Fabrication and Characterization of Inorganic Silver and Palladium Nanostructures within Hexagonal Cylindrical Channels of Mesoporous Carbon
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials
2.2. Mesoporous Carbons Templated by PEO-b-PCL Copolymers
2.3. Metal/Mesoporous Carbon Composites
2.4. Characterization
3. Results and Discussion
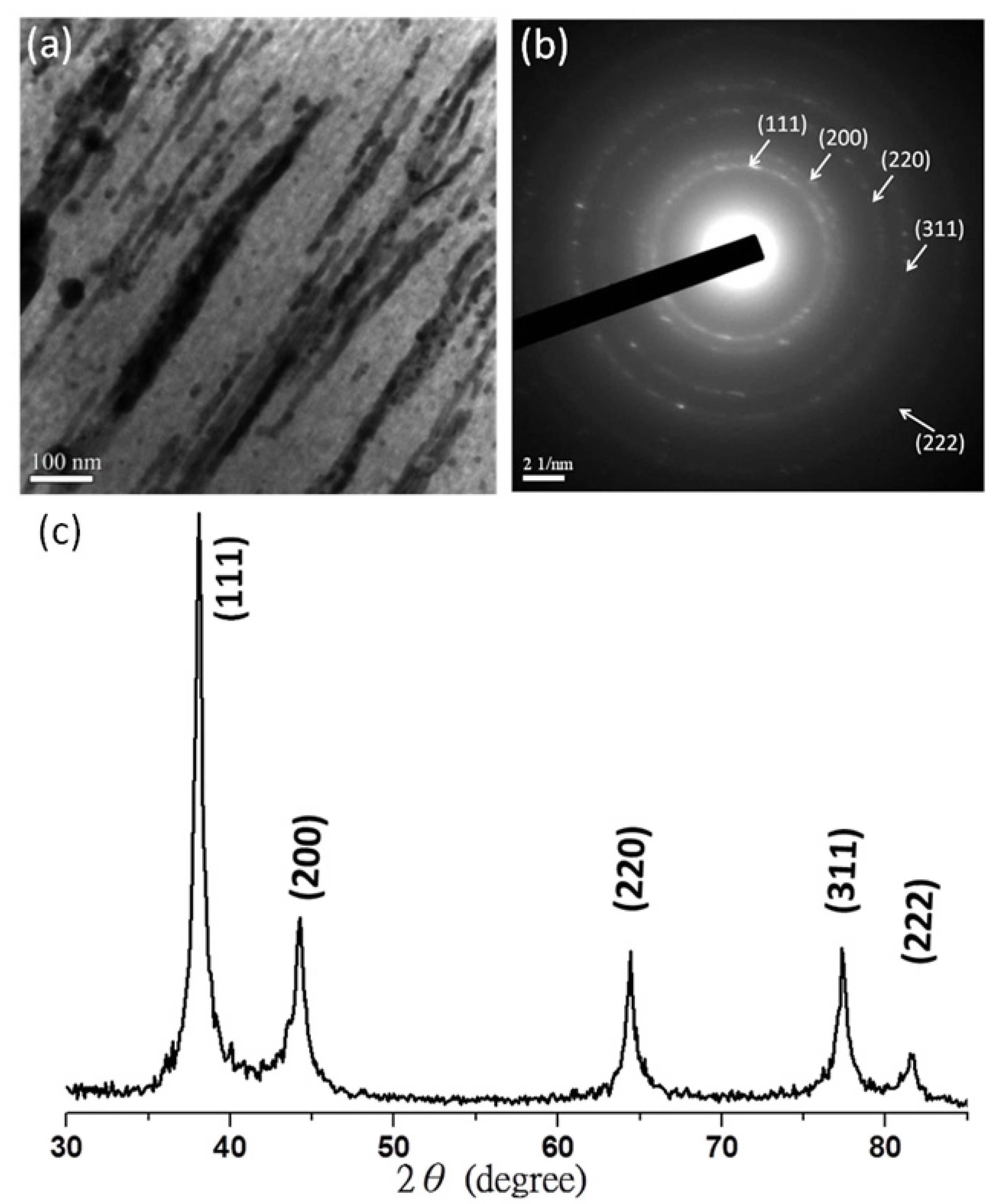
3.1. Silver Nanowires within Mesochannels of Hexagonal Cylindrical Mesoporous Carbon
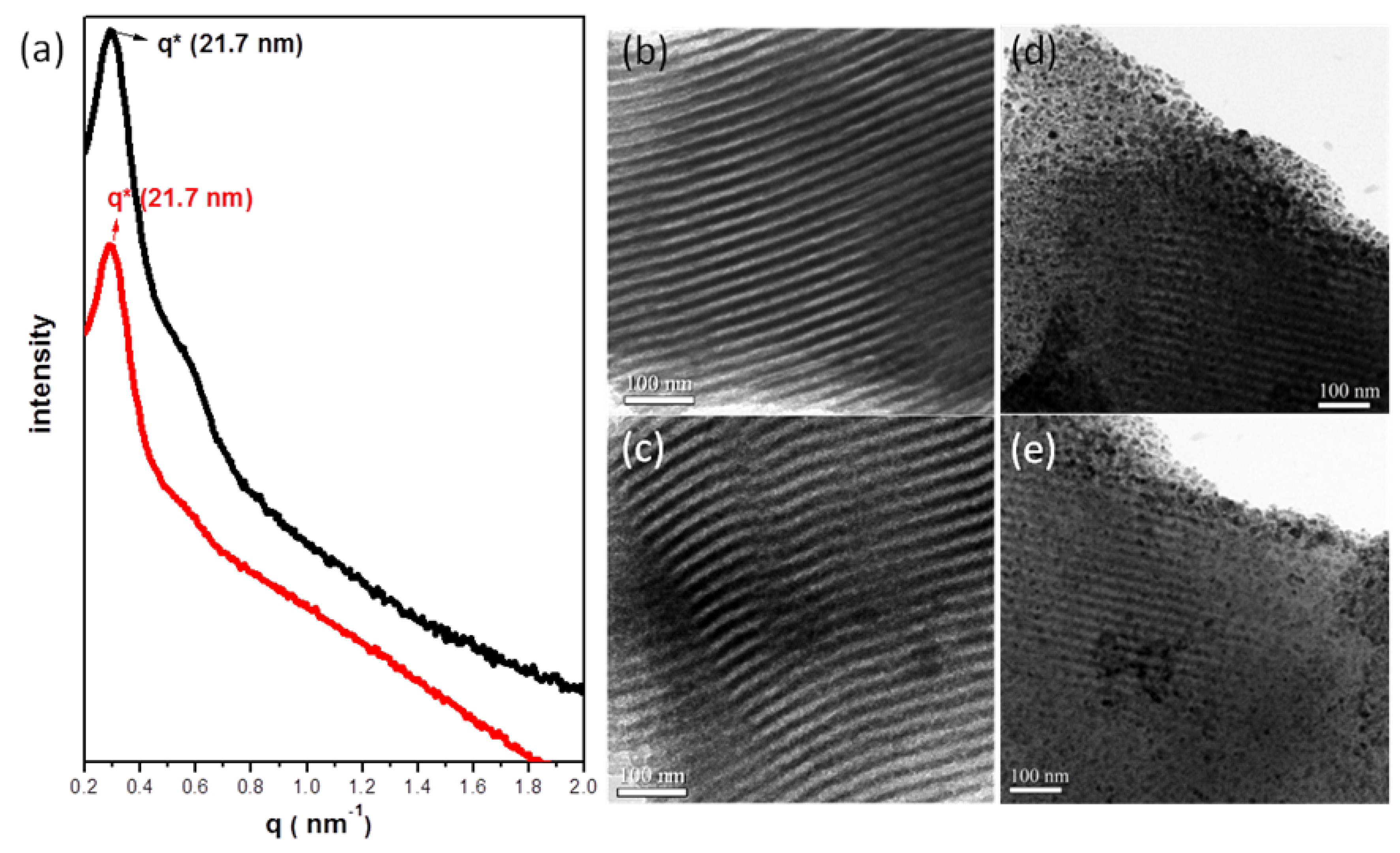
 , consistent with hexagonally packing. After impregnation of the Ag nanowires, the primary reflection peak underwent an apparent collapse, consistent with a certain degree of infilling of the Ag nanowires within the hexagonal mesochannels; in other words, imperfect infilling of the Ag precursors occurred during the impregnation step. For further confirmation of these phenomena, Figure 1b–e displays TEM images of the mesoporous carbon (Figure 1b,c) and Ag/mesoporous carbon (Figure 1d,e) viewed from the side of the hexagonal cylindrical structures (i.e., viewed from the (10) plane). Consistent with the SAXS data, the TEM image of the mesoporous carbon was typical of that expected for a side view of hexagonal packing (Figure 1b,c); on the other hand, some Ag nanowires were evident in Figure 1d,e, suggesting successful impregnation of the Ag nanowires within the hexagonal mesopores of the mesoporous carbon sample.
, consistent with hexagonally packing. After impregnation of the Ag nanowires, the primary reflection peak underwent an apparent collapse, consistent with a certain degree of infilling of the Ag nanowires within the hexagonal mesochannels; in other words, imperfect infilling of the Ag precursors occurred during the impregnation step. For further confirmation of these phenomena, Figure 1b–e displays TEM images of the mesoporous carbon (Figure 1b,c) and Ag/mesoporous carbon (Figure 1d,e) viewed from the side of the hexagonal cylindrical structures (i.e., viewed from the (10) plane). Consistent with the SAXS data, the TEM image of the mesoporous carbon was typical of that expected for a side view of hexagonal packing (Figure 1b,c); on the other hand, some Ag nanowires were evident in Figure 1d,e, suggesting successful impregnation of the Ag nanowires within the hexagonal mesopores of the mesoporous carbon sample. 
