthony, J.E.; Mourey, D.A. High mobility solution processed 6,13-bis(triisopropyl-silylethynyl) pentacene organic thin film transistors. Appl. Phys. Lett. 2007, 91, 063514:1–063514:3. [Google Scholar]
- He, Z.R.; Li, D.W.; Hensley, D.K.; Rondinone, A.J.; Chen, J.H. Switching phase separation mode by varying the hydrophobicity of polymer additives in solution-processed semiconducting small-molecule/polymer blends. Appl. Phys. Lett. 2013, 103, 113301:1–113301:5. [Google Scholar]
- Park, B.; Jeon, H.G.; Choi, J.; Kim, Y.K.; Lim, J.; Jung, J.; Cho, S.Y.; Lee, C. High-performance organic thin-film transistors with polymer-blended small-molecular semiconductor films, fabricated using a pre-metered coating process. J. Mater. Chem. 2012, 22, 5641–5646. [Google Scholar] [CrossRef]
- Lee, W.H.; Lim, J.A.; Kwak, D.; Cho, J.H.; Lee, H.S.; Choi, H.H.; Cho, K. Semiconductor-dielectric blends: A facile all solution route to flexible all-organic transistors. Adv. Mater. 2009, 21, 4243–4248. [Google Scholar] [CrossRef]
- Dickey, K.C.; Anthony, J.E.; Loo, Y.L. Improving organic thin-film transistor performance through solvent-vapor annealing of solution-processable triethylsilylethynyl anthradithiophene. Adv. Mater. 2006, 18, 1721–1726. [Google Scholar] [CrossRef]
- Lee, W.H.; Lim, J.A.; Kim, D.H.; Cho, J.H.; Jang, Y.; Kim, Y.H.; Han, J.I.; Cho, K. Room-temperature self-organizing characteristics of soluble acene field-effect transistors. Adv. Funct. Mater. 2008, 18, 560–565. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, D.H.; Cho, J.H.; Jang, Y.; Lim, J.A.; Kwak, D.; Choa, K. Change of molecular ordering in soluble acenes via solvent annealing and its effect on field-effect mobility. Appl. Phys. Lett. 2007, 91, 092105:1–092105:3. [Google Scholar]
- Lee, W.H.; Kwak, D.; Anthony, J.E.; Lee, H.S.; Choi, H.H.; Kim, D.H.; Lee, S.G.; Cho, K. The influence of the solvent evaporation rate on the phase separation and electrical performances of soluble acene-polymer blend semiconductors. Adv. Funct. Mater. 2012, 22, 267–281. [Google Scholar] [CrossRef]
- Subramanian, S.; Park, S.K.; Parkin, S.R.; Podzorov, V.; Jackson, T.N.; Anthony, J.E. Chromophore fluorination enhances crystallization and stability of soluble anthradithiophene semiconductors. J. Am. Chem. Soc. 2008, 130, 2706–2708. [Google Scholar]
- Goffri, S.; Muller, C.; Stingelin-Stutzmann, N.; Breiby, D.W.; Radano, C.P.; Andreasen, J.W.; Thompson, R.; Janssen, R.A.J.; Nielsen, M.M.; Smith, P.; et al. Multicomponent semiconducting polymer systems with low crystallization-induced percolation threshold. Nat. Mater. 2006, 5, 950–956. [Google Scholar] [CrossRef]
- Kjellander, B.K.C.; Smaal, W.T.T.; Anthony, J.E.; Gelinck, G.H. Inkjet printing of tips-pen on soluble polymer insulating films: A route to high-performance thin-film transistors. Adv. Mater. 2010, 22, 4612–4616. [Google Scholar] [CrossRef]
- Madec, M.B.; Crouch, D.; Llorente, G.R.; Whittle, T.J.; Geoghegan, M.; Yeates, S.G. Organic field effect transistors from ambient solution processed low molar mass semiconductor-insulator blends. J. Mater. Chem. 2008, 18, 3230–3236. [Google Scholar]
- Madec, M.B.; Smith, P.J.; Malandraki, A.; Wang, N.; Korvink, J.G.; Yeates, S.G. Enhanced reproducibility of inkjet printed organic thin film transistors based on solution processable polymer-small molecule blends. J. Mater. Chem. 2010, 20, 9155–9160. [Google Scholar] [CrossRef]
