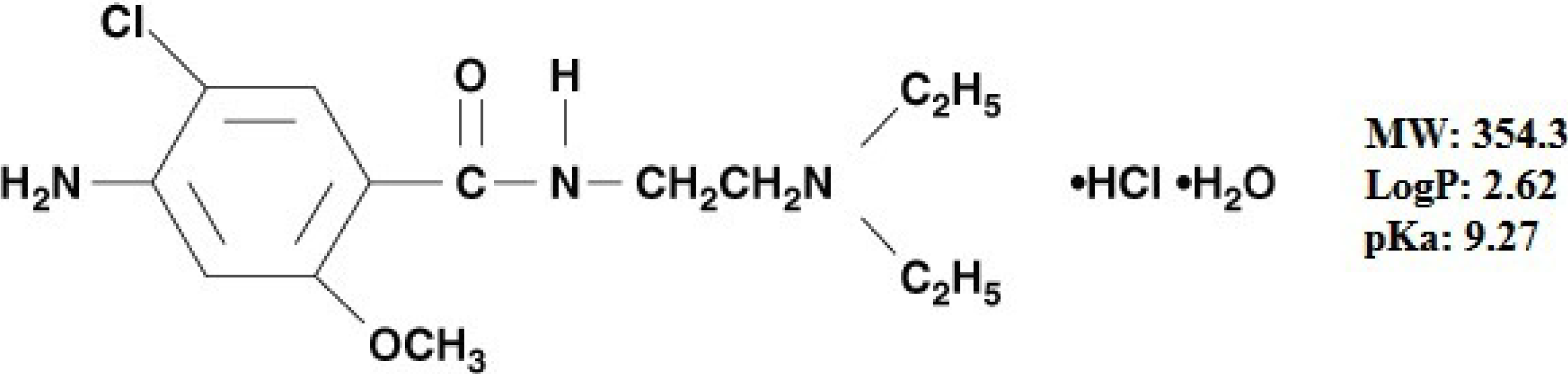
Optimization of Biopolymer Based Transdermal Films of Metoclopramide as an Alternative Delivery Approach
Abstract
:1. Introduction
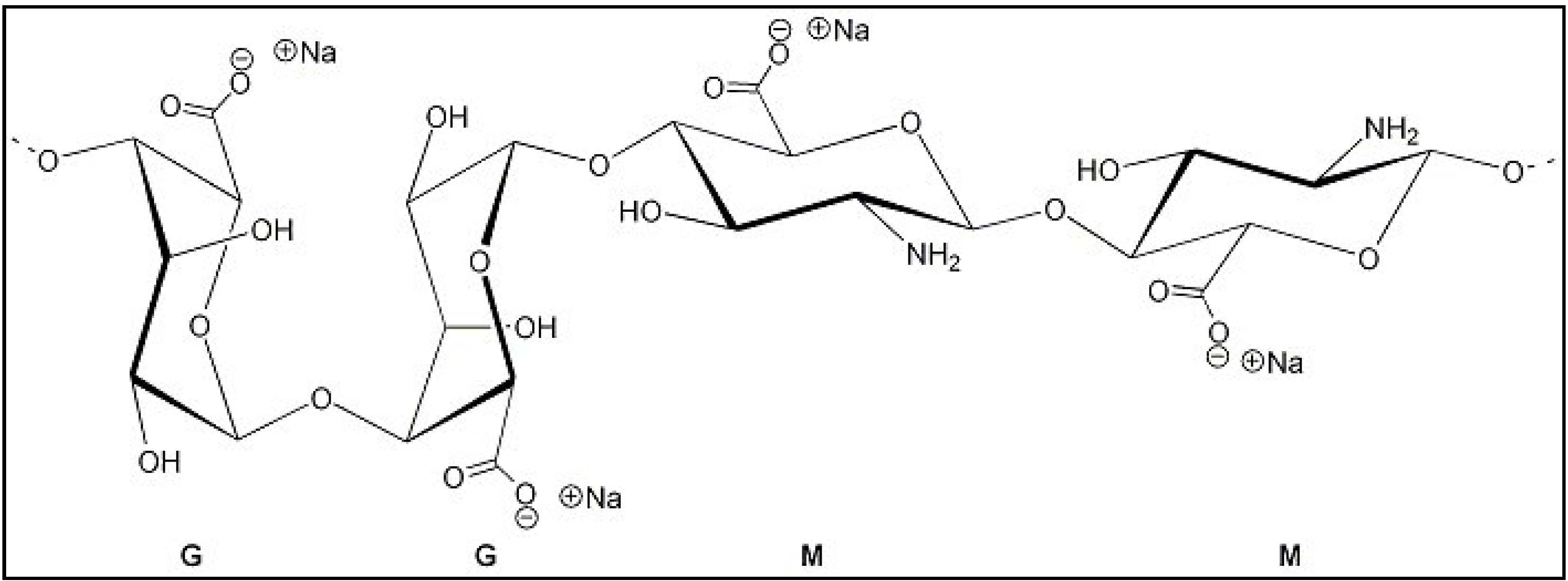
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Solubility of MTC in Terpenes and Co-Solvents
2.2.2. Formulation of Gels
| Compositions | Gel and Transdermal Formulations | |||||||
|---|---|---|---|---|---|---|---|---|
| G-NR | TF-NR | G-EU | TF-EU | G-TP | TF-TP | G-LM | TF-LM | |
| Sodium alginate | 4.5 | 4.5 | 4.5 | 4.5 | ||||
| Propylene glycol | 7.5 | 7.5 | 7.5 | 7.5 | ||||
| Nerolidol | 1.0 | – | – | – | ||||
| Eucalptol | – | 1.0 | – | – | ||||
| Terpinolene | – | – | 1.0 | – | ||||
| dl-Limonene | – | – | – | 1.0 | ||||
| Tween 80 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Distilled water | 86 | 86 | 86 | 86 | ||||
| Metoclopramide HCl | ||||||||
| % w/w a | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| mg/cm2 b | 3.3 | 3.3 | 3.3 | 3.3 | ||||
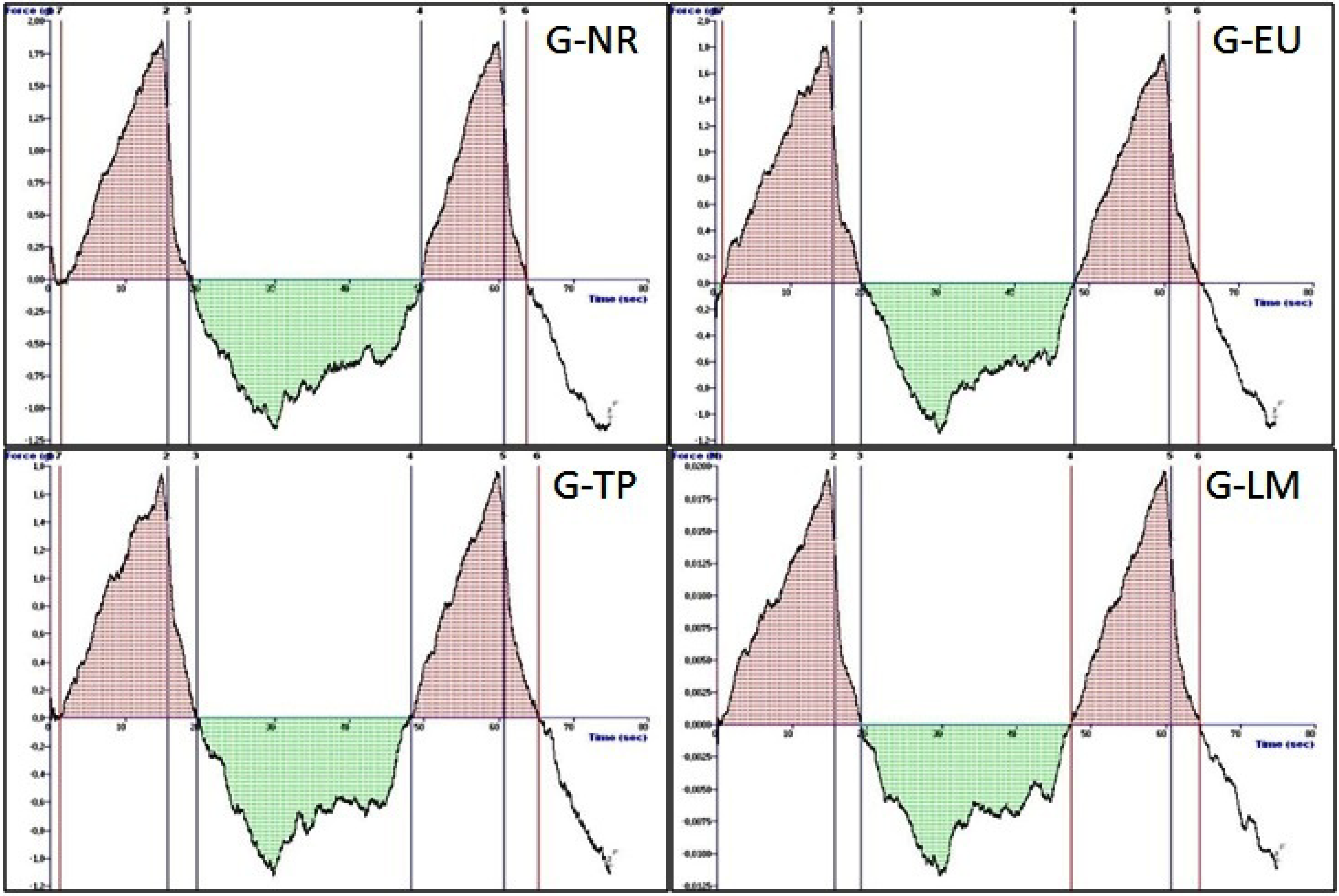
2.2.3. Mechanical Properties of the Sodium Alginate Gels-Texture Profile Analysis
2.2.4. Formulation of MTC Transdermal Films
2.2.5. Physicochemical Characteristics of MTC Transdermal Films
Organoleptic Examination
Thickness
Uniformity of Weight
Uniformity of Drug Content
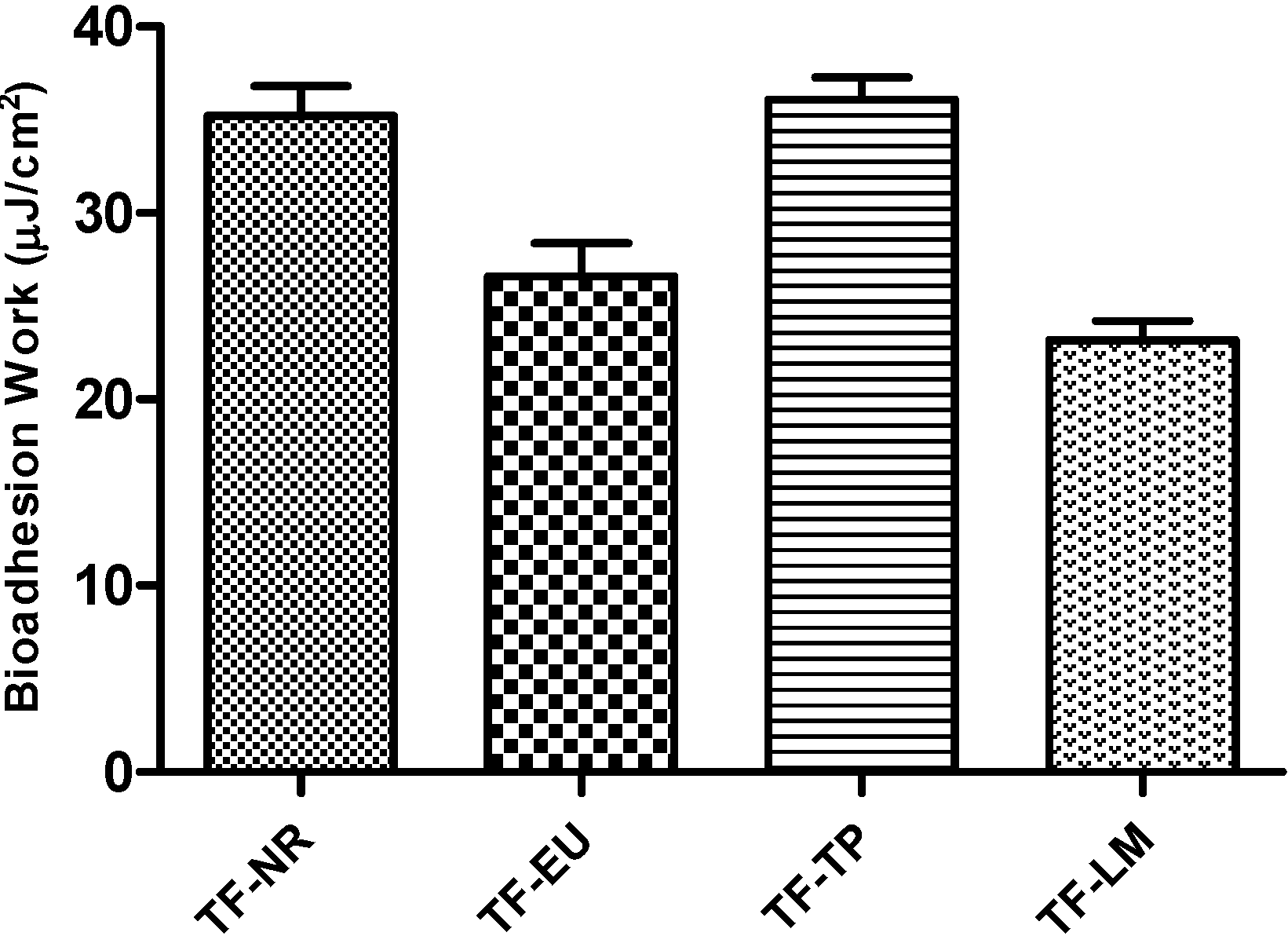
2.2.6. In Vitro Adhesion Test
2.2.7. In Vitro Release Studies of MTC from Transdermal Films
