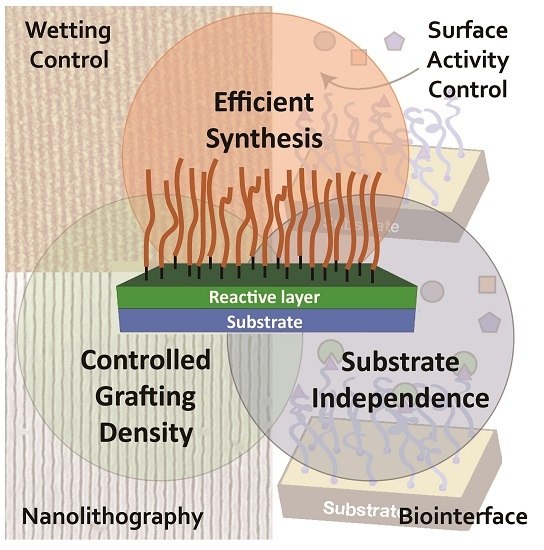
From Self-Assembled Monolayers to Coatings: Advances in the Synthesis and Nanobio Applications of Polymer Brushes
Abstract
:1. Introduction
2. Synthetic Approaches and Challenges

2.1. “Grafting-to” Approach
2.1.1. Solid State Grafting


2.1.2. Solution State Grafting

2.2. “Grafting-from” Approach
2.2.1. A Conventional Approach: Creating Initiator SAMs
2.2.2. Substrate Independent Initiator Immobilization Strategies


2.3. Micro- and Nano-Patterned Brushes


3. Challenges in Polymer Brush Characterizations
3.1. Chain Density

3.2. Determination of Molecular Weights of Polymer Brushes

3.3. Quantifying Surface Functional Groups: Initiator Density

4. Key Applications: Imparting Functionalities in Polymer Brushes for Biomedical Applications

4.1. Low Fouling Brushes for Controlling Material Interactions

4.2. Modification and Detection of Biological Components


4.3. Controlling Cell-Material Interactions with Modified Brushes

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wischerhoff, E.; Uhlig, K.; Lankenau, A.; Börner, H.G.; Laschewsky, A.; Duschl, C.; Lutz, J.-F. Controlled cell adhesion on PEG-based switchable surfaces. Angew. Chem. Int. Ed. 2008, 47, 5666–5668. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.; Liu, Y.; Huang, E.; Russell, T.P.; Hawker, C. Controlling polymer-surface interactions with random copolymer brushes. Science 1997, 275, 1458–1460. [Google Scholar] [CrossRef]
- Zhitenev, N.B.; Sidorenko, A.; Tennant, D.M.; Cirelli, R.A. Chemical modification of the electronic conducting states in polymer nanodevices. Nat. Nanotechnol. 2007, 2, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O. Polymer brushes here, there, and everywhere: Recent advances in their practical applications and emerging opportunities in multiple research fields. J. Polym. Sci. Part A 2012, 50, 3225–3258. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.E.; Ober, C.K. Characterization of polymer brush membranes via HF etch liftoff technique. ACS Macro Lett. 2013, 2, 241–245. [Google Scholar] [CrossRef]
- Lilge, I.; Schönherr, H. Covalently cross-linked poly(acrylamide) brushes on gold with tunable mechanical properties via surface-initiated atom transfer radical polymerization. Eur. Polym. J. 2013, 49, 1943–1951. [Google Scholar] [CrossRef]
- Amin, I.; Steenackers, M.; Zhang, N.; Beyer, A.; Zhang, X.; Pirzer, T.; Hugel, T.; Jordan, R.; Gölzhäuser, A. Polymer carpets. Small 2010, 6, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Steenackers, M.; Zhang, N.; Schubel, R.; Beyer, A.; Gölzhäuser, A.; Jordan, R. Patterned polymer carpets. Small 2011, 7, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Jennings, G.K.; Brantley, E.L. Physicochemical properties of surface-initiated polymer films in the modification and processing of materials. Adv. Mater. 2004, 16, 1983–1994. [Google Scholar] [CrossRef]
- Choi, I.S.; Langer, R. Surface-initiated polymerization of l-lactide: Coating of solid substrates with a biodegradable polymer. Macromolecules 2001, 34, 5361–5363. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, Y.-C. Grafting of homo- and block co-polypeptides on solid substrates by an improved surface-initiated vapor deposition polymerization. Langmuir 2002, 18, 9859–9866. [Google Scholar] [CrossRef]
- Harada, Y.; Girolami, G.S.; Nuzzo, R.G. Catalytic amplification of patterning via surface-confined ring-opening metathesis polymerization on mixed primer layers formed by contact printing. Langmuir 2003, 19, 5104–5114. [Google Scholar] [CrossRef]
