An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants
Abstract
:1. Introduction
2. Summary of Gas Phase Flame Retardant Activity

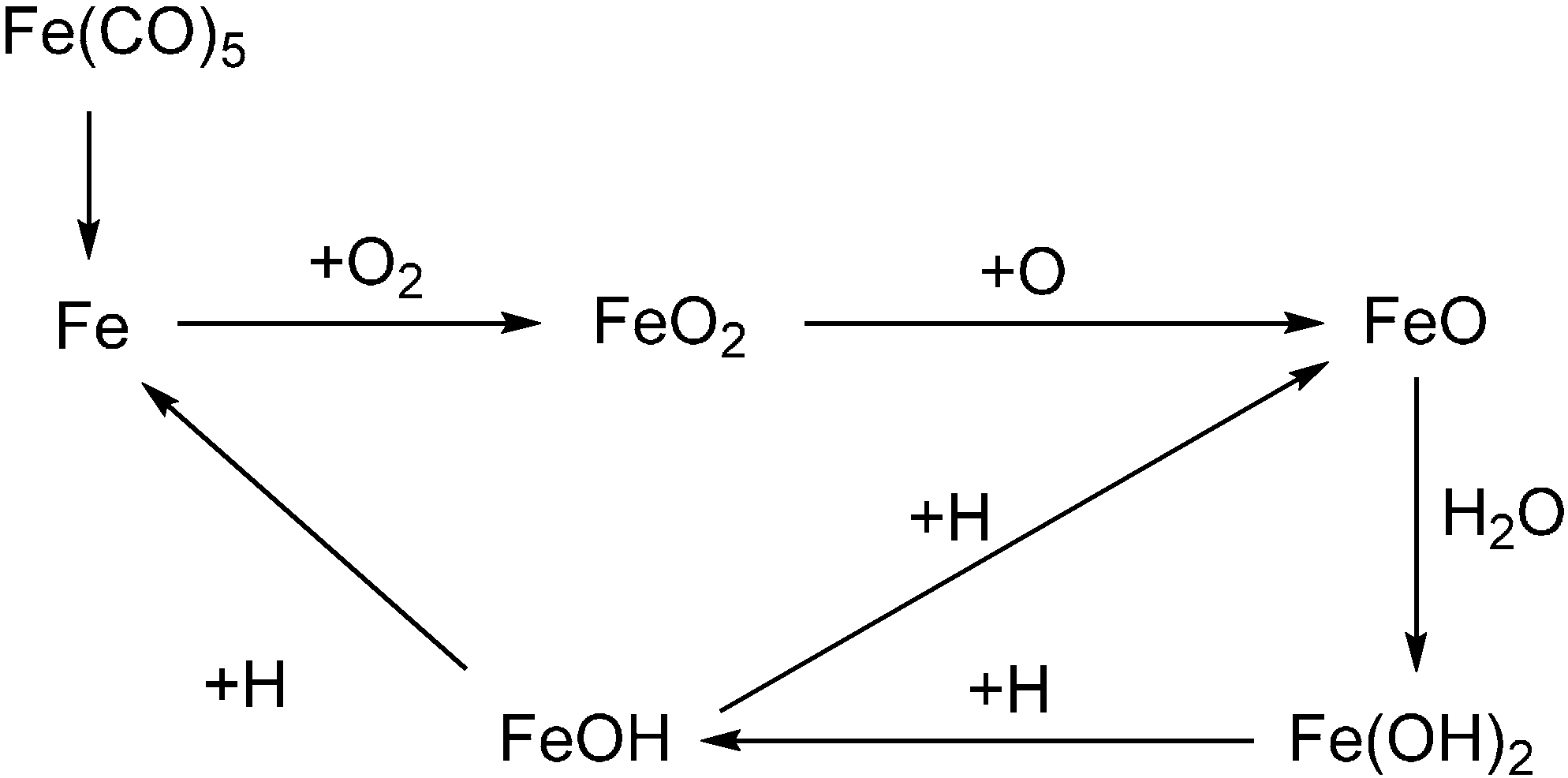
3. Gas Phase Active FRs for Polymeric Systems
3.1. Coupled Thermal Techniques (TGA-MS/FTIR)


3.2. Pyrolysis-Gas Chromatography (Py-GC)


3.3. Pyrolysis Combustion Flow Calorimetry (PCFC) and It Modifications

3.4. Detection of Active Radicals
3.4.1. Molecular Beam Mass Spectrometry (MBMS)


3.4.2. Chemiluminescence and Laser Induced Fluorescence
3.4.3. VUV Photoionization Coupled with Time of Flight MS


4. Conclusions
Conflicts of Interest
References
- Schartel, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Beach, M.W.; Rondan, N.G.; Froese, R.D.; Gerhart, B.B.; Green, J.G.; Stobby, B.G.; Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P. Studies of degradation enhancement of polystyrene by flame retardant additives. Polymer Degrad. Stab. 2008, 93, 1664–1673. [Google Scholar] [CrossRef]
- Beach, M.W.; Vozar, S.E.; Filipi, S.Z.; Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P.; Morgan, T.A.; Hu, T.I.; Sick, V. Screening approaches for gas-phase activity of flame retardants. Proc. Combust. Inst. 2009, 32, 2625–2632. [Google Scholar] [CrossRef]
- Benin, V.; Gardelle, B.; Morgan, A.B. Heat release of polyurethanes containing potential flame retardants based on boron and phosphorus chemistries. Polym. Degrad. Stab. 2014, 106, 108–121. [Google Scholar] [CrossRef]
- Bozi, J.; Czégény, Z.; Mészáros, E.; Blazsó, M. Thermal decomposition of flame retarded polycarbonates. J. Anal. Appl. Pyrolysis 2007, 79, 337–345. [Google Scholar] [CrossRef]
- Chen, D.-Q.; Wang, Y.-Z.; Hu, X.-P.; Wang, D.-Y.; Qu, M.-H.; Yang, B. Flame-retardant and anti-dripping effects of a novel char-forming flame retardant for the treatment of poly(ethylene terephthalate) fabrics. Polym. Degrad. Stab. 2005, 88, 349–356. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame retardant polymeric materials achieved by incorporation of styrene monomers containing both nitrogen and phosphorus. Polym. Degrad. Stab. 2012, 97, 2611–2618. [Google Scholar] [CrossRef]
- Feng, J.; Hao, J.; Du, J.; Yang, R. Using TGA/FTIR TGA/MS and cone calorimetry to understand thermal degradation and flame retardancy mechanism of polycarbonate filled with solid bisphenol a bis(diphenyl phosphate) and montmorillonite. Polym. Degrad. Stab. 2012, 97, 605–614. [Google Scholar] [CrossRef]
- Gaan, S.; Rupper, P.; Salimova, V.; Heuberger, M.; Rabe, S.; Vogel, F. Thermal decomposition and burning behavior of cellulose treated with ethyl ester phosphoramidates: Effect of alkyl substituent on nitrogen atom. Polym. Degrad. Stab. 2009, 94, 1125–1134. [Google Scholar] [CrossRef]
- Gibson, A.G. Flame retardant composites. In Fire Properties of Polymer Composite Materials; Gibson, A.G., Ed.; Springer: Dordrecht, The Netherland, 2007; pp. 237–241. [Google Scholar]