2.2.8. HPLC Analysis
2.2.9. Attenuated Total Reflectance Infrared (ATR-FTIR) Spectroscopy Studies
2.2.10. Statistical Analysis
3. Results and Discussion
| Code | Hardness (n) | Elasticity (mJ) | Cohesiveness | Adhesiveness (µj) | Compressibility (n·mm) |
|---|---|---|---|---|---|
| G-NR | 0.02 ± 0.01 | 0.71 ± 0.04 | 0.91 ± 0.04 | 104.33 ± 9.02 | 0.13 ± 0.03 |
| G-EU | 0.02 ± 0.01 | 0.87 ± 0.05 | 0.88 ± 0.03 | 57.33 ± 10.70 | 0.17 ± 0.02 |
| G-TP | 0.02 ± 0.01 | 0.80 ± 0.06 | 0.85 ± 0.12 | 85.33 ± 4.04 | 0.17 ± 0.05 |
| G-LM | 0.01 ± 0.02 | 0.79 ± 0.08 | 0.91 ± 0.04 | 84.67 ± 3.79 | 0.14 ± 0.02 |
| Transdermal Film | Thickness (mm) | Weight (mg·cm−2) | MTC Content (%) |
|---|---|---|---|
| TF-NR | 0.55 ± 0.05 | 60.12 ± 1.46 | 102.48 ± 0.73 |
| TF-EU | 0.67 ± 0.04 | 58.60 ± 1.51 | 101.56 ± 1.43 |
| TF-TP | 0.51 ± 0.02 | 63.59 ± 1.77 | 98.29 ± 1.76 |
| TF-LM | 0.47 ± 0.03 | 51.18 ± 1.16 | 92.70 ± 0.74 |
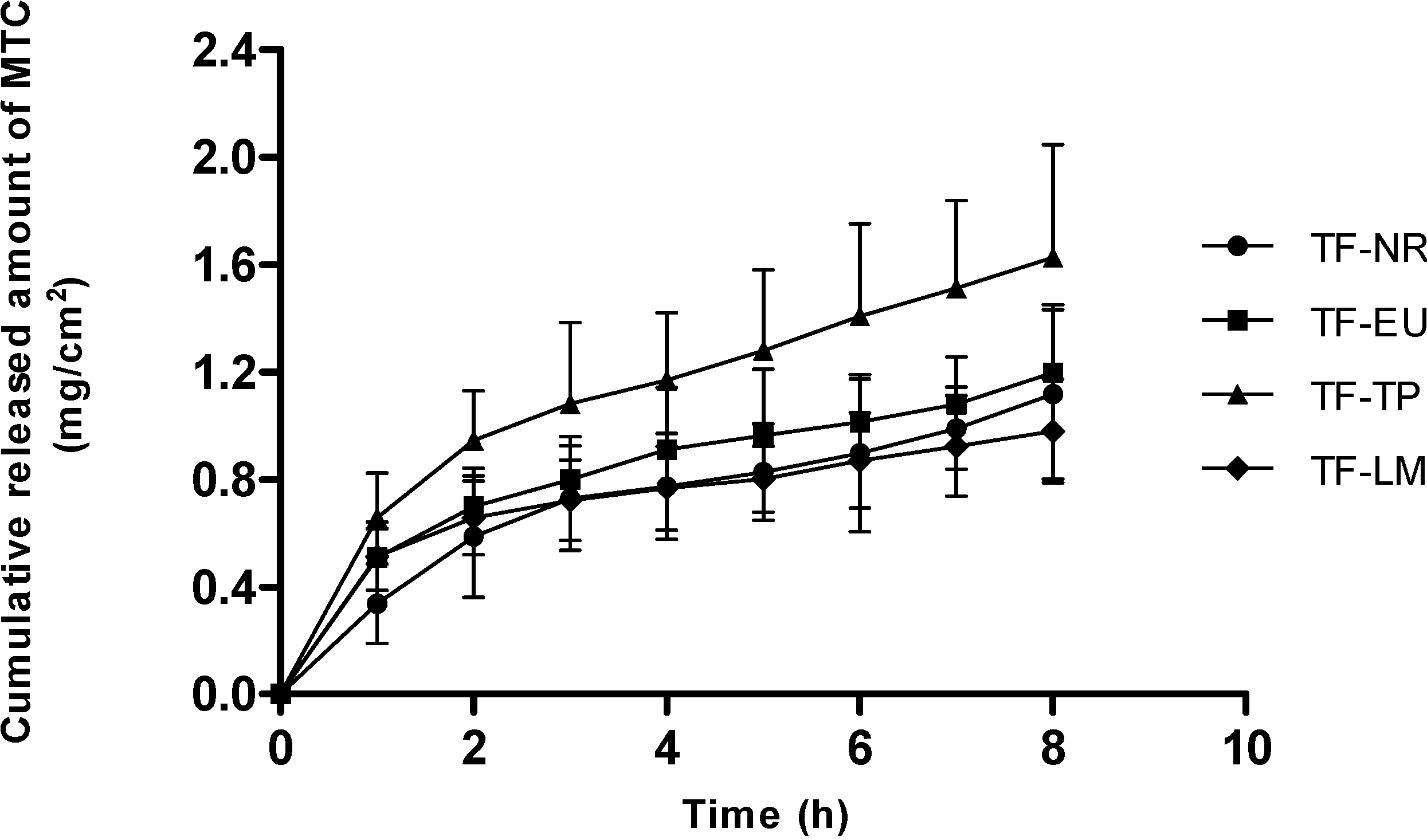
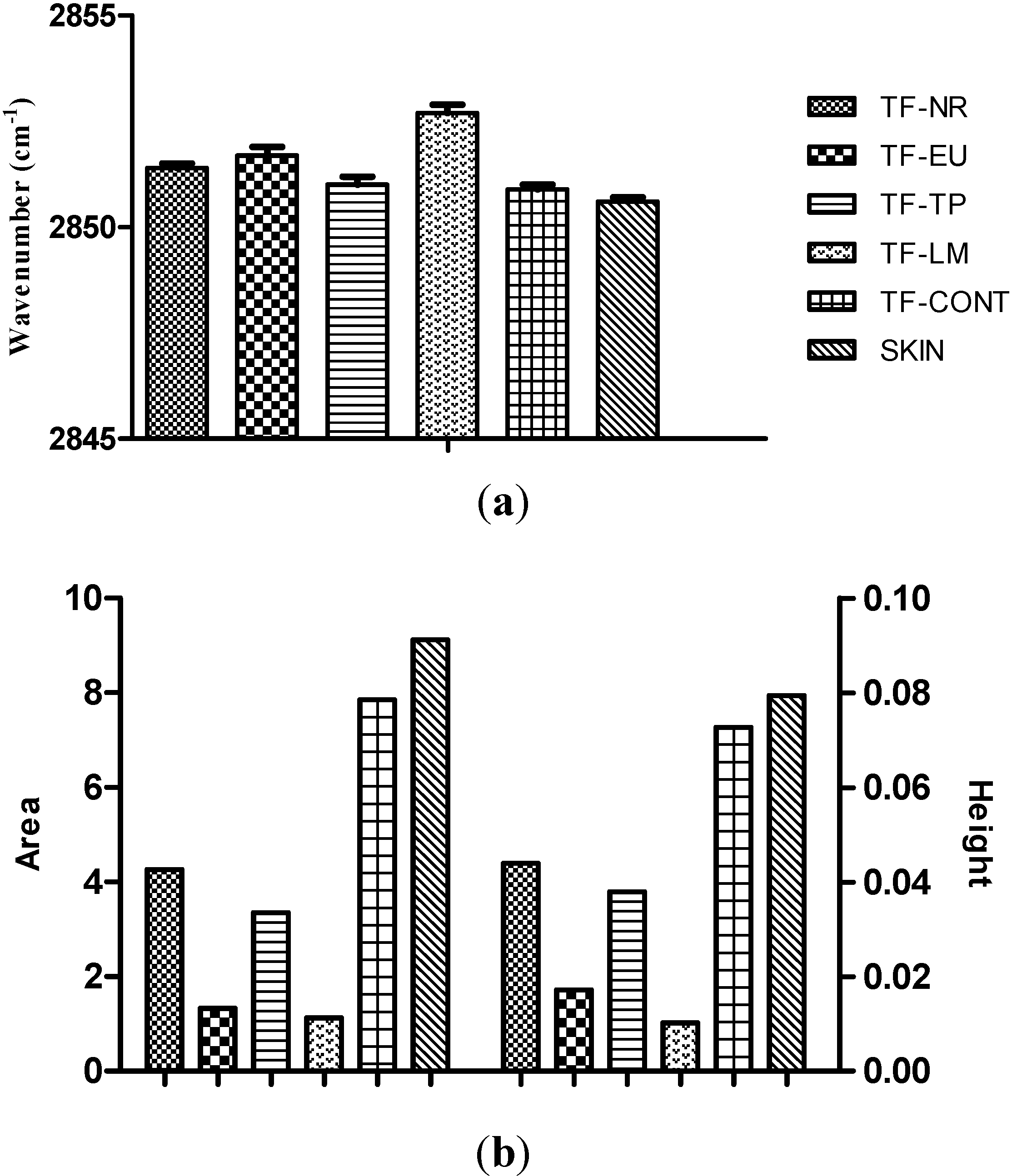
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Erdal, M.S.; Güngör, S.; Özsoy, Y. Biopolymers: Dermal and Transdermal Drug Delivery Systems. In Encyclopaedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M.K., Ed.; Taylor & Francis: New York, NY, USA, 2014; in press. [Google Scholar]
- Güngör, S.; Bektaş, A.; Alp, F.I.; Uydeş-Doğan, B.S.; Ozdemir, O.; Araman, A.; Özsoy, Y. Matrix-type transdermal patches of verapamil hydrochloride: In vitro permeation studies through excised rat skin and pharmacodynamic evaluation in rats. Pharm. Dev. Technol. 2008, 13, 283–289. [Google Scholar] [CrossRef]