- Kong, B.; Lee, J.K.; Choi, I.S. Surface-initiated, ring-opening metathesis polymerization: Formation of diblock copolymer brushes and solvent-dependent morphological changes. Langmuir 2007, 23, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.; Ulman, A.; Kang, J.F.; Rafailovich, M.H.; Sokolov, J. Surface-initiated anionic polymerization of styrene by means of self-assembled monolayers. J. Am. Chem. Soc. 1999, 121, 1016–1022. [Google Scholar] [CrossRef]
- Advincula, R.; Zhou, Q.; Park, M.; Wang, S.; Mays, J.; Sakellariou, G.; Pispas, S.; Hadjichristidis, N. Polymer brushes by living anionic surface initiated polymerization on flat silicon (SiOx) and gold surfaces: Homopolymers and block copolymers. Langmuir 2002, 18, 8672–8684. [Google Scholar] [CrossRef]
- Jordan, R.; Ulman, A. Surface initiated living cationic polymerization of 2-oxazolines. J. Am. Chem. Soc. 1998, 120, 243–247. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Synthesis of tethered polystyrene-block-poly(methyl methacrylate) monolayer on a silicate substrate by sequential carbocationic polymerization and atom transfer radical polymerization. J. Am. Chem. Soc. 1999, 121, 3557–3558. [Google Scholar] [CrossRef]
- Biesalski, M.; Rühe, J. Preparation and characterization of a polyelectrolyte monolayer covalently attached to a planar solid surface. Macromolecules 1999, 32, 2309–2316. [Google Scholar] [CrossRef]
- Huang, W.; Skanth, G.; Baker, G.L.; Bruening, M.L. Surface-initiated thermal radical polymerization on gold. Langmuir 2001, 17, 1731–1736. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Jones, R.G.; Moad, G. Terminology for reversible-deactivation radical polymerization previously called “controlled” radical or “living” radical polymerization. Pure Appl. Chem. 2010, 82, 483–491. [Google Scholar]
- Sedjo, R.A.; Mirous, B.K.; Brittain, W.J. Synthesis of polystyrene-block-poly(methyl methacrylate) brushes by reverse atom transfer radical polymerization. Macromolecules 2000, 33, 1492–1493. [Google Scholar] [CrossRef]
- Baum, M.; Brittain, W.J. Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique. Macromolecules 2002, 35, 610–615. [Google Scholar] [CrossRef]
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Husseman, M.; Malmström, E.E.; McNamara, M.; Mate, M.; Mecerreyes, D.; Benoit, D.G.; Hedrick, J.L.; Mansky, P.; Huang, E.; Russell, T.P.; et al. Controlled synthesis of polymer brushes by “living” free radical polymerization techniques. Macromolecules 1999, 32, 1424–1431. [Google Scholar] [CrossRef]
- Flynn, N.T.; Tran, T.N.T.; Cima, M.J.; Langer, R. Long-term stability of self-assembled monolayers in biological media. Langmuir 2003, 19, 10909–10915. [Google Scholar] [CrossRef]
- Strulson, M.K.; Johnson, D.M.; Maurer, J.A. Increased stability of glycol-terminated self-assembled monolayers for long-term patterned cell culture. Langmuir 2012, 28, 4318–4324. [Google Scholar] [CrossRef] [PubMed]
- Schwendel, D.; Dahint, R.; Herrwerth, S.; Schloerholz, M.; Eck, W.; Grunze, M. Temperature dependence of the protein resistance of poly- and oligo(ethylene glycol)-terminated alkanethiolate monolayers. Langmuir 2001, 17, 5717–5720. [Google Scholar] [CrossRef]
- Zorn, S.; Dettinger, U.; Skoda, M.W.A.; Jacobs, R.M.J.; Peisert, H.; Gerlach, A.; Chassé, T.; Schreiber, F. Stability of hexa(ethylene glycol) SAMs towards the exposure to natural light and repeated reimmersion. Appl. Surf. Sci. 2012, 258, 7882–7888. [Google Scholar] [CrossRef]
- Schmitt, S.K.; Murphy, W.L.; Gopalan, P. Crosslinked PEG mats for peptide immobilization and stem cell adhesion. J. Mater. Chem. B 2013, 1, 1349–1360. [Google Scholar] [CrossRef]
