Laccase Functionalization of Flax and Coconut Fibers
Abstract
:1. Introduction
2. Experimental Section
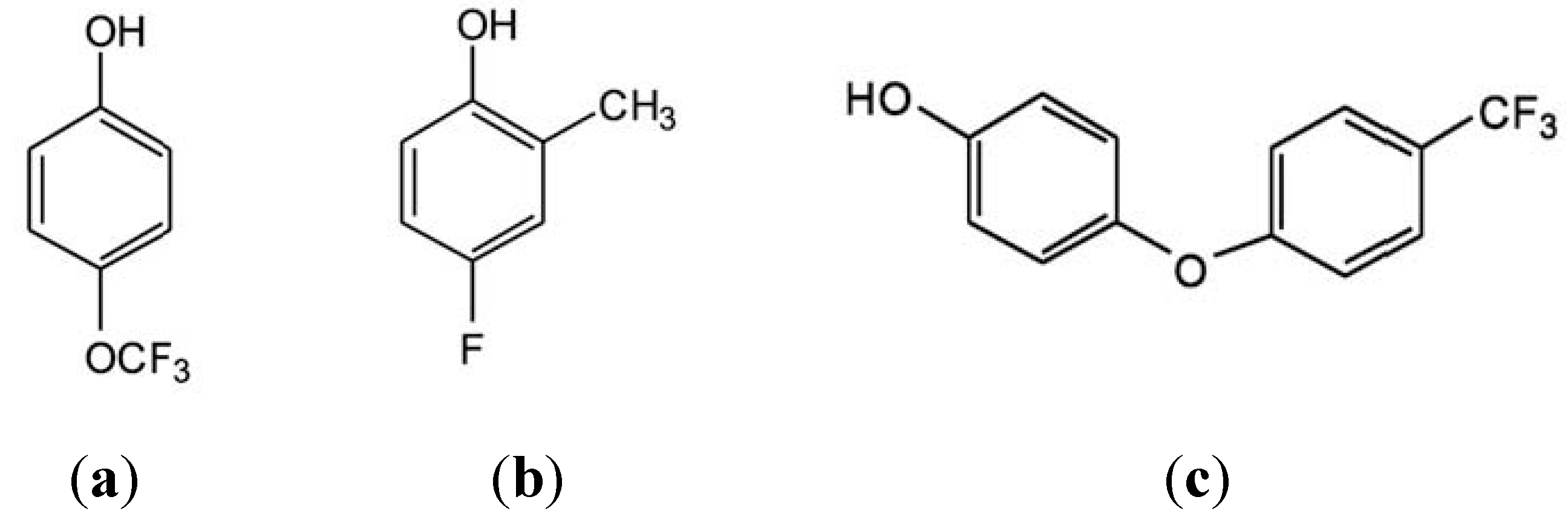
3. Results and Discussion
| Sample | % C | % F | % O | F/C | O/C |
|---|---|---|---|---|---|
| Flax + Laccase | 65.6 | – | 27.2 | – | 0.415 |
| Flax + Laccase + 4,2-FMP | 64.5 | – | 30.2 | – | 0.468 |
| Flax + Laccase + 4,-F3MP | 65.6 | 0.7 | 27.6 | 0.011 | 0.421 |
| Flax + Laccase + 4,4-F3MPP | 61.0 | 0.5 | 31.3 | 0.0082 | 0.513 |
| Sample | % C | % F | % O | F/C | O/C |
|---|---|---|---|---|---|
| Flax + Laccase | 76.8 | – | 20.6 | 0 | 0.268 |
| Flax + Laccase + 4,2-FMP | 76.1 | – | 20.3 | 0 | 0.267 |
| Flax + Laccase + 4,-F3MP | 70.4 | 4.7 | 22.1 | 0.067 | 0.314 |
| Flax + Laccase + 4,4-F3MPP | 72.8 | 0.3 | 24.3 | 0.004 | 0.334 |

| Sample | % C | % N | % O |
|---|---|---|---|
| Flax + Laccase | 72.5 | 3.9 | 23.3 |
| Flax + Laccase + DFA | 70.5 | 4.2 | 25 |
| Flax + Laccase + DFA+HBT | 73.7 | 4.4 | 21.5 |
| Flax + Laccase + DFA+ABTS | 71.9 | 4.5 | 23.3 |
| Flax + Laccase + DFA+TEMPO | 80.3 | 5.8 | 13.6 |

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kudanga, T.; Burton, S.; Nyanhongo, G.S.; Guebitz, G.M. Versatility of oxidoreductases in the remediation of environmental pollutants. Front. Biosci. 2012, 4, 1127–1149. [Google Scholar]
- Yaropolov, A.I.; Skorobogatko, O.V.; Vartanov, S.S.; Varfolomeyev, S.D. Laccase properties, catalytic mechanism, and applicability. Appl. Biochem. Biotechnol. 1994, 49, 257–280. [Google Scholar] [CrossRef]
- Burton, S.G. Laccases and phenol oxidase in organic synthesis—A review. Curr. Org. Chem. 2003, 7, 1317–1331. [Google Scholar] [CrossRef]
- Mikolasch, A.; Niedermeyer, T.H.J.; Lalk, M.; Hammer, E.; Seefeldt, S.; Hammer, E. Novel penicillins synthesized by biotransformation using laccase from Trametes spec. Chem. Pharm. Bull. 2006, 54, 632–638. [Google Scholar] [CrossRef]
- Agematu, H.; Tsuchida, T.; Kominato, K.; Shibamoto, N.; Yoshioka, T.; Nishida, H.; Okamoto, R.; Shin, T.; Murao, S. Enzymatic dimerization of penicillin X. J. Antibiot. 1993, 46, 141–148. [Google Scholar] [CrossRef]
- Zamorani, A.; Spettoli, P.; Lante, A.; Crapisi, A.; Pasini, G. Immobilized laccase and tyrosinase: An approach for wine stabilization. Ital. J. food Sci. 1993, 4, 409–414. [Google Scholar]
- Cantarelli, C.; Giovanelli, G. White wine stabilization treatments by enzymatic oxidation of polyphenols. Rev. Fr. Oenol. 1990, 127, 15–25. [Google Scholar]
- Almansa, E.; Kandelbauer, A.; Pereira, L.; Cavaco, P.; Guebitz, G.M. Influence of structure on dyedegradation with laccase mediator systems. Biocatal. Biotransform. 2004, 22, 315–324. [Google Scholar]
- Abadulla, E.; Robra, K.H.; Gubitz, G.; Silva, L.; Cavaco-Paulo, A. Enzymatic decolorization of textile dyeing effluents. Text. Res. J. 2000, 70, 409–414. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2-azinobis-(3-ethylbenzthiazoline-6-sulphonate). Appl. Microbiol. Biotechnol. 1992, 36, 823–827. [Google Scholar]