- Can, S.; Erdal, M.S.; Güngör, S.; Özsoy, Y. Optimization and characterization of chitosan films for transdermal delivery of ondansetron. Molecules 2013, 18, 5455–5471. [Google Scholar] [CrossRef]
- Bektaş, A.; Cevher, E.; Güngör, S.; Özsoy, Y. Design and evaluation of polysaccharide-based transdermal films for the controlled delivery of nifedipine. Chem. Pharm. Bull. 2014, 6, 1–9. [Google Scholar]
- Gruber, J.W. Polysaccharide based polymers in cosmetics. In Principles of Polymer Science and Technology in Cosmetics and Personal Care; Goddard, E.D., Gruber, J.V., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 339–402. [Google Scholar]
- Sinko, P.J.; Singh, Y. Martin’s Physical Pharmacy and Pharmaceutical Sciences, 6th ed.; Wolters Kluwer: Baltimore, MD, USA; pp. 492–515.
- Roy, S.K.; Prabhakar, B. Bioadhesive polymeric platforms for transmucosal drug delivery systems—A review. Trop. J. Pharm. Res. 2010, 9, 91–104. [Google Scholar]
- Andersen, T.; Strand, B.L.; Formo, K.; Alsberg, E.; Christensen, B.E. Alginates as biomaterials in tissue engineering. Carbohydr. Chem. 2012, 37, 227–258. [Google Scholar]
- Kitagawa, S.; Li, H.; Sato, S. Skin permeation of parabens in excised guinea pig dorsal skin, its modification by penetration enhancers and their relationship with n-octanol/water partition coefficients. Chem. Pharm. Bull. 1997, 45, 1354–1357. [Google Scholar] [CrossRef]