- Tong, Y.; Tyrode, E.; Osawa, M.; Yoshida, N.; Watanabe, T.; Nakajima, A.; Ye, S. Preferential adsorption of amino-terminated silane in a binary mixed self-assembled monolayer. Langmuir 2011, 27, 5420–5426. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.L.; Lehnert, R.J.; Schonherr, H.; Vancso, J. Factors affecting the preparation of permanently end-grafted polystyrene layers. Polymer 1999, 40, 525–530. [Google Scholar] [CrossRef]
- Ruiz, R.; Kang, H.; Detcheverry, F.A.; Dobisz, E.; Kercher, D.S.; Albrecht, T.R.; de Pablo, J.J.; Nealey, P.F. Density multiplication and improved lithography by directed block copolymer assembly. Science 2008, 321, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Bojko, A.; Andreatta, G.; Montagne, F.; Renaud, P.; Pugin, R. Fabrication of thermo-responsive nano-valve by grafting-to in melt of poly(N-isopropylacrylamide) onto nanoporous silicon nitride membranes. J. Membr. Sci. 2014, 468, 118–125. [Google Scholar] [CrossRef]
- Widin, J.M.; Kim, M.; Schmitt, A.K.; Han, E.; Gopalan, P.; Mahanthappa, M.K. Bulk and thin film morphological behavior of broad dispersity poly(styrene-b-methyl methacrylate) block copolymers. Macromolecules 2013, 46, 4472–4480. [Google Scholar] [CrossRef]
- Gianotti, V.; Antonioli, D.; Sparnacci, K.; Laus, M.; Giammaria, T.J.; Lupi, F.F.; Seguini, G.; Perego, M. On the thermal stability of PS-b-PMMA block and P(S-r-MMA) random copolymers for nanopatterning applications. Macromolecules 2013, 46, 8224–8234. [Google Scholar] [CrossRef]
- Guo, R.; Kim, E.; Gong, J.; Choi, S.; Ham, S.; Ryu, D.Y. Perpendicular orientation of microdomains in PS-b-PMMA thin films on the PS brushed substrates. Soft Matter 2011, 7, 6920–6925. [Google Scholar] [CrossRef]
- Kim, M.; Han, E.; Sweat, D.P.; Gopalan, P. Interplay of surface chemical composition and film thickness on graphoepitaxial assembly of asymmetric block copolymers. Soft Matter 2013, 9, 6135. [Google Scholar] [CrossRef]
- Park, S.-M.; Liang, X.; Harteneck, B.D.; Pick, T.E.; Hiroshiba, N.; Wu, Y.; Helms, B.A.; Olynick, D.L. Sub-10 nm nanofabrication via nanoimprint directed self-assembly of block copolymers. ACS Nano 2011, 5, 8523–8531. [Google Scholar] [CrossRef] [PubMed]
- Mansky, P.; Russell, T.P.; Hawker, C.J.; Mays, J.; Cook, D.C.; Satija, S.K. Interfacial segregation in disordered block copolymers: Effect of tunable surface potentials. Phys. Rev. Lett. 1997, 79, 237–240. [Google Scholar] [CrossRef]
- Huang, E.; Pruzinsky, S.; Russell, T.P. Neutrality conditions for block copolmyer systems on random copolymer brush surfaces. Macromolecules 1999, 32, 5299–5303. [Google Scholar] [CrossRef]
- Bai, J.; Zhong, X.; Jiang, S.; Huang, Y.; Duan, X. Graphene nanomesh. Nat. Nanotechnol. 2010, 5, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Safron, N.S.; Kim, M.; Gopalan, P.; Arnold, M.S. Barrier-guided growth of micro- and nano-structured graphene. Adv. Mater. 2012, 24, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Safron, N.S.; Han, E.; Arnold, M.S.; Gopalan, P. Fabrication and characterization of large-area, semiconducting nanoperforated graphene materials. Nano Lett. 2010, 10, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Jung, Y.-S.; Wu, S.; Ismach, A.; Olynick, D.L.; Cabrini, S.; Bokor, J. Formation of bandgap and subbands in graphene nanomeshes with Sub-10 nm ribbon width fabricated via nanoimprint lithography. Nano Lett. 2010, 10, 2454–2460. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Safron, N.S.; Han, E.; Arnold, M.S.; Gopalan, P. Electronic transport and raman scattering in size-controlled nanoperforated graphene. ACS Nano 2012, 6, 9846–9854. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wi, S. Transport characteristics of multichannel transistors made from densely aligned Sub-10 nm half-pitch graphene nanoribbons. ACS Nano 2012, 6, 9700–9710. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, M.; Safron, N.S.; Arnold, M.S.; Gopalan, P. Transfer of pre-assembled block copolymer thin film to nanopattern unconventional substrates. ACS Appl. Mater. Interfaces 2014, 6, 9442–9448. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, M.; Safron, N.S.; Han, E.; Arnold, M.S.; Gopalan, P. A Facile route for fabricating graphene nanoribbon array transistors using graphoepitaxy of a symmetric block copolymer. SPIE Adv. Lithogr. 2015, 9428, 94280T. [Google Scholar]
- Ham, S.; Shin, C.; Kim, E.; Ryu, D.Y.; Jeong, U.; Russell, T.P.; Hawker, C.J. Microdomain orientation of PS-b-PMMA by controlled interfacial interactions. Macromolecules 2008, 41, 6431–6437. [Google Scholar] [CrossRef]
- Han, E.; Stuen, K.O.; La, Y.-H.; Nealey, P.F.; Gopalan, P. Effect of composition of substrate-modifying random copolymers on the orientation of symmetric and asymmetric diblock copolymer domains. Macromolecules 2008, 41, 9090–9097. [Google Scholar] [CrossRef]
- Chai, J.; Buriak, J.M. Using cylindrical domains of block copolymers to self-assemble and align metallic nanowires. ACS Nano 2008, 2, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Kang, H.; Liu, C.C.; Nealey, P.F.; Gopalan, P. Graphoepitaxial assembly of symmetric block copolymers on weakly preferential substrates. Adv. Mater. 2010, 22, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Stoykovich, M.P.; Ruiz, R.; Zhang, Y.; Black, C.T.; Nealey, P.F. Directed assembly of lamellae-forming block copolymers by using chemically and topographically patterned substrates. Adv. Mater. 2007, 19, 607–611. [Google Scholar] [CrossRef]
- Liu, C.-C.; Thode, C.J.; Delgadillo, P.A.R.; Craig, G.S.W.; Nealey, P.F.; Gronheid, R. Towards an all-track 300 mm process for directed self-assembly. J. Vac. Sci. Technol. B 2011, 29, 06F203. [Google Scholar] [CrossRef]
- Lupi, F.F.; Giammaria, T.J.; Seguini, G.; Ceresoli, M.; Perego, M.; Antonioli, D.; Gianotti, V.; Sparnacci, K.; Laus, M. Flash grafting of functional random copolymers for surface neutralization. J. Mater. Chem. C 2014, 2, 4909–4917. [Google Scholar] [CrossRef]
- Zdyrko, B.; Kinnan, M.K.; Chumanov, G.; Luzinov, I. Fabrication of optically active flexible polymer films with embedded chain-like arrays of silver nanoparticles. Chem. Commun. 2008, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Zdyrko, B.; Hoy, O.; Kinnan, M.K.; Chumanov, G.; Luzinov, I. Nano-patterning with polymer brushes via solvent-assisted polymer grafting. Soft Matter 2008, 4, 2213–2219. [Google Scholar] [CrossRef]
- Zdyrko, B.; Hoy, O.; Luzinov, I. Toward protein imprinting with polymer brushes. Biointerphases 2009, 4, FA17–FA21. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, A.; Synytska, A.; Uhlmann, P.; Stamm, M.; Kremer, F. Tuning the adhesion of silica microparticles to a poly(2-vinyl pyridine) brush: An AFM force measurement study. Langmuir 2012, 28, 15555–15565. [Google Scholar] [CrossRef] [PubMed]
- Popelka, Š.; Houska, M.; Havlíková, J.; Proks, V.; Kučkaa, J.; Šturcová, A.; Bačáková, L.; Rypáčeka, F. Poly(ethylene oxide) brushes prepared by the “grafting to” method as a platform for the assessment of cell receptor-ligand binding. Eur. Polym. J. 2014, 58, 11–22. [Google Scholar]
