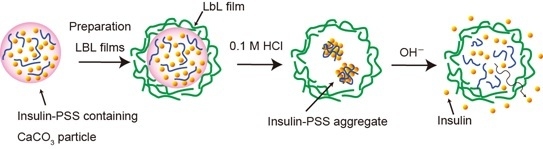
pH-Dependent Release of Insulin from Layer-by-Layer-Deposited Polyelectrolyte Microcapsules
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of LbL Microcapsules
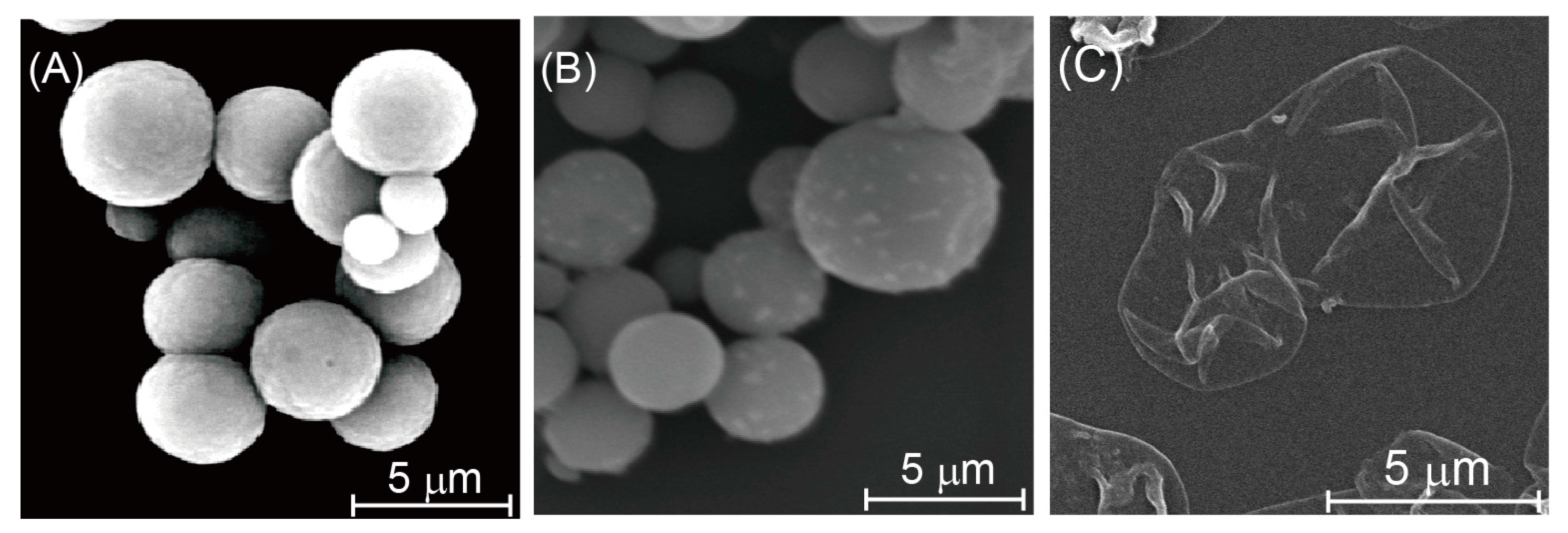
2.3. AFM and SEM Images of CaCO3 Particles and Microcapsules
2.4. ζ-Potential of LbL Film-Coated CaCO3 Particles
2.5. Evaluation of Insulin Release from Microcapsules
3. Results and Discussion
3.1. Preparation of Insulin-Containing Microcapsules



3.2. pH-Dependent Release of Insulin from Microcapsules


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Decher, G.; Hong, J.D. Building up ultrathin multilayer films by a self-assembly process: II. Consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Ber. Bunsenges. Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Decher, G. Fussy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Pavlukhina, S.; Sukhishvili, S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv. Drug Deliv. Rev. 2011, 63, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Sato, K.; Anzai, J. Layer-by-layer assembled thin films composed of carboxyl-terminated poly(amidoamine) dendrimers as a pH-sensitive nano-device. J. Colloid Interface Sci. 2008, 326, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Egawa, Y.; Mizukawa, Y.; Hoshi, T.; Anzai, J. Construction of positively-charged layered assemblies assisted by cyclodextrin complexation. Chem. Commun. 2002, 2, 164–165. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Gao, C. Stepwise assembly of the same polyelectrolytes using host-guest interaction to obtain microcapsules with multiresponsive properties. Chem. Mater. 2008, 20, 4194–4199. [Google Scholar] [CrossRef]
- Pallarola, D.; von Bildering, C.; Pietrasanta, L.I.; Queralto, N.; Knoll, W.; Battaglini, F.; Azzaroni, O. Recognition-driven layer-by-layer construction of multiprotein assemblies on surfaces: A biomolecular toolkit for building up chemoresponsive bioelectrochemical interfaces. Phys. Chem. Chem. Phys. 2012, 14, 11027–11039. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.; DeRosa, M.C. Development of a biocompatible layer-by-layer film systems using aptamer technology for smart materials applications. Polymers 2014, 6, 1631–1654. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Kolasiňska-Sojka, M.; Warszyňski, P. The electroactive multilayer films of polyelectrolytes and Prussian blue nanoparticles and their application for H2O2 sensors. Colloid Polym. Sci. 2014, 292, 455–465. [Google Scholar] [CrossRef]
- Hoshi, T.; Akase, S.; Anzai, J. Preparation of multilayer thin films containing avidin through sugar-lectin interactions and their binding properties. Langmuir 2002, 18, 7024–7028. [Google Scholar] [CrossRef]
- Volodkin, D.; von Klitzing, R.; Moehwald, H. Polyelectrolyte multilayers: Toward single cell studies. Polymers 2014, 6, 1502–1527. [Google Scholar] [CrossRef]
- Martins, G.V.; Mano, J.F.; Alves, N.M. Nanostructured self-assembled films containing chitosan fabricated at neutral pH. Carbohydr. Polym. 2010, 80, 570–573. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Gregurec, D.; Olszyna, M.; Politakos, N.; Yate, L.; Dahne, L.; Moya, S.E. Stability of polyelectrolyte multilayers in oxidizing media: A critical issue for the development of multilayer based membranes for nanofiltration. Colloid Polym. Sci. 2015, 293, 381–388. [Google Scholar] [CrossRef]