- Li, T.; Ren, C.; Wang, M.; Zhao, L.; Wang, X.; Fang, L. Optimized preparation and evaluation of indomethacin transdermal patch. Asian J. Pharm. Sci. 2007, 2, 249–259. [Google Scholar]
- Krisnaiah, Y.S.R.; Raju, V.; Kumar, M.S.; Rama, B.; Raghumurthy, V.; Ramana Murthy, K.V. Studies on optimizing in vitro transdermal permeation of ondansetron hydrochloride using nerodilol, carvone, and limonene as penetration enhancers. Pharm. Dev. Technol. 2008, 13, 177–185. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. In vivo skin permeation of sodium naproxen formulated in pluronic F-127 gels: Effect of Azone and Transcutol. Drug Dev. Ind. Pharm. 2005, 31, 447–454. [Google Scholar] [CrossRef]
- Benson, H.A.E. Transdermaldrug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef]
- Levison, K.K.; Takayama, K.; Isowa, K.; Okabe, K.; Nagai, T. Formulation optimization of indomethacin gels containing a combination of three kinds of cyclic monoterpenes as percutaneous penetration enhancers. J. Pharm. Sci. 1994, 83, 1367–1372. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef]
- Kang, L.; Yap, C.W.; Lim, P.F.C.; Chen, Y.Z.; Ho, P.C.; Chan, Y.W., Wong; Chan, S.Y. Formulation development of transdermal dosage forms: Quantitative structure-activity relationship model for predicting activities of terpenes that enhance drug penetration through human skin. J. Control. Release 2007, 120, 211–219. [Google Scholar] [CrossRef]