- Motornov, M.; Sheparovych, R.; Katz, E.; Minko, S. Chemical gating with nanostructured responsive polymer brushes: Mixed brush versus homopolymer brush. ACS Nano 2008, 2, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Bittrich, E.; Burkert, S.; Müller, M.; Eichhorn, K.-J.; Stamm, M.; Uhlmann, P. Temperature-sensitive swelling of poly(N-isopropylacrylamide) brushes with low molecular weight and grafting density. Langmuir 2012, 28, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, N.; Winkler, R.; Tress, M.; Uhlmann, P.; Reiche, M.; Kipnusu, W.K.; Kremer, F. Glassy dynamics of poly(2-vinyl-pyridine) brushes with varying grafting density. Soft Matter 2015, 11, 3062–3066. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Eichhorn, K.-J.; Kuckling, D.; Stamm, M.; Uhlmann, P. Chain extension of stimuli-responsive polymer brushes: A general strategy to overcome the drawbacks of the “grafting-to” approach. Adv. Func. Mater. 2013, 23, 5675–5681. [Google Scholar] [CrossRef]
- Damiron, D.; Mazzolini, J.; Cousin, F.; Boisson, C.; D’Agosto, F.; Drockenmuller, E. Poly(ethylene) brushes grafted to silicon substrates. Polym. Chem. 2012, 3, 1838–1845. [Google Scholar] [CrossRef]
- Flavel, B.S.; Jasieniak, M.; Velleman, L.; Ciampi, S.; Luais, E.; Peterson, J.R.; Griesser, H.J.; Shapter, J.G.; Gooding, J.J. Grafting of poly(ethylene glycol) on click chemistry modified Si(100) surfaces. Langmuir 2013, 29, 8355–8362. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Brown, P.; Gengenbach, T.; Griesser, H.J.; Meagher, L. End terminal, poly(ethylene oxide) graft layers: Surface forces and protein adsorption. Langmuir 2009, 25, 9149–9156. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, G.; Schoch, R.L.; Feuz, L.; Höök, F.; Lim, R.Y.H.; Dahlin, A.B. Strongly stretched protein resistant poly(ethylene glycol) brushes prepared by grafting-to. ACS Appl. Mater. Interfaces 2015, 7, 7505–7515. [Google Scholar] [CrossRef] [PubMed]
- Koutsos, V.; van der Vegte, E.W.; Hadziioannou, G. Direct view of structural regimes of end-grafted polymer monolayers: A scanning force microscopy study. Langmuir 1999, 32, 1233–1236. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, H. Cleavage of diblock copolymer brushes in a selective solvent and fusion of vesicles self-assembled by pinned micelles. Langmuir 2015, 31, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Z.-W.; Zhao, H. Patchy micelles based on coassembly of block copolymer chains and block copolymer brushes on silica particles. Langmuir 2015, 31, 4129–4136. [Google Scholar] [CrossRef] [PubMed]
- Abbou, J.; Anne, A.; Demaille, C. Probing the structure and dynamics of end-grafted flexible polymer chain layers by combined atomic force-electrochemical microscopy. Cyclic voltammetry within nanometer-thick macromolecular poly(ethylene glycol) layers. J. Am. Chem. Soc. 2004, 126, 10095–10108. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Rodriguez-Emmenegger, C.; Preuss, C.M.; Pop-Georgievski, O.; Verveniotis, E.; Trouillet, V.; Rezek, B.; Barner-Kowollik, C. A facile avenue to conductive polymer brushes via cyclopentadiene–maleimide Diels–Alder ligation. Chem. Commun. 2013, 49, 8623–8625. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Alonzo, J.; Yu, X.; Hong, K.; Messman, J.M.; Ivanov, I.; Lavrik, N.V.; Banerjee, M.; Rathore, R.; Sun, Z.; et al. Grafting density effects, optoelectrical properties and nano-patterning of poly(para-phenylene) brushes. J. Mater. Chem. A 2013, 1, 13426–13432. [Google Scholar] [CrossRef]
- Paoprasert, P.; Spalenka, J.W.; Peterson, D.L.; Ruther, R.E.; Hamers, R.J.; Evans, P.G.; Gopalan, P. Grafting of poly(3-hexylthiophene) brushes on oxides using click chemistry. J. Mater. Chem. 2010, 20, 2651–2658. [Google Scholar] [CrossRef]
- Ostaci, R.-V.; Damiron, D.; Akhrass, S.A.; Grohens, Y.; Drockenmuller, E. Poly(ethylene glycol) brushes grafted to silicon substrates by click chemistry: Influence of PEG chain length, concentration in the grafting solution and reaction time. Polym. Chem. 2011, 2, 348–354. [Google Scholar] [CrossRef]