- Hardalupas, Y.; Orain, M.; Panoutsos, C.S.; Taylor, A.M.K.P.; Olofsson, J.; Seyfried, H.; Richter, M.; Hult, J.; Aldén, M.; Hermann, F.; et al. Chemiluminescence sensor for local equivalence ratio of reacting mixtures of fuel and air (flameseek). Appl. Therm. Eng. 2004, 24, 1619–1632. [Google Scholar] [CrossRef]
- Higgins, B.; McQuay, M.Q.; Lacas, F.; Rolon, J.C.; Darabiha, N.; Candel, S. Systematic measurements of oh chemiluminescence for fuel-lean, high-pressure, premixed, laminar flames. Fuel 2001, 80, 67–74. [Google Scholar] [CrossRef]
- Horacek, H.; Grabner, W. Nitrogen based flame retardants for nitrogen containing polymers. Makromol. Chem. Macromol. Symp. 1993, 74, 271–276. [Google Scholar] [CrossRef]
- Jakab, E.; Uddin, M.A.; Bhaskar, T.; Sakata, Y. Thermal decomposition of flame-retarded high-impact polystyrene. J. Anal. Appl. Pyrolysis 2003, 68–69, 83–99. [Google Scholar] [CrossRef]
- Jayaweera, T.M.; Melius, C.F.; Pitz, W.J.; Westbrook, C.K.; Korobeinichev, O.P.; Shvartsberg, V.M.; Shmakov, A.G.; Rybitskaya, I.V.; Curran, H.J. Flame inhibition by phosphorus-containing compounds over a range of equivalence ratios. Combust. Flame 2005, 140, 103–115. [Google Scholar] [CrossRef]
- Klatt, M. Nitrogen-based flame retardants. In The Non-Halogenated Flame Retardant Handbook; Morgan, A.B., Wilkie, C.A., Eds.; Scrivener Publishing: Beverly, MA, USA, 2014. [Google Scholar]
- König, A.; Kroke, E. Methyl-DOPO—A new flame retardant for flexible polyurethane foam. Polym. Adv. Technol. 2011, 22, 5–13. [Google Scholar] [CrossRef]
- König, A.; Kroke, E. Flame retardancy working mechanism of methyl-DOPO and MPPP in flexible polyurethane foam. Fire Mater. 2012, 36, 1–15. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P.; Beach, M.W.; Hu, T.I.; Morgan, T.A. Structure of a freely propagating rich CH4/air flame containing triphenylphosphine oxide and hexabromocyclododecane. Combust. Flame 2007, 149, 384–391. [Google Scholar] [CrossRef]
- Vora, N.; Siow, J.E.; Laurendeau, N.M. Chemical scavenging activity of gaseous suppressants by using laser-induced fluorescence measurements of hydroxyl. Combust. Flame 2001, 126, 1393–1401. [Google Scholar] [CrossRef]
- Hirschler, M.M. Smoke toxicity measurements made so that the results can be used for improved fire safety. J. Fire Sci. 1991, 9, 330–347. [Google Scholar] [CrossRef]
- Molyneux, S.; Stec, A.A.; Hull, T.R. The effect of gas phase flame retardants on fire effluent toxicity. Polym. Degrad. Stab. 2014, 106, 36–46. [Google Scholar] [CrossRef]
- Schartel, B.; Pawlowski, K.H.; Lyon, R.E. Pyrolysis combustion flow calorimeter: A tool to assess flame retarded pc/abs materials? Thermochim. Acta 2007, 462, 1–14. [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 31–68. [Google Scholar]
- Linteris, G. Influence of flame properties on the effectiveness of gas-phase fire retardants. In Proceedings of the 243rd ACS National Meeting & Exposition, San Diego, CA, USA, 25–29 March 2012.
- Rigolo, M.; Woodhams, R.T. Basic magnesium carbonate flame retardants for polypropylene. Polym. Eng. Sci. 1992, 32, 327–334. [Google Scholar] [CrossRef]