- Rizwan, M.; Aqil, M.; Ahad, A.; Sultana, Y.; Ali, M.M. Transdermal delivery of valsartan: I. Effect of various terpenes. Drug Dev. Ind. Pharm. 2008, 34, 618–626. [Google Scholar] [CrossRef]
- Williams, A.C.; Edwards, H.G.M.; Lawson, E.E.; Barry, B.W. Molecular interactions between the penetration enhancer 1,8-cineole and human skin. J. Raman Spectrosc. 2006, 37, 361–366. [Google Scholar] [CrossRef]
- Babita, K.; Kumar, V.; Rana, V.; Jain, S.; Tiwory, A.K. Thermotropic and spectroscopic behavior of skin: Relationship with percutaneous permeation enhancement. Curr. Drug Deliv. 2006, 3, 95–113. [Google Scholar] [CrossRef]
- Chang, J.S.; Tsai, Y.H.; Wu, P.C.; Huang, Y.B. The effect of mixed-solvent and terpenes on percutaneous absorption of meloxicam gel. Drug Dev. Ind. Pharm. 2007, 33, 984–989. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Terpenes and the lipid-protein partition theory of skin penetration enhancement. Pharm. Res. 1991, 8, 17–24. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. The enhancement index concept applied to terpene penetration enhancers for human skin and model lipophilic (oestradiol) and hydrophilic (5-fluorouracil) drugs. Int. J. Pharm. 1991, 74, 157–168. [Google Scholar] [CrossRef]