- Camarero, S.; Garcia, O.; Vidal, T.; Colom, J.; del Rio, J.C.; Gutierrez, A.; Gras, J.M.; Monje, R.; Martinez, M.J.; Martinez, A.T. Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microbiol. Technol. 2004, 35, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Aracri, E.; Fillat, A.; Colom, J.F.; Gutiérrez, A.; del Río, J.C.; Martínez, Á.T.; Vidal, T. Enzymatic grafting of simple phenols on flax and sisal pulp fibres using laccases. Bioresour. Technol. 2010, 101, 8211–8216. [Google Scholar] [CrossRef]
- Chandra, R.P.; Ragauskas, A.J. Evaluating laccase-facilitated coupling of phenolic acids to high-yield kraft pulps. Enzyme Microb. Technol. 2002, 30, 855–861. [Google Scholar] [CrossRef]
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzyme Microb. Technol. 2007, 43, 3–37. [Google Scholar]
- Barzyk, D.; Page, D.; Ragauskas, A.J. Acidic group topochemistry and fiber-to-fiber specific bond strength. J. Pulp Pap. Sci. 1997, 23, 59–61. [Google Scholar]
- Kudanga, T.; Prasetyo, E.N.; Widsten, P.; Kandelbauer, A.; Jury, S.; Heathcote, C.; Sipila, J.; Weber, H.; Nyanhongo, G.S.; Guebitz, G.M. Laccase catalyzed covalent coupling of fluorophenols increases lignocellulose surface hydrophobicity. Bioresour. Technol. 2010, 101, 2793–2799. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipila, J.; Guebitz, G.M.; Nyanhongo, G.S. Reactivity of long chain alkylamines to lignin moieties: Implications on hydrophobicity of lignocellulose materials. J. Biotechnol. 2010, 149, 81–87. [Google Scholar] [CrossRef]
- Nabi Saheb, D.; Pog, J.P. Natural fiber polymer composites: A review. Adv. Polym. Technol. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Greimel, K.J.; Perz, V.; Koren, K.; Feola, R.; Temel, A.; Sohar, C.; Herrero Acero, E.; Klimant, I.; Guebitz, G.M. Banning toxic heavy-metal catalysts from paints: Enzymatic cross-linking of alkyd resins. Green Chem. 2013, 15, 381–388. [Google Scholar] [CrossRef]
- Nyanhongo, G.S.; Nugroho Prasetyo, E.; Herrero Acero, E.; Guebitz, G.M. Engineering strategies for successful development of functional polymers using oxidative enzymes. Chem. Eng. Technol. 2012, 35, 1359–1372. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipila, J.; Nyanhongo, G.S.; Guebitz, G.M. Enzymatic grafting of functional molecules to the lignin model dibenzodioxocin and lignocellulose material. Enzyme Microb. Technol. 2010, 46, 272–280. [Google Scholar] [CrossRef]
- Schultz, A.; Jonas, U.; Hammer, E.; Schauer, F. Dehalogenation of chlorinated hydroxybiphenyls by fungal laccase. Appl. Environ. Microbiol. 2001, 67, 4377–4381. [Google Scholar] [CrossRef]
- Jonas, U.; Hammer, E.; Haupt, E.T.K.; Schauer, F. Characterisation of coupling products formed by biotransformation of biphenyl and diphenyl ether by the white rot fungus Pycnoporus cinnabarinus. Arch. Microbiol. 2000, 174, 393–398. [Google Scholar] [CrossRef]
- Božič, M.; Gorgieva, S.; Kokol, V. Laccase-mediated functionalization of chitosan by caffeic and galic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [Google Scholar] [CrossRef]