- Sawada, Y.; Yamaguchi, J.; Sakurai, O.; Uematsu, K.; Mizutani, N.; Kato, M. Thermogravimetric study on the decomposition of hydromagnesite 4MgCO3·Mg(OH)2·4H2O. Thermochim. Acta 1979, 33, 127–140. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; John, M.J.; Jacobs, V.; Luyt, A.S. Review on flammability of biofibres and biocomposites. Carbohydr. Polym. 2014, 111, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linteris, G.T.; Meier, O.C.; Pagliaro, J.L. Flame inhibition by CF3CHCl2 (HCFC-123). Combust. Sci. Technol. 2014, 186, 792–814. [Google Scholar] [CrossRef]
- Bocchini, S.; Camino, G. Halogen-containing flame retardants. In Fire Retardancy of Polymeric Materials, 2nd ed.; Wilkie, C.A., Morgan, A.B., Eds.; Taylor & Francis Group: Abingdon, UK, 2009; pp. 79–82. [Google Scholar]
- Wang, M.Y.; Horrocks, A.R.; Horrocks, S.; Hall, M.E.; Pearson, J.S.; Clegg, A.S. Flame retardant textile back-coatings. Part 1: Antimony-halogen system interactions and the effect of replacement by phosphorus-containing agents. J. Fire Sci. 2000, 18, 265–294. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Acierno, D.; Russo, P. Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides. Eur. Polym. J. 2011, 47, 1390–1401. [Google Scholar] [CrossRef]
- Si, M.; Feng, J.; Hao, J.; Xu, L.; Du, J. Synergistic flame retardant effects and mechanisms of nano-Sb2O3 in combination with aluminum phosphinate in poly(ethylene terephthalate). Polym. Degrad. Stab. 2014, 100, 70–78. [Google Scholar] [CrossRef]
- Miller, D.R.; Evers, R.L.; Skinner, G.B. Effects of various inhibitors on hydrogen-air flame speeds. Combust. Flame 1963, 7, 137–142. [Google Scholar] [CrossRef]
- Werner, J.H.; Cool, T.A. Kinetic model for the decomposition of dmmp in a hydrogen/oxygen flame. Combust. Flame 1999, 117, 78–98. [Google Scholar] [CrossRef]
- Twarowski, A. The influence of phosphorus oxides and acids on the rate of H + OH recombination. Combust. Flame 1993, 94, 91–107. [Google Scholar] [CrossRef]
- Twarowski, A. Photometric determination of the rate of H2O formation from H and OH in the presence of phosphine combustion products. Combust. Flame 1993, 94, 341–348. [Google Scholar] [CrossRef]
- Twarowski, A. Reduction of a phosphorus oxide and acid reaction set. Combust. Flame 1995, 102, 41–54. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Doering, M. Recent developments in halogen free flame retardants for epoxy resins for electrical and electronic applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef]
- Rasmussen, C.L.; Glarborg, P.; Marshall, P. Mechanisms of radical removal by SO2. Proc. Combust. Inst. 2007, 31, 339–347. [Google Scholar] [CrossRef]
- Zachariah, M.R.; Smith, O.I. Experimental and numerical studies of sulfur chemistry in H2/O2/SO2 flames. Combust. Flame 1987, 69, 125–139. [Google Scholar] [CrossRef]
- Bulewicz, E.M.; Padley, P.J. Catalytic effect of metal additives on free radical recombination rates in H2+O2+N2 flames. Symp. Int. Combust. 1971, 13, 73–80. [Google Scholar] [CrossRef]
- Babushok, V.; Tsang, W. Inhibitor rankings for alkane combustion. Combust. Flame 2000, 123, 488–506. [Google Scholar] [CrossRef]