- Moghimi, H.R.; Williams, A.C.; Barry, B.W. Enhancement by terpenes of 5-fluororuracil permeation through the stratum corneum: model solvent approach. J. Pharm. Pharmacol. 1998, 50, 955–964. [Google Scholar] [CrossRef]
- Hori, M.; Satoh, S.; Maibach, H.I.; Guy, R.H. Enhancement of propranolol hydrochloride and diazepam skin absorption in vitro: Effect of enhancer lipophilicity. J. Pharm. Sci. 1991, 80, 32–35. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awaad, G.A.S.; Mortada, N.M.; Abd El Hady, S.S. Rapid-onset intranasal delivery of metoclopramide hydrochloride. Part I. Influence of formulation variables on drug absorption in anesthetized rats. Int. J. Pharm. 2006, 327, 89–96. [Google Scholar] [CrossRef]
- Zaki, N.M.; Mortada, N.M.; Awaad, G.A.S.; Abd El Hady, S.S. Rapid-onset intranasal delivery of metoclopramide hydrochloride Part II: Safety of various absorption enhancers and pharmacokinetic evaluation. Int. J. Pharm. 2006, 327, 97–103. [Google Scholar] [CrossRef]
- Pitrè, D.; Stradi, R. Metoclopramide hydrochloride. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: San Diego, CA, USA, 1987; Volume 16, pp. 327–360. [Google Scholar]
- Stosik, A.G.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Metoclopramide hydrochloride. J. Pharm. Sci. 2008, 97, 3700–3708. [Google Scholar] [CrossRef]
- Cevher, E.; Sensoy, D.; Taha, M.A.M. Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS PharmSciTech 2008, 9, 953–965. [Google Scholar] [CrossRef]