- Kang, J.; Shin, N.; Jang, D.Y.; Prabhu, V.M.; Yoon, D.Y. Structure and properties of small molecule-polymer blend semiconductors for organic thin film transistors. J. Am. Chem. Soc. 2008, 130, 12273–12275. [Google Scholar] [CrossRef]
- Ohe, T.; Kuribayashi, M.; Yasuda, R.; Tsuboi, A.; Nomoto, K.; Satori, K.; Itabashi, M.; Kasahara, J. Solution-processed organic thin-film transistors with vertical nanophase separation. Appl. Phys. Lett. 2008, 93, 053303:1–053303:3. [Google Scholar]
- Shin, N.; Kang, J.; Richter, L.J.; Prabhu, V.M.; Kline, R.J.; Fischer, D.A.; DeLongchamp, D.M.; Toney, M.F.; Satija, S.K.; Gundlach, D.J.; et al. Vertically segregated structure and properties of small molecule-polymer blend semiconductors for organic thin-film transistors. Adv. Funct. Mater. 2013, 23, 366–376. [Google Scholar] [CrossRef]
- Kim, Y.H.; Anthony, J.E.; Park, S.K. Polymer blended small molecule organic field effect transistors with improved device-to-device uniformity and operational stability. Org. Electron. 2012, 13, 1152–1157. [Google Scholar] [CrossRef]
- Jo, S.B.; Lee, W.H.; Qiu, L.Z.; Cho, K. Polymer blends with semiconducting nanowires for organic electronics. J. Mater. Chem. 2012, 22, 4244–4260. [Google Scholar] [CrossRef]
- Qiu, L.Z.; Lee, W.H.; Wang, X.H.; Kim, J.S.; Lim, J.A.; Kwak, D.; Lee, S.; Cho, K. Organic thin-film transistors based on polythiophene nanowires embedded in insulating polymer. Adv. Mater. 2009, 21, 1349–1353. [Google Scholar] [CrossRef]
- Qiu, L.Z.; Xu, Q.; Lee, W.H.; Wang, X.H.; Kang, B.; Lv, G.Q.; Cho, K. Organic thin-film transistors with a photo-patternable semiconducting polymer blend. J. Mater. Chem. 2011, 21, 15637–15642. [Google Scholar] [CrossRef]
- Qiu, L.Z.; Wang, X.; Lee, W.H.; Lim, J.A.; Kim, J.S.; Kwak, D.; Cho, K. Organic thin-film transistors based on blends of poly(3-hexylthiophene) and polystyrene with a solubility-induced low percolation threshold. Chem. Mater. 2009, 21, 4380–4386. [Google Scholar] [CrossRef]
- Kim, F.S.; Jenekhe, S.A. Charge transport in poly(3-butylthiophene) nanowires and their nanocomposites with an insulating polymer. Macromolecules 2012, 45, 7514–7519. [Google Scholar] [CrossRef]
- Kramer, T.J.; Chew, A.R.; Schiros, T.; Kymissis, I.; Herman, I.P. Poly-(3)hexylthiophene nanowire networks for versatile fabrication of bulk heterojunctions with increased active volume. J. Vac. Sci. Technol. B 2013, 31, 031209:1–031209:8. [Google Scholar]
- Lim, J.A.; Kim, J.H.; Qiu, L.; Lee, W.H.; Lee, H.S.; Kwak, D.; Cho, K. Inkjet-printed single-droplet organic transistors based on semiconductor nanowires embedded in insulating polymers. Adv. Funct. Mater. 2010, 20, 3292–3297. [Google Scholar] [CrossRef]
- Lu, G.H.; Blakesley, J.; Himmelberger, S.; Pingel, P.; Frisch, J.; Lieberwirth, I.; Salzmann, I.; Oehzelt, M.; di Pietro, R.; Salleo, A.; et al. Moderate doping leads to high performance of semiconductor/insulator polymer blend transistors. Nat. Commun. 2013, 4, 1588:1–1588:8. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, W.H.; Park, Y.D. Organic Semiconductor/Insulator Polymer Blends for High-Performance Organic Transistors. Polymers 2014, 6, 1057-1073. https://doi.org/10.3390/polym6041057
Lee WH, Park YD. Organic Semiconductor/Insulator Polymer Blends for High-Performance Organic Transistors. Polymers. 2014; 6(4):1057-1073. https://doi.org/10.3390/polym6041057
Chicago/Turabian StyleLee, Wi Hyoung, and Yeong Don Park. 2014. "Organic Semiconductor/Insulator Polymer Blends for High-Performance Organic Transistors" Polymers 6, no. 4: 1057-1073. https://doi.org/10.3390/polym6041057