- Ostaci, R.-V.; Damiron, D.; Capponi, S.; Vignaud, G.; Léger, L.; Grohens, Y.; Drockenmuller, E. Polymer brushes grafted to “passivated” silicon substrates using click chemistry. Langmuir 2008, 24, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Hansson, S.; Trouillet, V.; Tischer, T.; Goldmann, A.S.; Carlmark, A.; Barner-Kowollik, C.; Malmström, E. Grafting efficiency of synthetic polymers onto biomaterials: A comparative study of grafting-from versus grafting-to. Biomacromolecules 2013, 14, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sun, J.; Jiang, S.; Zeng, Y.; Li, Y.; Yan, S.; Geng, J.; Huang, Y. Grafting P3HT brushes on GO sheets: Distinctive properties of the GO/P3HT composites due to different grafting approaches. J. Mater. Chem. 2012, 22, 21583–21591. [Google Scholar] [CrossRef]
- Yang, K.; Huang, X.; Zhu, M.; Xie, L.; Tanaka, T.; Jiang, P. Combining RAFT polymerization and thiol–ene click reaction for core–shell structured polymer@BaTiO3 nanodielectrics with high dielectric constant, low dielectric loss, and high energy storage capability. ACS Appl. Mater. Interfaces 2014, 6, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Kedracki, D.; Chekini, M.; Maroni, P.; Schlaad, H.; Nardin, C. Synthesis and self-assembly of a DNA molecular brush. Biomacromolecules 2014, 15, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Averick, S.; Roth, E.; Luebke, D.; Nulwala, H.; Matyjaszewski, K. Clickable poly(ionic liquid)s for modification of glass and silicon surfaces. Polymer 2014, 55, 3330–3338. [Google Scholar] [CrossRef]
- Deng, J.; Liu, X.; Shi, W.; Cheng, C.; He, C.; Zhao, C. Light-triggered switching of reversible and alterable biofunctionality via β-cyclodextrin/azobenzene-based host−guest interaction. ACS Macro Lett. 2014, 3, 1130–1133. [Google Scholar] [CrossRef]
- Welch, M.E.; Xu, Y.; Chen, H.; Smith, N.; Tague, M.E.; Abruña, H.D.; Baird, B.; Ober, C.K. Polymer brushes as functional, patterned surfaces for nanobiotechnology. J. Photopolym. Sci. Technol. 2013, 25, 53–56. [Google Scholar] [CrossRef] [PubMed]
- He, R.-X.; Zhang, M.; Tan, F.; Leung, P.H.M.; Zhao, X.-Z.; Chan, H.L.W.; Yang, M.; Yan, F. Detection of bacteria with organic electrochemical transistors. J. Mater. Chem. 2012, 22, 22072–22076. [Google Scholar] [CrossRef]
- Welch, M.E.; Doublet, T.; Bernard, C.; Malliaras, G.G.; Ober, C.K. A Glucose sensor via stable immobilization of the GOx enzyme on an organic transistor using a polymer brush. J. Polym. Sci. A 2015, 53, 372–377. [Google Scholar] [CrossRef]
- Steenackers, M.; Gigler, A.M.; Zhang, N.; Deubel, F.; Seifert, M.; Hess, L.H.; Lim, C.H.Y.X.; Loh, K.P.; Garrido, J.A.; Jordan, R.; et al. Polymer brushes on graphene. J. Am. Chem. Soc. 2011, 133, 10490–10498. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, M.; Küller, A.; Stoycheva, S.; Grunze, M.; Jordan, R. Structured and gradient polymer brushes from biphenylthiol self-assembled monolayers by self-initiated photografting and photopolymerization (SIPGP). Langmuir 2009, 25, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Hess, L.H.; Lyuleeva, A.; Blaschke, B.M.; Sachsenhauser, M.; Seifert, M.; Garrido, J.A.; Deubel, F. Graphene transistors with multifunctional polymer brushes for biosensing applications. ACS Appl. Mater. Interfaces 2014, 6, 9705–9710. [Google Scholar] [CrossRef] [PubMed]
- von Werne, T.A.; Germack, D.S.; Hagberg, E.C.; Sheares, V.V.; Hawker, C.J.; Carter, K.R. A versatile method for tuning the chemistry and size of nanoscopic features by living free radical polymerization. J. Am. Chem. Soc. 2003, 125, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Koylu, D.; Carter, K.R. Stimuli-responsive surfaces utilizing cleavable polymer brush layers. Macromolecules 2009, 42, 8655–8660. [Google Scholar] [CrossRef]