- Barneto, A.G.; Aracri, E.; Andreu, G.; Vidal, T. Investigating the structure-effect relationships of various natural phenols used as laccase mediators in the biobleaching of kenaf and sisal pulps. Bioresour. Technol. 2012, 112, 327–335. [Google Scholar] [CrossRef]
- Schroeder, M.; Fatarella, E.; Kovac, J.; Guebitz, G.M.; Kokol, V. Laccase-induced grafting on plasma-preatreated polypropylene. Biomacromolecules 2008, 9, 2735–2741. [Google Scholar] [CrossRef]
- Witayakran, S.; Ragauskas, A.J. Modification of high-lignin softwood kraft pulp with laccase and amino acids. Enzyme Microb. Technol. 2009, 44, 176–181. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Mikolasch, A.; Lalk, M. Nuclear amination catalyzed by fungal laccases: Reaction products of p-hydroquinones and primary aromatic amines. J. Org. Chem. 2005, 70, 2002–2008. [Google Scholar] [CrossRef]
- Sampaio, S.; Taddei, P.; Monti, P.; Buchert, J.; Freddi, G. Enzymatic grafting of chitosan onto Bombyx mori silk fibroin: Kinetic and IR vibrational studies. J. Biotechnol. 2005, 116, 21–33. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Cantero, G.; Arbelaiz, A.; Llano-Ponte, R.; Mondragon, I. Effects of fibre treatment on wettability and mechanical behavior of flax/polypropylene composites. Compos. Sci. Technol. 2003, 63, 1247–1254. [Google Scholar] [CrossRef]
- Chen, T.H.; Small, D.A.; Wu, L.Q.; Rubloff, G.W.; Ghodssi, R.; Vazquez-Duhalt, R.; Bentley, W.E.; Payne, G.F. Nature-inspired creation of protein-polysaccharide conjugate and its subsequent assembly onto a patterned surface. Langmuir 2003, 19, 9382–9386. [Google Scholar] [CrossRef]
- Acero, E.H.; Ribitsch, D.; Rodriguez, R.D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Greimel, K.J.; Schroeder, M.; Kandelbaueret, A.; Heumann, S.; et al. Two-step enzymatic functionalisation of polyamide with phenolics. J. Mol. Catal. Enzym. 2012, 79, 54–60. [Google Scholar] [CrossRef]
- Nady, N.; Schroën, K.; Franssen, M.C.; Lagen, B.V.; Murali, S.; Boom, R.M.; Mohyeldin, M.S.; Zuilhof, H. Mild and highly flexible enzyme-catalyzed modification of poly(ethersulfone) membranes. ACS Appl. Mater. Interfaces 2011, 3, 801–810. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Acero, E.H.; Kudanga, T.; Ortner, A.; Kaluzna, I.; De Wildeman, S.; Nyanhongo, G.S.; Guebitz, G.M. Laccase Functionalization of Flax and Coconut Fibers. Polymers 2014, 6, 1676-1684. https://doi.org/10.3390/polym6061676
Acero EH, Kudanga T, Ortner A, Kaluzna I, De Wildeman S, Nyanhongo GS, Guebitz GM. Laccase Functionalization of Flax and Coconut Fibers. Polymers. 2014; 6(6):1676-1684. https://doi.org/10.3390/polym6061676
Chicago/Turabian StyleAcero, Enrique Herrero, Tukayi Kudanga, Andreas Ortner, Iwona Kaluzna, Stefaan De Wildeman, Gibson S. Nyanhongo, and Georg M. Guebitz. 2014. "Laccase Functionalization of Flax and Coconut Fibers" Polymers 6, no. 6: 1676-1684. https://doi.org/10.3390/polym6061676




