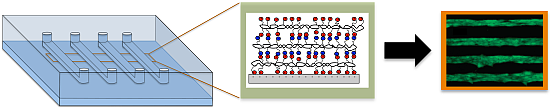
Polyelectrolyte Multilayers in Microfluidic Systems for Biological Applications
Abstract
:1. Introduction
2. Biosensors
2.1. Enzymatic Biochips

2.2. Fluorescently-Based Biosensors
2.3. Electronically-Based Biosensors


3. Patterning with PEMs
3.1. Cellular Interfaces and Patterning

3.2. PEMS with Surface Gradients

3.3. DNA-Based PEMs


4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interf. Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G.; Schmitt, J. Fine-tuning of the film thickness of ultrathin multilayer films composed of consecutively alternating layers of anionic and cationic polyelectrolytes. In Trends in Colloid and Interface Science VI; Helm, C., Lösche, M., Möhwald, H., Eds.; Steinkopff: Dresden, Germany, 1992; pp. 160–164. [Google Scholar]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef]
- Schönhoff, M. Self-assembled polyelectrolyte multilayers. Curr. Opin. Colloid Interf. Sci. 2003, 8, 86–95. [Google Scholar] [CrossRef]
- Klitzing, R.V.; Wong, J.E.; Jaeger, W.; Steitz, R. Short range interactions in polyelectrolyte multilayers. Curr. Opin. Colloid Interf. Sci. 2004, 9, 158–162. [Google Scholar]
- Hammond, P.T. Form and function in multilayer assembly: New applications at the nanoscale. Adv. Mater. 2004, 16, 1271–1293. [Google Scholar] [CrossRef]
- Lavalle, P.J.; Voegel, C.; Vautier, D.; Senger, B.; Schaaf, P.; Ball, V. Dynamic aspects of films prepared by a sequential deposition of species: Perspectives for smart and responsive materials. Adv. Mater. 2011, 23, 1191–1221. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Kharlampieva, E.; Izumrudov, V. Where polyelectrolyte multilayers and polyelectrolyte complexes meet. Macromolecules 2006, 39, 8873–8881. [Google Scholar] [CrossRef]
- Reyes, D.R.; Iossifidis, D.; Auroux, P.A.; Manz, A. Micro total analysis systems. 1. Introduction, theory, and technology. Anal. Chem. 2002, 74, 2623–2636. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 57, 181–189. [Google Scholar] [CrossRef]
- Dertinger, S.K.W.; Chiu, D.T.; Jeon, N.L.; Whitesides, G.M. Generation of gradients having complex shapes using microfluidic networks. Anal. Chem. 2001, 73, 1240–1246. [Google Scholar] [CrossRef]
- Jeon, N.L.; Dertinger, S.K.W.; Chiu, D.T.; Choi, I.S.; Stroock, A.D.; Whitesides, G.M. Generation of solution and surface gradients using microfluidic systems. Langmuir 2000, 16, 8311–8316. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, W.; Meng, S.; Kong, J.; Lu, H.; Yang, P.; Girault, H.H.; Liu, B. Assembly-controlled biocompatible interface on a microchip: Strategy to highly efficient proteolysis. Chem. Eur. J. 2006, 12, 6585–6591. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Y.; Ji, J.; Chen, X.; Kong, J.; Yang, P.; Girault, H.H.; Liu, B. Gold nanoparticle assembly microfluidic reactor for efficient on-line proteolysis. Mol. Cell. Proteomics 2007, 6, 1428–1436. [Google Scholar] [CrossRef]
- Linder, V.; Verpoorte, E.; Thormann, W.; de Rooij, N.F.; Sigrist, H. Surface biopassivation of replicated poly(dimethylsiloxane) microfluidic channels and application to heterogeneous immunoreaction with on-chip fluorescence detection. Anal. Chem. 2001, 73, 4181–4189. [Google Scholar] [CrossRef]
- Sung, W.C.; Chang, C.C.; Makamba, H.; Chen, S.H. Long-term affinity modification on poly(dimethylsiloxane) substrate and its application for ELISA analysis. Anal. Chem. 2008, 80, 1529–1535. [Google Scholar] [CrossRef]
- Weng, C.H.; Huang, T.B.; Huang, C.C.; Yeh, C.S.; Lei, H.Y.; Lee, G.B. A suction-type microfluidic immunosensing chip for rapid detection of the dengue virus. Biomed. Microdevices 2011, 13, 585–595. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Li, Y.; Shi, Y.; Su, X. Multicolor quantum dot-encoded microspheres for the detection of biomolecules. Talanta 2007, 72, 1446–1452. [Google Scholar] [CrossRef]