- Yameen, B.; Khan, H.U.; Knoll, W.; Förch, R.; Jonas, U. Surface initiated polymerization on pulsed plasma deposited polyallylamine: A polymer substrate-independent strategy to soft surfaces with polymer brushes. Macromol. Rapid Commun. 2011, 32, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Lua, Y.; Meagher, L. A substrate-independent method for surface grafting polymer layers by atom transfer radical polymerization: Reduction of protein adsorption. Acta Biomater. 2012, 8, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lin, L.; Dalsin, J.L.; Messersmith, P.B. Biomimetic Anchor for surface-initiated polymerization from metal substrates. J. Am. Chem. Soc. 2005, 127, 15843–15847. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, W.C.; Xua, F.J.; Zhang, L.Q.; Yang, W.T. Preparation of pH-sensitive membranes via dopamine-initiated atom transfer radical polymerization. J. Membr. Sci. 2011, 367, 7–13. [Google Scholar] [CrossRef]
- Hu, H.; Yu, B.; Ye, Q.; Gu, Y.; Zhou, F. Modification of carbon nanotubes with a nanothin polydopamine layer and polydimethylamino-ethyl methacrylate brushes. Carbon 2010, 48, 2347–2353. [Google Scholar] [CrossRef]
- Wang, W.-C.; Wang, J.; Liao, Y.; Zhang, L.; Cao, B.; Song, G.; She, X. Surface initiated ATRP of acrylic acid on dopamine-functionalized AAO membranes. J. Appl. Polym. Sci. 2010, 117, 534–541. [Google Scholar] [CrossRef]
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for surface-initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar] [CrossRef]
- Kuang, J.; Messersmith, P.B. Universal surface-initiated polymerization of antifouling zwitterionic brushes using a mussel-mimetic peptide initiator. Langmuir 2012, 28, 7258–7266. [Google Scholar] [CrossRef] [PubMed]
- Zobrist, C.; Sobocinski, J.; Lyskawa, J.; Fournier, D.; Miri, V.; Traisnel, M.; Jimenez, M.; Woisel, P. Functionalization of titanium surfaces with polymer brushes prepared from a biomimetic RAFT agent. Macromolecules 2011, 44, 5883–5892. [Google Scholar] [CrossRef]
- Wang, X.; Ye, Q.; Gao, T.; Liu, J.; Zhou, F. Self-assembly of catecholic macroinitiator on various substrates and surface-initiated polymerization. Langmuir 2012, 28, 2574–2581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Klep, V.; Zdyrko, B.; Luzinov, I. Polymer grafting via ATRP initiated from macroinitiator synthesized on surface. Langmuir 2004, 20, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Sweat, D.P.; Kim, M.; Yu, X.; Gopalan, P. A single-component inimer containing cross-linkable ultrathin polymer coating for dense polymer brush growth. Langmuir 2013, 29, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Sweat, D.P.; Kim, M.; Yu, X.; Schmitt, S.K.; Han, E.; Choi, J.W.; Gopalan, P. A dual functional layer for block copolymer self-assembly and the growth of nanopatterned polymer brushes. Langmuir 2013, 29, 12858–12865. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from surfaces for “everyone”: ARGET ATRP in the presence of air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Amin, I.; Jordan, R. Patterned Polymer Brushes. Chem. Soc. Rev. 2012, 41, 3280–3296. [Google Scholar] [CrossRef] [PubMed]
- Olivier, A.; Meyer, F.; Raquez, J.-M.; Damman, P.; Dubois, P. Surface-initiated controlled polymerization as a convenient method for designing functional polymer brushes: From self-assembled monolayers to patterned surfaces. Prog. Polym. Sci. 2012, 37, 157–181. [Google Scholar] [CrossRef]