- Vanpee, M.; Shirodkar, P.P. A study of flame inhibition by metal compounds. Symp. Int. Combust. 1979, 17, 787–795. [Google Scholar] [CrossRef]
- Jensen, D.E.; Jones, G.A. Catalysis of radical recombination in flames by iron. J. Chem. Phys. 1974, 60, 3421–3425. [Google Scholar] [CrossRef]
- Rumminger, M.D.; Reinelt, D.; Babushok, V.; Linteris, G.T. Numerical study of the inhibition of premixed and diffusion flames by iron pentacarbonyl. Combust. Flame 1999, 116, 207–219. [Google Scholar] [CrossRef]
- Linteris, G.T.; Katta, V.R.; Takahashi, F. Experimental and numerical evaluation of metallic compounds for suppressing cup-burner flames. Combust. Flame 2004, 138, 78–96. [Google Scholar] [CrossRef]
- Linteris, G.T.; Knyazev, V.D.; Babushok, V.I. Inhibition of premixed methane flames by manganese and tin compounds. Combust. Flame 2002, 129, 221–238. [Google Scholar] [CrossRef]
- Sepe, M.P. Thermal Analysis of Polymers; iSmithers Rapra Publishing: Singpore, 1997. [Google Scholar]
- Liu, S.; Ye, H.; Zhou, Y.; He, J.; Jiang, Z.; Zhao, J.; Huang, X. Study on flame-retardant mechanism of polycarbonate containing sulfonate-silsesquioxane-fluoro retardants by TGA and FTIR. Polym. Degrad. Stab. 2006, 91, 1808–1814. [Google Scholar] [CrossRef]
- Lin, H.; Han, L.; Dong, L. Thermal degradation behavior and gas phase flame-retardant mechanism of polylactide/PCPP blends. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, M.; Mispreuve, H.; Naescher, R.; Gaan, S. Flame retardancy and thermal decomposition of flexible polyurethane foams: Structural influence of organophosphorus compounds. Polym. Degrad. Stab. 2012, 97, 2428–2440. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Vázquez-Torres, H.; Licea-Claveríe, A. TGA/FTIR study on thermal degradation of polymethacrylates containing carboxylic groups. Polym. Degrad. Stab. 2006, 91, 3312–3321. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Irusta, L.; Fernandez-Berridi, M.J. Application of TGA/FTIR to the study of the thermal degradation mechanism of silanized poly(ether-urethanes). Polym. Degrad. Stab. 2012, 97, 1671–1679. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Espinosa, J.I.M.; Cauich-Rodríguez, J.V.; Ávila-Ortega, A.; Vázquez-Torres, H.; Marcos-Fernández, A.; San Román, J. TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym. Degrad. Stab. 2009, 94, 1666–1677. [Google Scholar] [CrossRef]
- Hurley, S.L.; Mittleman, M.L.; Wilkie, C.A. Preparation and thermal degradation of copolymers of 2-sulfoethyl methacrylate and methyl methacrylate. Polym. Degrad. Stab. 1993, 39, 345–354. [Google Scholar] [CrossRef]
- Wilkie, C.A. TGA/FTIR: An extremely useful technique for studying polymer degradation. Polym. Degrad. Stab. 1999, 66, 301–306. [Google Scholar] [CrossRef]
- Chandrasiri, J.A.; Wilkie, C.A. Thermal degradation of diphenyl disulfide and a blend of diphenyl disulfide with poly(methyl methacrylate). Polym. Degrad. Stab. 1994, 46, 275–284. [Google Scholar] [CrossRef]