- Crivat, G.; Da Silva, S.M.; Reyes, D.R.; Locascio, L.E.; Gaitan, M.; Rosenzweig, N.; Rosenzweig, Z. Quantum dot FRET-based probes in thin films grown in microfluidic channels. J. Am. Chem. Soc. 2010, 132, 1460–1461. [Google Scholar] [CrossRef]
- Kartalov, E.P.; Quake, S.R. Microfluidic device reads up to four consecutive base pairs in DNA sequencing by synthesis. Nucleic Acids Res. 2004, 32, 2873–2879. [Google Scholar] [CrossRef]
- Mandal, S.; Goddard, J.M.; Erickson, D. A multiplexed optofluidic biomolecular sensor for low mass detection. Lab Chip 2009, 9, 2924–2932. [Google Scholar] [CrossRef]
- Durstock, M.F.; Rubner, M.F. Dielectric properties of polyelectrolyte multilayers. Langmuir 2001, 17, 7865–7872. [Google Scholar] [CrossRef]
- Ghafar-Zadeh, E.; Sawan, M. A Core-CBCM sigma delta capacitive sensor array dedicated to lab-on-chip applications. Sensors Actuators Phys. 2008, 144, 304–313. [Google Scholar] [CrossRef]
- Hou, C.S.J.; Milovic, N.; Godin, M.; Russo, P.R.; Chakrabarti, R.; Manalis, S.R. Label-free microelectronic PCR quantification. Anal. Chem. 2006, 78, 2526–2531. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Cheng, Y.; Kim, E.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F. Coupling electrodeposition with layer-by-layer assembly to address proteins within microfluidic channels. Adv. Mater. 2011, 23, 5817–5821. [Google Scholar] [CrossRef]
- Gammoudi, I.; Raimbault, V.; Tarbague, H.; Moroté, F.; Grauby-Heywang, C.; Othmane, A.; Kalfat, R.; Moynet, D.; Rebière, D.; Dejous, C.; et al. Enhanced bio-inspired microsensor based on microfluidic/bacteria/love wave hybrid structure for continuous control of heavy metals toxicity in liquid medium. Sensors Actuators B Chem. 2014, 198, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Gammoudi, I.; Tarbague, H.; Othmane, A.; Moynet, D.; Rebière, D.; Kalfat, R.; Dejous, C. Love-wave bacteria-based sensor for the detection of heavy metal toxicity in liquid medium. Biosens. Bioelectron. 2010, 26, 1723–1726. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Habibi-Rezaei, M.; Meghrous, J.; Xiao, C.; Male, K.B.; Kamen, A. Monitoring motility, spreading, and mortality of adherent insect cells using an impedance sensor. Anal. Chem. 2001, 73, 1844–1848. [Google Scholar] [CrossRef]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell–substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef]
- Arndt, S.; Seebach, J.; Psathaki, K.; Galla, H.J.; Wegener, J. Bioelectrical impedance assay to monitor changes in cell shape during apoptosis. Biosens. Bioelectron. 2004, 19, 583–594. [Google Scholar] [CrossRef]
- Mijares, G.; Reyes, D.R.; Geist, J.; Gaitan, M. Polyelectrolyte multilayer-treated electrodes for real-time electronic sensing of cell proliferation. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 61–73. [Google Scholar] [CrossRef]
- Wang, A.J.; Xu, J.J.; Chen, H.Y. Proteins modification of poly(dimethylsiloxane) microfluidic channels for the enhanced microchip electrophoresis. J. Chromatogr. A 2006, 1107, 257–264. [Google Scholar] [CrossRef]
- Reyes, D.R.; Perruccio, E.M.; Becerra, S.P.; Locascio, L.E.; Gaitan, M. Micropatterning neuronal cells on polyelectrolyte multilayers. Langmuir 2004, 20, 8805–8811. [Google Scholar] [CrossRef]
- Forry, S.P.; Reyes, D.R.; Gaitan, M.; Locascio, L.E. Facilitating the culture of mammalian nerve cells with polyelectrolyte multilayers. Langmuir 2006, 22, 5770–5775. [Google Scholar] [CrossRef]
- Forry, S.P.; Reyes, D.R.; Gaitan, M.; Locascio, L.E. Cellular immobilization within microfluidic microenvironments: Dielectrophoresis with polyelectrolyte multilayers. J. Am. Chem. Soc. 2006, 128, 13678–13679. [Google Scholar]
- Reyes, D.R.; Hong, J.S.; Elliott, J.T.; Gaitan, M. Hybrid cell adhesive material for instant dielectrophoretic cell trapping and long-term cell function assessment. Langmuir 2011, 27, 10027–10034. [Google Scholar] [CrossRef]