- Dou, Y.; Han, J.; Wang, T.; Wei, M.; Evans, D.G.; Duan, X. Temperature-controlled electrochemical switch based on layered double hydroxide/poly(N-isopropylacrylamide) ultrathin films fabricated via layer-by-layer assembly. Langmuir 2012, 28, 9535–9542. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J. H2O2-induced decomposition of layer-by-layer films consisting of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol). Langmuir 2014, 30, 9247–9250. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, Q.; Chu, T.; Zhang, X.; Wu, Z.; Yu, D. Glucose-sensitive polyelectrolyte nanocapsules based on layer-by-layer technique for protein drug delivery. J. Mater. Sci. 2014, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer deposited nano- and micro-assemblies for insulin delivery: A review. Mater. Sci. Eng. 2014, 34, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Antipov, A.A.; Sukhorukov, G.B.; Leporatti, S.; Radtchenko, I.L.; Donath, E.; Möhwald, H. Polyelectrolyte multilayer capsule permeability control. Colloid Surf. A 2002, 535–541. [Google Scholar] [CrossRef]
- Qi, W.; Yan, X.; Fei, J.; Wang, A.; Cui, Y.; Li, J. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials 2009, 30, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Dam, H.H.; Caruso, F. Formation and degradation of layer-by-layer-assembled polyelectrolyte polyrotaxane capsules. Langmuir 2013, 29, 7203–7208. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological applications of LBL multilayer capsules: From drug delivery to sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, I.; Yashchenok, A.; Borodina, T.; Bukreeva, T.; Konrad, M.; Mohwald, H.; Skirtach, A. Controlled enzyme-catalyzed degradation of polymeric capsules template on CaCO3: Influence of the number of LbL layers, conditions of degradation, and disassembly of multicompartments. J. Control. Release 2012, 162, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-by-layer-assembled capsules and films for therapeutic delivery. Small 2010, 6, 1836–1852. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Lu, J.; Hong, A.; Zhong, D.; Xie, S.; Liu, Q.; Huang, Y.; Shi, Y.; Xue, W. Preparation and characterization of PEM-coated alginate microgels for controlled release of protein. Biomed. Mater. 2012, 7, 035012. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Nouri, A.; Fernandes, T.; Rodrigues, J.; Tomás, H. Gene delivery using biodegradable polyelectrolyte microcapsules prepared through the layer-by-layer technique. Biotechnol. Prog. 2012, 28, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sato, K.; Anzai, J. Layer-by-layer polyelectrolyte films containing insulin for pH-triggered release. J. Mater. Chem. 2010, 20, 1546–1552. [Google Scholar] [CrossRef]
- Yoshida, K.; Hashide, R.; Ishii, T.; Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer films composed of poly(allylamine) and insulin for pH-triggered release of insulin. Colloid Surf. B 2012, 91, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Hashide, R.; Yoshida, K.; Kotaki, K.; Watanabe, T.; Watahiki, R.; Takahashi, S.; Sato, K.; Anzai, J. Use of anionic polysaccharides for the preparation of insulin-containing layer-by-layer films and their pH stability. Polym. Bull. 2012, 69, 229–239. [Google Scholar] [CrossRef]