- Jones, D.S.; Irwin, C.R.; Woolfson, A.D.; Djokic, J.; Adams, V. Physicochemical characterization and preliminary in vivo efficiacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J. Pharm. Sci. 1999, 88, 592–598. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Bommannan, D.; Potts, R.O.; Guy, R.H. Examination of stratum corneum barrier function in vivo by infrared spectroscopy. J. Investig. Dermatol. 1990, 95, 403–408. [Google Scholar]
- Gorcea, M.; Hadgraft, J.; Moore, D.J.; Lane, M.E. In vivo barrier challenge and initial recovery in human facial skin. Skin Res. Technol. 2013, 19, e375–e382. [Google Scholar] [CrossRef]
- Baloğlu, E.; Karavana, S.Y.; Hysein, I.Y.; Köse, T. Design and formulation of mebeverine HCl semisolid formulations for intraorally administration. AAPS PharmSciTech 2010, 11, 181–188. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J.; Coulter, W.A. Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm. Res. 1996, 13, 1734–1738. [Google Scholar] [CrossRef]
- Cevher, E.; Taha, M.A.M.; Orlu, M.; Araman, A. Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F.; O’Neill, M.J. Mucoadhesive, syringeable drug delivery systems for controlled application of metronidazole to the periodontal pocket: In vitro release kinetics, syringeability, mechanical and mucoadhesive properties. J. Control. Release 1997, 49, 71–79. [Google Scholar] [CrossRef]
- The United States Pharmacopeia 31, 26th ed.; U.S. Pharmacopeial Convention: Twinbrook Parkway, Rockwille, MD, USA, 2008; p. 683.