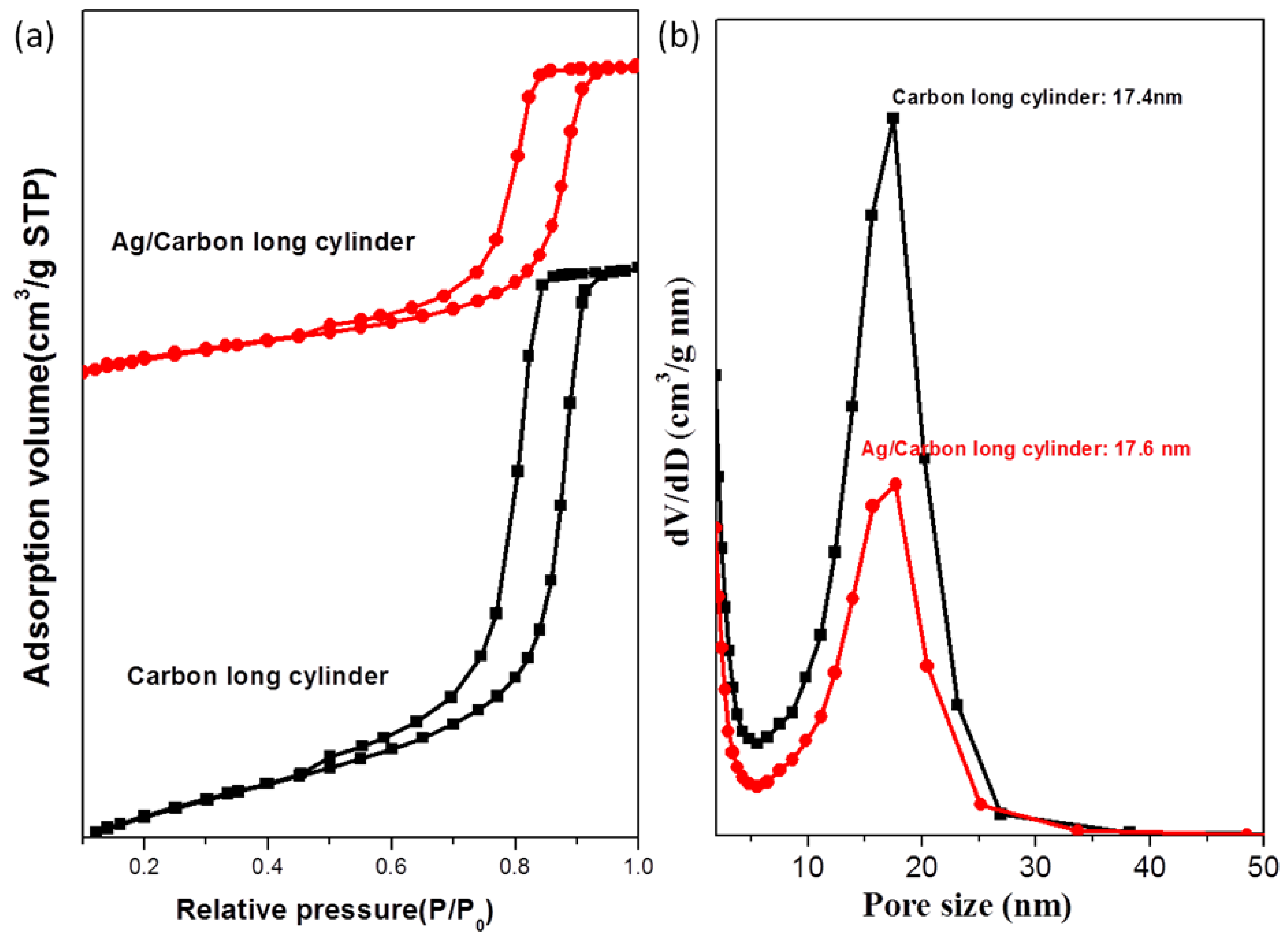
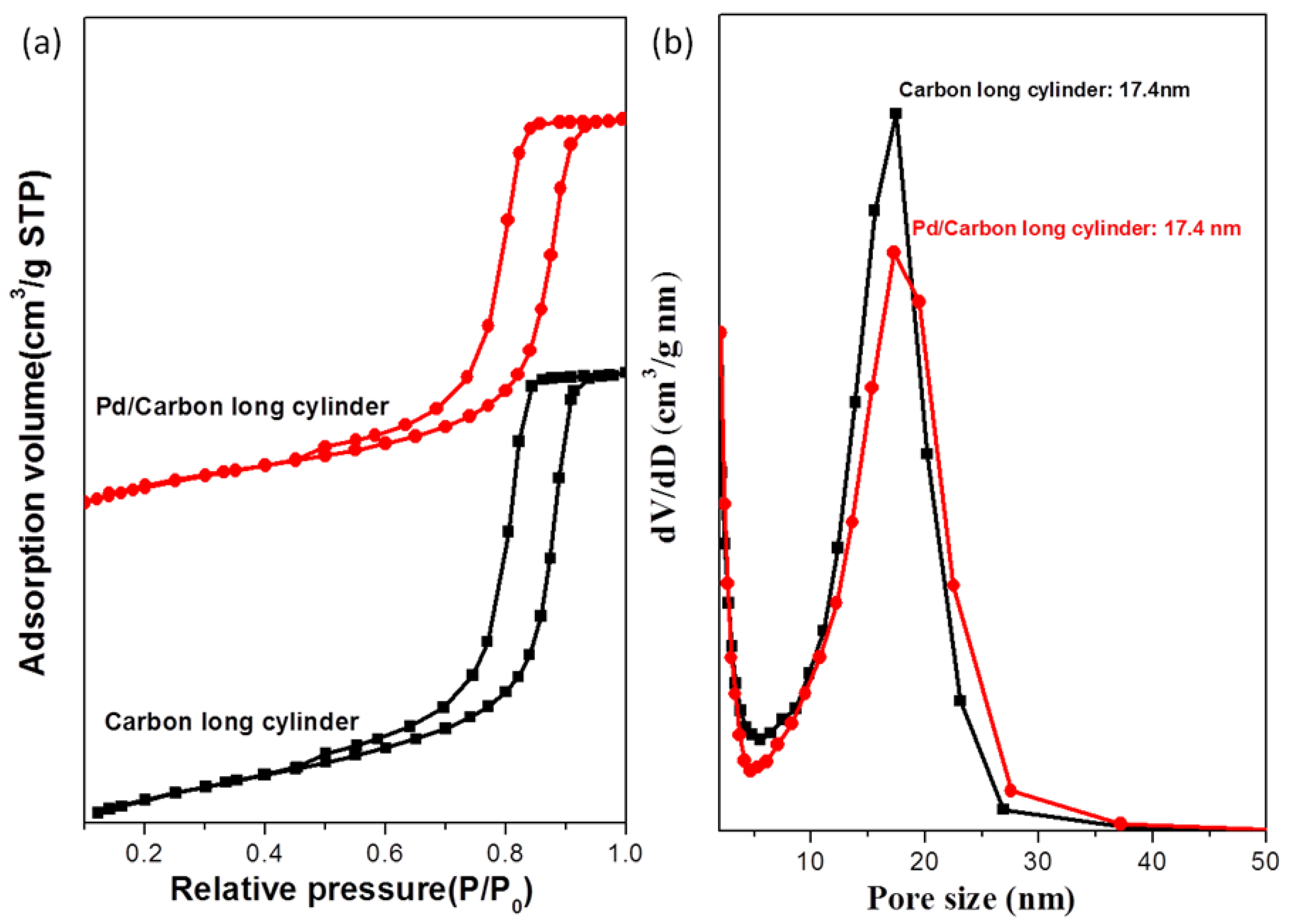
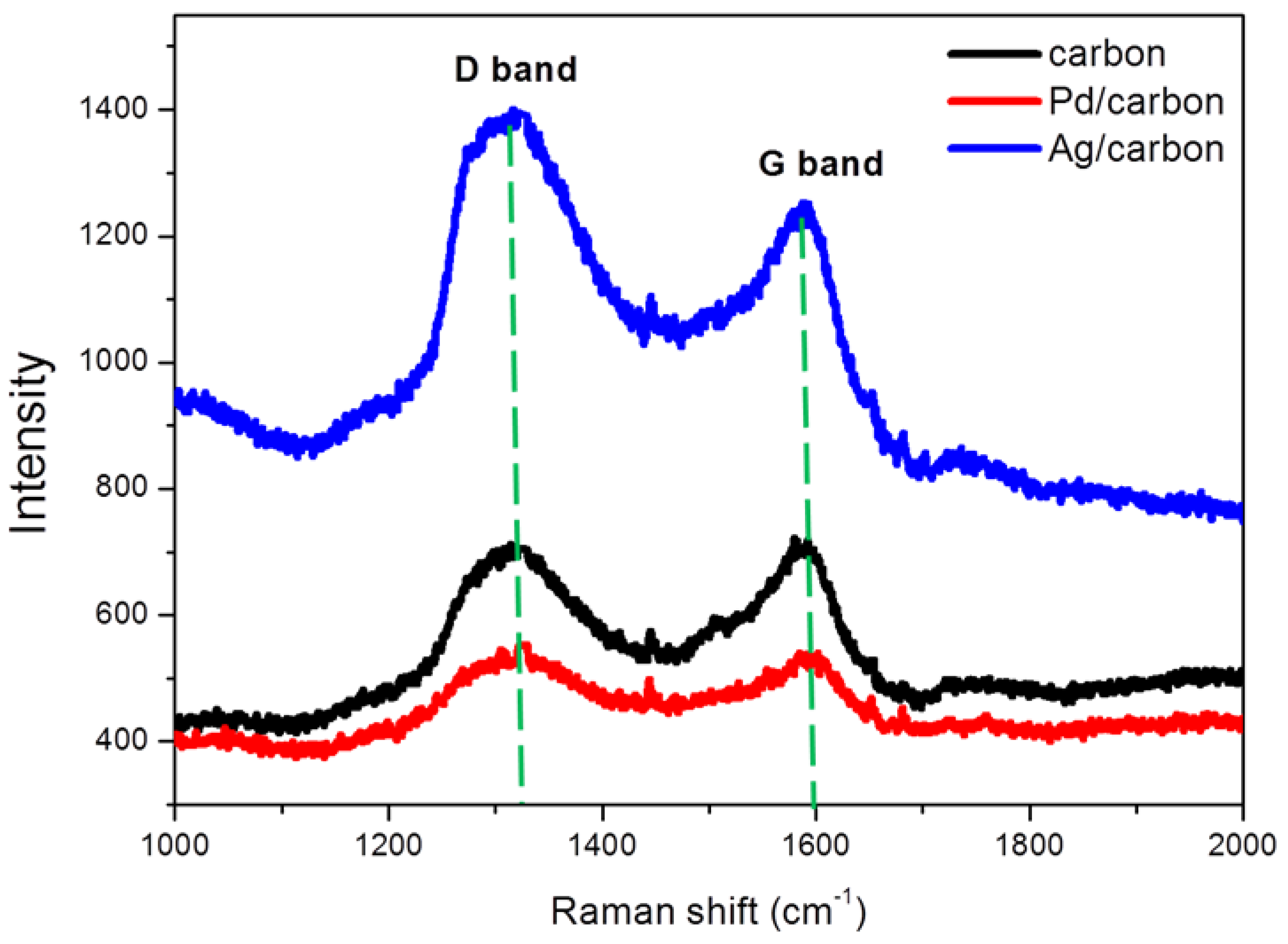
| Sample | d (nm) a | Pore size (nm) | SBET (m2/g) b | SM (m2/g) b | Pore volume (cm3/g) | Micropore volume (cm3/g) | Note | ID/IG c | Infilling ratio (%) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 21.7 | 17.4 | 729 | 515 | 0.70 | 0.239 | Cylindrical mesoporous carbon | 0.996 | – |
| 2 | 21.7 | 17.6 | 409 | 286 | 0.39 | 0.133 | Ag/mesoporous carbon | 1.116 | 44 |
| 3 | 21.7 | 17.4 | 660 | 468 | 0.62 | 0.218 | Pd/mesoporous carbon | 1.009 | 11 |
3.2. Palladium Nanometals within Mesochannels of Hexagonal Cylindrical Mesoporous Carbon
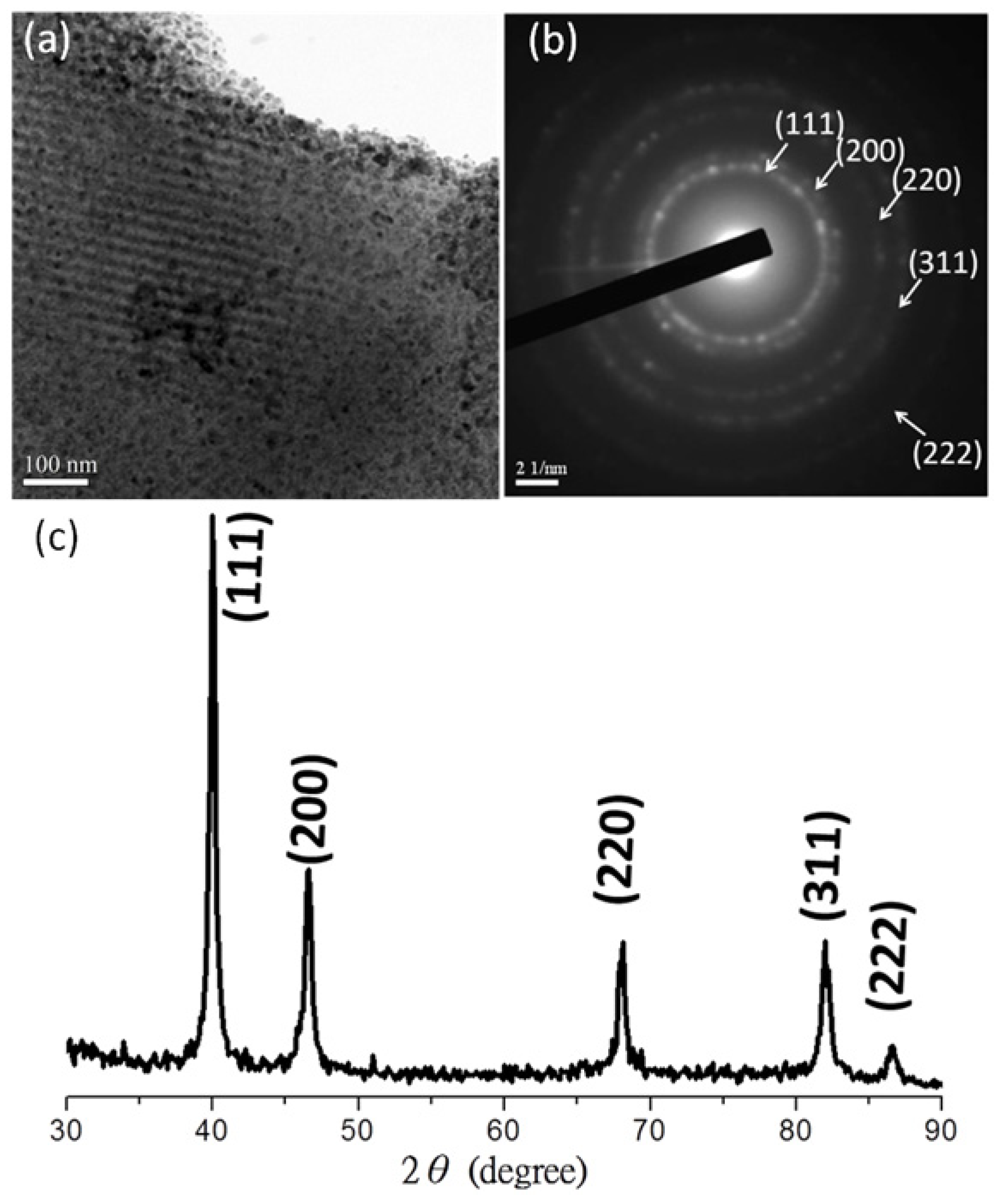
3.3. Raman Spectra of Mesoporous Carbon and Nanometal/Mesoporous Carbon Samples
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Soler-Illia, G.; Crepaldi, E.L.; Grosso, D.; Sanchez, C. Block copolymer-templated mesoporous oxides. Curr. Opin. Coll. Interface Sci. 2003, 8, 109–126. [Google Scholar] [CrossRef]
- Deng, Y.H.; Yu, T.; Wan, Y.; Shi, Y.F.; Meng, Y.; Gu, D.; Zhang, L.J.; Huang, Y.; Liu, C.; Wu, X.J.; et al. Ordered mesoporous silicas and carbons with large accessible pores templated from amphiphilic diblock copolymer poly(ethylene oxide)-b-polystyrene. J. Am. Chem. Soc. 2007, 129, 1690–1697. [Google Scholar] [CrossRef]
- Valkama, S.; Nykanen, A.; Kosonen, H.; Ramani, R.; Tuomisto, F.; Engelhardt, P.; ten Brinke, G.; Ikkala, O.; Ruokolainen, J. Hierarchical porosity in self-assembled polymers: Post-modification of block copolymer–phenolic resin complexes by pyrolysis allows the control of micro- and mesoporosity. Adv. Funct. Mater. 2007, 17, 183–190. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Deng, Y.H.; Wei, J.; Sun, Z.K.; Gu, D.; Bongard, H.; Liu, C.; Wu, H.H.; Tu, B.; Schuth, F.; et al. Design of amphiphilic ABC triblock copolymer for templating synthesis of large-pore ordered mesoporous carbons with tunable pore wall thickness. Chem. Mater. 2009, 21, 3996–4005. [Google Scholar] [CrossRef]
- Li, J.G.; Kuo, S.W. Phase behavior of mesoporous nanostructures templated by amphiphilic crystalline–crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). RSC Adv. 2011, 1, 1822–1833. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, Y.D.; Kuo, S.W. From Microphase separation to self-organized mesoporous phenolic resin through competitive hydrogen bonding with double-crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). Macromolecules 2011, 44, 9295–9309. [Google Scholar] [CrossRef]