- Chandrasiri, J.A.; Wilkie, C.A. The thermolysis of poly(methyl methacrylate) in the presence of phenyltin chlorides PhxSnCl4−x (x = 1–3). Polym. Degrad. Stab. 1994, 45, 83–89. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Thomsen, J.R.; Mittleman, M.L. Interaction of poly(methyl methacrylate) and nafions. J. Appl. Polym. Sci. 1991, 42, 901–909. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C.; Qu, H.; Ma, H.; Jiao, Y.; Xie, J. Investigation on the thermal degradation of flexible poly(vinyl chloride) filled with ferrites as flame retardant and smoke suppressant using TGA–FTIR and TGA–MS. Polym. Degrad. Stab. 2013, 98, 1506–1514. [Google Scholar] [CrossRef]
- Wang, F.C.-Y. Polymer additive analysis by pyrolysis-gas chromatography: II. Flame retardants. J. Chromatogr. A 2000, 886, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Ye, L.; Qiu, Y.; Qu, S. Thermal degradation behavior of the compound containing phosphaphenanthrene and phosphazene groups and its flame retardant mechanism on epoxy resin. Polymer 2011, 52, 5486–5493. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-phase flame-retardant effect of hexa-phenoxy-cyclotriphosphazene on rigid polyurethane foams containing expandable graphite. Polymer 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Wang, F.C.-Y.; Hasha, D.L. Reaction of methylamine with acetonylacetone and its application in polymer analysis. Anal. Chem. 1999, 71, 1131–1137. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Sun, N.; Xu, M.; Xu, G.; Xin, F.; Chen, Y. Pyrolysis route of a novel flame retardant constructed by phosphaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Zhu, P.; Sui, S.; Wang, B.; Sun, K.; Sun, G. A study of pyrolysis and pyrolysis products of flame-retardant cotton fabrics by DSC, TGA, and PY–GC–MS. J. Anal. Appl. Pyrolysis 2004, 71, 645–655. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I. Screening flame retardants for plastics using microscale combustion calorimetry. Polym. Eng. Sci. 2007, 47, 1501–1510. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I. Thermal analysis of flammability. J. Therm. Anal. Calorim. 2007, 89, 441–448. [Google Scholar] [CrossRef]
- Ménard, R.; Negrell-Guirao, C.; Ferry, L.; Sonnier, R.; David, G. Synthesis of new flame-retardants by radical chain transfer copolymerization of glycidyl methacrylate and dimethoxy-phosphorylmethyl methacrylate. Eur. Polym. J. 2014, 57, 109–120. [Google Scholar] [CrossRef]
- Buczko, A.; Stelzig, T.; Bommer, L.; Rentsch, D.; Heneczkowski, M.; Gaan, S. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stab. 2014, 107, 158–165. [Google Scholar] [CrossRef]
- Sonnier, R.; Dorez, G.; Vahabi, H.; Longuet, C.; Ferry, L. FTIR-PCFC coupling: A new method for studying the combustion of polymers. Combust. Flame 2014, 161, 1398–1407. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Crowley, S. In gas phase combustion studies of flame retardant polymer in the microscale combustion calorimeter. In Proceedings of the 24th Annual Conference on Recent Advances in Flame Retardancy of Polymeric Materials, Stamford, CT, USA, 20–22 May 2013.