- Gutschke, E.; Bracht, S.; Nagel, S.; Weitschies, W. Adhesion testing of transdermal matrix patches with a probe tack test—In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2010, 75, 399–404. [Google Scholar] [CrossRef]
- Wokovich, A.M.; Prodduturi, S.; Doub, W.H.; Hussain, A.S.; Buhse, L.F. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar] [CrossRef]
- Güngör, S.; Erdal, M.S.; Özsoy, Y. Plasticizers in Transdermal Drug Delivery Systems. In Recent Advances in Plasticizers; Luqman, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 91–112. [Google Scholar]
- Costa, P.; Lobo, S.J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Bhatt, D.C.; Dhake, A.S.; Khar, R.K.; Mishra, D.N. Development and in vitro evaluation of transdermal matrix films of metoprolol tartrate. Yakugaku Zasshi. 2008, 128, 1325–1331. [Google Scholar] [CrossRef]
- Mamatha, T.; Venkateswara Rao, J.; Mukkanti, K.; Ramesh, G. Development of matrix type transdermal patches of lercanidipine hydrochloride: physicochemical and in vitro characterization. Daru. J. Pharm. Sci. 2010, 18, 9–16. [Google Scholar]
- Tirunagari, M.; Jangala, V.R.; Khagga, M.; Gannu, R. Transdermal therapeutic system of isradipine: Effect of hydrophilic and hydrophobic matrix on in vitro and ex vivo characteristics. Arch. Pharm. Res. 2010, 33, 1025–1033. [Google Scholar] [CrossRef]
- Melero, A.; Garrigues, T.M.; Almudever, P.; Villodre, A.M.; Lehr, C.M.; Schaefer, U. Nortriptyline hydrochloride skin absorption: Development of a transdermal patch. Eur. J. Pharm. Biopharm. 2008, 69, 588–596. [Google Scholar] [CrossRef]
- Zhao, K.; Singh, J. Mechanism(s) of in vitro percutaneous absorption enhancement of Tamoxifen by Enhancers. J. Pharm. Sci. 2000, 89, 771–780. [Google Scholar] [CrossRef]
- Levi, K.; Kwan, A.; Rhines, A.S.; Gorcea, M.; Moore, D.J.; Dauskardt, R.H. Emollient molecule effects on the drying stresses in human stratum corneum. Br. J. Dermatol. 2010, 163, 695–703. [Google Scholar] [CrossRef]
- Erdal, M.S.; Peköz Yıldız, A.; Aksu, B.; Araman, A. Impacts of chemical enhancers on skin permeation and deposition of terbinafine. Pharm. Dev. Technol. 2014, 19, 565–570. [Google Scholar] [CrossRef]
- Zhao, K.; Singh, J. Mechanisms of percutaneous absorption of tamoxifen by terpenes: Eugenol, d-limonene and menthone. J. Control. Rel. 1998, 55, 253–260. [Google Scholar] [CrossRef]
- Takahashi, K.; Sakano, H.; Yoshida, M.; Numata, N.; Mizuno, N. Characterization of the influence of polyol fatty acid esters on the permeation of diclofenac through rat skin. J. Control. Rel. 2001, 73, 351–358. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hwang, T.L.; Leu, Y.L. Effect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogels. Int. J. Pharm. 2003, 250, 313–325. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aktar, B.; Erdal, M.S.; Sagirli, O.; Güngör, S.; Özsoy, Y. Optimization of Biopolymer Based Transdermal Films of Metoclopramide as an Alternative Delivery Approach. Polymers 2014, 6, 1350-1365. https://doi.org/10.3390/polym6051350
Aktar B, Erdal MS, Sagirli O, Güngör S, Özsoy Y. Optimization of Biopolymer Based Transdermal Films of Metoclopramide as an Alternative Delivery Approach. Polymers. 2014; 6(5):1350-1365. https://doi.org/10.3390/polym6051350
Chicago/Turabian StyleAktar, Betül, Meryem Sedef Erdal, Olcay Sagirli, Sevgi Güngör, and Yıldız Özsoy. 2014. "Optimization of Biopolymer Based Transdermal Films of Metoclopramide as an Alternative Delivery Approach" Polymers 6, no. 5: 1350-1365. https://doi.org/10.3390/polym6051350