- Li, J.G.; Chang, Y.H.; Lin, Y.S.; Kuo, S.W. Templating amphiphilic poly(ethylene oxide-b-ε-caprolactone) diblock copolymers provides ordered mesoporous silicas with large tunable pores. RSC Adv. 2012, 2, 12973–12982. [Google Scholar] [CrossRef]
- Li, J.G.; Chen, W.C.; Kuo, S.W.S. Phase behavior of mesoporous silicas templated by the amphiphilic diblock copolymer poly(ethylene-b-ethylene oxide). Microporous Mesoporous Mater. 2012, 163, 34–41. [Google Scholar] [CrossRef]
- Li, J.G.; Chung, C.Y.; Kuo, S.W. Transformations and enhanced long-range ordering of mesoporous phenolic resin templated by poly(ethylene oxide-b-ε-caprolactone) block copolymers blended with star poly(ethylene oxide)-functionalized silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 18583–18595. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, R.B.; Kuo, S.W. Hierarchical mesoporous silica fabricated from an ABC triblock terpolymer as a single template. Macromol. Rapid Commun. 2012, 33, 678–682. [Google Scholar] [CrossRef]
- Chu, W.C.; Li, J.G.; Kuo, S.W. From flexible to mesoporous polybenzoxazine resins templated by poly(ethylene oxide-b-ε-caprolactone) copolymer through reaction induced microphase separation mechanism. RSC Adv. 2013, 3, 6485–6498. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Phenolic resin-based carbons with ultra-large mesopores prepared in the presence of poly(ethylene oxide)–poly(butylene oxide)–poly(ethylene oxide) triblock copolymer and trimethyl benzene. Carbon 2013, 51, 45–51. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.Q.; Shi, Y.F.; Yang, H.F.; Li, Z.; Yu, C.Z.; Tu, B.; Zhao, D.Y. Ordered mesoporous polymers and homologous carbon frameworks: Amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.Q.; Shi, Y.F.; Cheng, L.; Feng, D.; Wu, Z.X.; Chen, Z.X.; Wan, Y.; Stein, A.; et al. A Family of highly ordered mesoporous polymer resin and carbon structures from organic–organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Lee, Y.F.; Chang, K.H.; Chu, C.Y.; Chen, H.L.; Hu, C.C. Microstructure tuning of mesoporous silica prepared by evaporation-induced self-assembly processes: Interactions among solvent evaporation, micelle formation/packing and sol condensation. RSC Adv. 2011, 1, 401–407. [Google Scholar] [CrossRef]