- Hashide, R.; Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J. Insulin-containing layer-by-layer films deposited on poly(lactic acid) microbeads for pH-controlled release of insulin. Colloid Surf. B 2012, 89, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Puhl, S.; Meinel, L.; Germershaus, O. Silk fibroin layer-by-layer microcapsules for localized gene delivery. Biomaterials 2014, 35, 7929–7939. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Gao, C.; Möhwald, H. Manipulating the properties of polyelectrolyte microcapsules by glutaraldehyde cross-linking. Chem. Mater. 2005, 17, 4610–4616. [Google Scholar] [CrossRef]
- Nayak, S.R.; McShane, M.J. Encapsulation of peroxidase by polymerized acrylic acid monomers in “clean” polyelectrolyte microcapsules. J. Biomed. Nanotechnol. 2007, 3, 170–177. [Google Scholar] [CrossRef]
- Cui, F.; Shi, K.; Zhang, L.; Tao, A.; Kawashima, Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J. Control. Release 2006, 114, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, R.; Moya, S.; Hin, M.; Mitlöhner, R.; Donath, E.; Kiesewetter, H.; Möhwald, H.; Baumler, H. Permeation of macromolecules into polyelectrolyte microcapsules. Biomacromolecules. 2002, 3, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Mauser, T.; Déjugnat, C.; Sukhorukov, G.B. Reversible pH-dependent properties of multilayer microcapsules made of weak polyelectrolytes. Macromol. Rapid Commun. 2004, 25, 1781–1785. [Google Scholar] [CrossRef]
- Ye, S.; Wang, C.; Liu, X.; Tong, Z.; Ren, B.; Zeng, F. New loading process and release properties of insulin from polysaccharide microcapsules fabricated through layer-by-layer assembly. J. Control. Release 2006, 112, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.; Schaaf, P.; Voegel, J.; Picart, C. Layer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspects. Langmuir 2004, 20, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jewell, C.M.; Fredin, N.J.; Lynn, D.M. Assembly of multilayered films using well-defined, end-labeled poly(acrylic acid): Influence of molecular weight on exponential growth in a synthetic weak polyelectrolyte system. Langmuir 2007, 23, 8452–8459. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Ono, T.; Kashiwagi, Y.; Takahashi, S.; Sato, K.; Anzai, J.-i. pH-Dependent Release of Insulin from Layer-by-Layer-Deposited Polyelectrolyte Microcapsules. Polymers 2015, 7, 1269-1278. https://doi.org/10.3390/polym7071269
Yoshida K, Ono T, Kashiwagi Y, Takahashi S, Sato K, Anzai J-i. pH-Dependent Release of Insulin from Layer-by-Layer-Deposited Polyelectrolyte Microcapsules. Polymers. 2015; 7(7):1269-1278. https://doi.org/10.3390/polym7071269
Chicago/Turabian StyleYoshida, Kentaro, Tetsuya Ono, Yoshitomo Kashiwagi, Shigehiro Takahashi, Katsuhiko Sato, and Jun-ichi Anzai. 2015. "pH-Dependent Release of Insulin from Layer-by-Layer-Deposited Polyelectrolyte Microcapsules" Polymers 7, no. 7: 1269-1278. https://doi.org/10.3390/polym7071269