- Raffan, F.; Ding, X.; Kraemer, R.H.; Stoliarov, S.I. Milligram-scale flame calorimeter: A novel instrument for flammability asessment using MG-sized samples. In Proceedings of the 25th Annual Conference on Recent Advances in Flame Retardancy of Polymeric Materials, Stamford, CT, USA, 19–21 May 2014.
- Raffan-Montoya, F.; Ding, X.; Kraemer, R.H.; Stoliarov, S.I. Flaming combustion calorimeter: A novel instrument for flammability assessment using MG-sized samples. In Proceedings of the 25th Annual Conference on Recent Advances in Flame Retardancy of Polymeric Materials, Stamford, CT, USA, 19–21 May 2014.
- Evans, R.J.; Milne, T.A. Molecular characterization of the pyrolysis of biomass. Energy Fuels 1987, 1, 123–137. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Deutch, S.P.; French, R.J. Quantitative measurement of biomass gasifier tars using a molecular-beam mass spectrometer: Comparison with traditional impinger sampling. Energy Fuels 2007, 21, 3036–3043. [Google Scholar] [CrossRef]
- Crichton, E. Construction of A Molecular Beam Mass Spectrometer for in Situ Probing of a Diamond Chemical Vapour Deposition Environment; University of Bristol: Bristol, UK, 2007. [Google Scholar]
- Shmakov, A.G.; Shvartsberg, V.M.; Korobeinichev, O.P.; Beach, M.W.; Hu, T.I.; Morgan, T.A. On the mechanism of action of phosphorus-containing retardants. Mendeleev Commun. 2007, 17, 186–187. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Bolshova, T.A.; Shvartsberg, V.M.; Chernov, A.A. Inhibition and promotion of combustion by organophosphorus compounds added to flames of CH4 or H2 in O2 and Ar. Combust. Flame 2001, 125, 744–751. [Google Scholar] [CrossRef]
- Hardalupas, Y.; Orain, M. Local measurements of the time-dependent heat release rate and equivalence ratio using chemiluminescent emission from a flame. Combust. Flame 2004, 139, 188–207. [Google Scholar] [CrossRef]
- Vora, N.; Laurendeau, N.M. Analysis of CF3Br flame suppression activity using quantitative laser-induced fluorescence measurements of the hydroxyl radical. Combust. Sci. Technol. 2001, 166, 15–39. [Google Scholar] [CrossRef]
- Siow, J.E.; Laurendeau, N.M. Flame inhibition activity of phosphorus-containing compounds using laser-induced fluorescence measurements of hydroxyl. Combust. Flame 2004, 136, 16–24. [Google Scholar] [CrossRef]
- Bodi, A.; Johnson, M.; Gerber, T.; Gengeliczki, Z.; Sztáray, B.; Baer, T. Imaging photoelectron photoion coincidence spectroscopy with velocity focusing electron optics. Rev. Sci. Instrum. 2009, 80, 034101. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Grützmacher, H.; Levalois-Grützmacher, J.; Gaan, S. Elucidating the thrmal decomposition of dimethyl methyl phosphonate by VUV photoionization: Pathways to the po radical, a key species in flame retardant mechanism. Chem. Eur. J. 2015, 21, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504-526. https://doi.org/10.3390/polym7030504
Salmeia KA, Fage J, Liang S, Gaan S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers. 2015; 7(3):504-526. https://doi.org/10.3390/polym7030504
Chicago/Turabian StyleSalmeia, Khalifah A., Julien Fage, Shuyu Liang, and Sabyasachi Gaan. 2015. "An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants" Polymers 7, no. 3: 504-526. https://doi.org/10.3390/polym7030504