- Wei, H.; Lv, Y.Y.; Han, L.; Tu, B.; Zhao, D.Y. Facile synthesis of transparent mesostructured composites and corresponding crack-free mesoporous carbon/silica monoliths. Chem. Mater. 2011, 23, 2353–2360. [Google Scholar]
- De Los Cobos, O.; Fousseret, B.; Lejeune, M.; Rossignol, F.; Dutreilh-Colas, M.; Carrion, C.; Boissiere, C.; Ribot, F.; Sanchez, C.; Cattoen, X.; et al. Tunable multifunctional mesoporous silica microdots arrays by combination of inkjet printing, EISA, and click chemistry. Chem. Mater. 2012, 24, 4337–4342. [Google Scholar] [CrossRef]
- Vaid, C.; Murugavel, S.; Kashayap, R.; Tandon, R.P. Synthesis and in vitro bioactivity of surfactant templated mesoporous sodium silicate glasses. Microporous Mesoporous Mater. 2012, 159, 17–23. [Google Scholar] [CrossRef]
- Ba, J.H.; Polleux, J.; Antonietti, M.; Niederberger, M. Non-aqueous synthesis of tin oxide nanocrystals and their assembly into ordered porous mesostructures. Adv. Mater. 2005, 17, 2509. [Google Scholar] [CrossRef]
- Das, S.K.; Bhunia, M.K.; Bhaumik, A. Self-assembled TiO2 nanoparticles: Mesoporosity, optical and catalytic properties. Dalton Trans. 2010, 39, 4382–4390. [Google Scholar] [CrossRef]
- Soler-Illia, G.; Louis, A.; Sanchez, C. Synthesis and characterization of mesostructured titania-based materials through evaporation-induced self-assembly. Chem. Mater. 2002, 14, 750–759. [Google Scholar] [CrossRef]
- Chaichanawong, J.; Yamamoto, T.; Kataoka, S.; Endo, A.; Ohmori, T. Synthesis of monodisperse carbonaceous beads with ordered mesoporous structure. Carbon 2009, 47, 929–932. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Z.; Zeng, K.; Zheng, S. From self-organized novolac resins to ordered nanoporous carbons. Macromolecules 2010, 43, 2960–2969. [Google Scholar] [CrossRef]
- Huang, C.H.; Doong, R.A. Sugarcane bagasse as the scaffold for mass production of hierarchically porous carbon monoliths by surface self-assembly. Microporous. Mesoporous Mater. 2012, 147, 47–52. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Han, S.J.; Hyeon, T. Simple silica-particle template synthesis of mesoporous carbons. Chem. Commun. 1999, 1955–1956. [Google Scholar]