- Hanke, C.; Dittrich, P.S.; Reyes, D.R. Dielectrophoretic cell capture on polyester membranes. ACS Appl. Mater. Interfaces 2012, 4, 1878–1882. [Google Scholar] [CrossRef]
- Johann, R.M.; Baiotto, C.; Renaud, P. Micropatterned surfaces of PDMS as growth templates for HEK 293 cells. Biomed. Microdevices 2007, 9, 475–485. [Google Scholar] [CrossRef]
- Monge, C.; Ren, K.; Berton, K.; Guillot, R.; Peyrade, D.; Picart, C. Engineering muscle tissues on microstructured polyelectrolyte multilayer films. Tissue Eng. A 2012, 18, 1664–1676. [Google Scholar]
- Lee, J.H.; Kim, H.E.; Im, J.H.; Bae, Y.M.; Choi, J.S.; Huh, K.M.; Lee, C.S. Preparation of orthogonally functionalized surface using micromolding in capillaries technique for the control of cellular adhesion. Colloids Surface B Biointerf. 2008, 64, 126–134. [Google Scholar] [CrossRef]
- Palamà, I.E.; D’Amone, S.; Coluccia, A.M.L.; Gigli, G. Micropatterned polyelectrolyte nanofilms promote alignment and myogenic differentiation of C2C12 cells in standard growth media. Biotechnol. Bioeng. 2013, 110, 586–596. [Google Scholar] [CrossRef]
- Toh, Y.C.; Zhang, C.; Zhang, J.; Khong, Y.; Chang, M.S.; Samper, V.D.; van Noort, D.; Hutmacher, D.W.; Yu, H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 2007, 7, 302–309. [Google Scholar] [CrossRef]
- Mehta, G.; Kiel, M.J.; Lee, J.W.; Kotov, N.; Linderman, J.J.; Takayama, S. Polyelectrolyte-clay-protein layer films on microfluidic PDMS bioreactor surfaces for primary murine bone marrow culture. Adv. Funct. Mater. 2007, 17, 2701–2709. [Google Scholar] [CrossRef]
- Schmolke, H.; Demming, S.; Edlich, A.; Magdanz, V.; Buttgenbach, S.; Franco-Lara, E.; Krull, R.; Klages, C.P. Polyelectrolyte multilayer surface functionalization of poly(dimethylsiloxane) (PDMS) for reduction of yeast cell adhesion in microfluidic devices. Biomicrofluidic 2010, 4. [Google Scholar] [CrossRef]
- Almodóvar, J.; Crouzier, T.; Selimović, Š.; Boudou, T.; Khademhosseini, A.; Picart, C. Gradients of physical and biochemical cues on polyelectrolyte multilayer films generated via microfluidics. Lab Chip 2013, 13, 1562–1570. [Google Scholar] [CrossRef]
- Almodóvar, J.; Guillot, R.; Monge, C.; Vollaire, J.; Selimović, Š.; Coll, J.L.; Khademhosseini, A.; Picart, C. Spatial patterning of BMP-2 and BMP-7 on biopolymeric films and the guidance of muscle cell fate. Biomaterials 2014, 35, 3975–3985. [Google Scholar] [CrossRef]
- Kirchhof, K.; Andar, A.; Yin, H.B.; Gadegaard, N.; Riehle, M.O.; Groth, T. Polyelectrolyte multilayers generated in a microfluidic device with pH gradients direct adhesion and movement of cells. Lab Chip 2011, 11, 3326–3335. [Google Scholar] [CrossRef]
- Dootz, R.; Nie, J.; Du, B.; Herminghaus, S.; Pfohl, T. Raman and surface enhanced raman microscopy of microstructured polyethylenimine/DNA multilayers. Langmuir 2006, 22, 1735–1741. [Google Scholar] [CrossRef]
- Lee, H.J.; Wark, A.W.; Corn, R.M. Utilizing ultrathin DNA/poly-lysine multilayer films to create liquid/liquid interfaces: Spectroscopic characterization, interfacial reactions and nanoparticle adsorption. J. Phys. Condens. Matter. 2007, 19. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Minnikanti, S.; Gangopadhyay, A.; Reyes, D.R. Polyelectrolyte Multilayers in Microfluidic Systems for Biological Applications. Polymers 2014, 6, 2100-2115. https://doi.org/10.3390/polym6082100
Minnikanti S, Gangopadhyay A, Reyes DR. Polyelectrolyte Multilayers in Microfluidic Systems for Biological Applications. Polymers. 2014; 6(8):2100-2115. https://doi.org/10.3390/polym6082100
Chicago/Turabian StyleMinnikanti, Saugandhika, Aveek Gangopadhyay, and Darwin R. Reyes. 2014. "Polyelectrolyte Multilayers in Microfluidic Systems for Biological Applications" Polymers 6, no. 8: 2100-2115. https://doi.org/10.3390/polym6082100