- Fuertes, A.B. Template synthesis of mesoporous carbons with a controlled particle size. J. Mater. Chem. 2003, 13, 3085–3088. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Synthesis of mesoporous carbons using ordered and disordered mesoporous silica templates and polyacrylonitrile as carbon precursor. J. Phys. Chem. B 2005, 109, 9216–9225. [Google Scholar] [CrossRef]
- Lu, A.H.; Li, W.C.; Schmidt, W.; Schuth, F. Template synthesis of large pore ordered mesoporous carbon. Microporous. Mesoporous Mater. 2005, 80, 117–128. [Google Scholar]
- Yang, C.M.; Weidenthaler, C.; Spliethoff, B.; Mayanna, M.; Schuth, F. Facile template synthesis of ordered mesoporous carbon with polypyrrole as carbon precursor. Chem. Mater. 2005, 17, 355–358. [Google Scholar] [CrossRef]
- Zhuang, X.; Wan, Y.; Feng, C.M.; Shen, Y.; Zhao, D.Y. Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem. Mater. 2009, 21, 706–716. [Google Scholar]
- Liang, C.D.; Li, Z.J.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar]
- Saha, D.; Deng, S.G. Hydrogen adsorption on ordered mesoporous carbons doped with Pd, Pt, Ni, and Ru. Langmuir 2009, 25, 12550–12560. [Google Scholar] [CrossRef]
- Yin, Y.Y.; Zhou, S.X.; Min, C.; Wu, L.M. Preparation of rattle-type magnetic mesoporous carbon spheres and their highly efficient adsorption and separation. J. Colloid. Interface Sci. 2011, 361, 527–533. [Google Scholar] [CrossRef]
- Wang, G.Q.; Xing, W.; Zhuo, S.P. Application of mesoporous carbon to counter electrode for dye-sensitized solar cells. J. Power Sources 2009, 194, 568–573. [Google Scholar] [CrossRef]
- Numao, S.; Judai, K.; Nishijo, J.; Mizuuchi, K.; Nishi, N. Synthesis and characterization of mesoporous carbon nano-dendrites with graphitic ultra-thin walls and their application to supercapacitor electrodes. Carbon 2009, 47, 306–312. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Liang, C.D.; Dai, S.; Jaroniec, M. Mesoporous carbon materials with ultra-thin pore walls and highly dispersed nickel nanoparticles. Eur. J. Inorg. Chem. 2009, 2009, 605–612. [Google Scholar]
- Su, F.B.; Zhao, X.S.; Wang, Y.; Zeng, J.H.; Zhou, Z.C.; Lee, J.Y. Synthesis of graphitic ordered macroporous carbon with a three-dimensional interconnected pore structure for electrochemical applications. J. Phys. Chem. B 2005, 109, 20200–20206. [Google Scholar] [CrossRef]
- Chi, Y.; Zhao, L.; Yuan, Q.; Yan, X.; Li, Y.J.; Li, N.; Li, X.T. In situ auto-reduction of silver nanoparticles in mesoporous carbon with multifunctionalized surfaces. J. Mater. Chem. 2012, 22, 13571–13577. [Google Scholar]
- Huwe, H.; Froba, M. Synthesis and characterization of transition metal and metal oxide nanoparticles inside mesoporous carbon CMK-3. Carbon 2007, 45, 304–314. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Pang, J.B.; Jiang, N.; Hampsey, J.E.; Lu, Y.F. Direct synthesis of palladium-containing mesoporous carbon. Microporous Mesoporous Mater. 2005, 81, 149–154. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.Y.; Zhao, Q.F.; Klingstedt, M.; Terasaki, O.; Zhao, D.Y. Ordered mesoporous Pd/silica−carbon as a highly active heterogeneous catalyst for coupling reaction of chlorobenzene in aqueous media. J. Am. Chem. Soc. 2009, 131, 4541–4550. [Google Scholar] [CrossRef]
- Su, F.B.; Zeng, J.H.; Bao, X.Y.; Yu, Y.S.; Lee, J.Y.; Zhao, X.S. Preparation and characterization of highly ordered graphitic mesoporous carbon as a Pt catalyst support for direct methanol fuel cells. Chem. Mater. 2005, 17, 3960–3967. [Google Scholar] [CrossRef]
- Panels, J.E.; Lee, J.; Park, K.Y.; Kang, S.Y.; Marquez, M.; Wiesner, U.; Joo, Y.L. Synthesis and characterization of magnetically active carbon nanofiber/iron oxide composites with hierarchical pore structures. Nanotechnology 2008, 19, 455612. [Google Scholar] [CrossRef]
- Andrada, D.M.; Vieira, H.S.; Oliveira, M.M.; Santos, A.P.; Yin, L.C.; Saito, R.; Pimenta, M.A.; Fantini, C.; Furtado, C.A. Dramatic increase in the Raman signal of functional groups on carbon nanotube surfaces. Carbon 2013, 56, 235–242. [Google Scholar]
- Hirschmann, T.C.; Araujo, P.T.; Muramatsu, H.; Zhang, X.; Nielsch, K.; Kim, Y.A.; Dresselhaus, M.S. Characterization of bundled and individual triple-walled carbon nanotubes by resonant Raman spectroscopy. ACS Nano 2013, 7, 2381–2387. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; Takahashi, M.; Mori, T.; Balasubramanian, V.V.; Ariga, K. Controlling the textural parameters of mesoporous carbon materials. Microporous Mesoporous Mater. 2007, 100, 20–26. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.X.; Sun, X.; Guo, Y.X.; Guo, H.; Tang, J.; Xue, H.R.; Liu, M.Z.; Zhang, X.X.; Zhu, L.; et al. Synthesis of ordered mesoporous boron-containing carbon films and their corrosion behavior in simulated proton exchange membrane fuel cells environment. J. Power Sources 2012, 212, 1–12. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Pushkarev, V.V.; Cherepanova, S.V.; Chuvilin, A.L.; Likholobov, V.A. Catalytic filamentous carbon: Structural and textural properties. Carbon 2003, 41, 1605–1615. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, C.L.; Zhang, Z.Y.; Zhang, M.Y.; Mu, J.B.; Guo, Z.C.; Liu, Y.C. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar] [CrossRef]
- Sterk, L.; Górka, J.; Vinu, A.; Jaroniec, M. Soft-templating synthesis of ordered mesoporous carbons in the presence of tetraethyl orthosilicate and silver salt. Microporous Mesoporous Mater. 2012, 156, 121–126. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Wang, L. Ordered mesoporous carbon-reduced graphene oxide composites decorating with Ag nanoparticles for surface enhanced Raman scattering. Mater. Lett. 2013, 97, 173–176. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, F.; Qin, D.; Wang, Y.; Zhang, J.; Liu, J.; Feng, Y.; Lu, X. One-pot synthesis of silver nanoparticle catalysts supported on N-doped ordered mesoporous carbon and application in the detection of nitrobenzene. Carbon 2014, 69, 481–489. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.-G.; Tsai, C.-Y.; Kuo, S.-W. Fabrication and Characterization of Inorganic Silver and Palladium Nanostructures within Hexagonal Cylindrical Channels of Mesoporous Carbon. Polymers 2014, 6, 1794-1809. https://doi.org/10.3390/polym6061794
Li J-G, Tsai C-Y, Kuo S-W. Fabrication and Characterization of Inorganic Silver and Palladium Nanostructures within Hexagonal Cylindrical Channels of Mesoporous Carbon. Polymers. 2014; 6(6):1794-1809. https://doi.org/10.3390/polym6061794
Chicago/Turabian StyleLi, Jheng-Guang, Cheng-Ying Tsai, and Shiao-Wei Kuo. 2014. "Fabrication and Characterization of Inorganic Silver and Palladium Nanostructures within Hexagonal Cylindrical Channels of Mesoporous Carbon" Polymers 6, no. 6: 1794-1809. https://doi.org/10.3390/polym6061794