- Onses, M.S.; Ramirez-Hernandez, A.; Hur, S.-M.; Sutanto, E.; Williamson, L.; Alleyne, A.G.; Nealey, P.F.; de Pablo, J.J.; Rogers, J.A. Block copolymer assembly on nanoscale patterns of polymer brushes formed by electrohydrodynamic jet printing. ACS Nano 2014, 8, 6606–6613. [Google Scholar] [CrossRef] [PubMed]
- Onses, M.S.; Song, C.; Williamson, L.; Sutanto, E.; Ferreira, P.M.; Alleyne, A.G.; Nealey, P.F.; Ahn, H.; Rogers, J.A. Hierarchical patterns of three-dimensional block-copolymer films formed by electrohydrodynamic jet printing and self-assembly. Nat. Nanotechnol. 2013, 8, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, C.C.; Liu, G.; Nealey, P.F. Molecular transfer printing using block copolymers. ACS Nano 2010, 4, 559–609. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Kim, M.; Gopalan, P. Chemical patterns from surface grafted resists for directed assembly of block copolymers. ACS Nano 2012, 6, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Leolukman, M.; Kim, M.; Gopalan, P. Resist free patterning of nonpreferential buffer layers for block copolymer lithography. ACS Nano 2010, 4, 6527–6534. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Park, M.Y.; Tanaka, M.; Ober, C.K. Direct patterning of intrinsically electron beam sensitive polymer brushes. ACS Nano 2010, 4, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Paik, M.Y.; Xu, Y.; Rastogi, A.; Tanaka, M.; Yi, Y.; Ober, C.K. Patterning of polymer brushes. A direct approach to complex, sub-surface structures. Nano Lett. 2010, 10, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Binder, K.; Milchev, A. Polymer brushes on flat and curved surfaces: How computer simulations can help to test theories and to interpret experiments. J. Polym. Sci. Part B 2012, 50, 1515–1555. [Google Scholar] [CrossRef]
- Moh, L.C.H.; Losego, M.D.; Braun, P.V. Solvent quality effects on scaling behavior of poly(methyl methacrylate) brushes in the moderate- and high-density regimes. Langmuir 2011, 27, 3698–3702. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Tirrell, M.; Lodge, T.P. Tethered chains in polymer microstructures. Adv. Polym. Sci. 1992, 100, 31–71. [Google Scholar]
- De Gennes, P.G. Conformations of polymers attached to an interface. Macromolecules 1980, 13, 1069–1075. [Google Scholar] [CrossRef]
- Lai, P.Y.; Halperin, A. Polymer brush at high coverage. Macromolecules 1991, 24, 4981–4982. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V.; Pryamitsyn, V.A.; Birshtein, T.M. Coil-globule type transitions in polymers. 1. Collapse of layers of grafted polymer chains. Macromolecules 1991, 24, 140–149. [Google Scholar] [CrossRef]
- Wu, T.; Efimenko, K.; Genzer, J. Combinatorial study of the mushroom-to-brush crossover in surface anchored polyacrylamide. J. Am. Chem. Soc. 2002, 124, 9394–9395. [Google Scholar] [CrossRef] [PubMed]
- Auroy, P.; Auvray, L. Collapse-stretching transition for polymer brushes: Preferential solvation. Macromolecules 1992, 25, 4134–4141. [Google Scholar] [CrossRef]
- Patil, R.R.; Turgman-Cohen, S.; Šrogl, J.; Kiserow, D.; Genzer, J. On-demand degrafting and the study of molecular weight and grafting density of poly(methyl methacrylate) brushes on flat silica substrates. Langmuir 2015, 31, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Crockett, R.M.; Spencer, N.D. Molecular-weight determination of polymer brushes generated by SI-ATRP on flat surfaces. Macromolecules 2014, 47, 269–275. [Google Scholar] [CrossRef]
- Gorman, C.B.; Petrie, R.J.; Genzer, J. Effect of substrate geometry on polymer molecular weight and polydispersity during surface-initiated polymerization. Macromolecules 2008, 41, 4856–4865. [Google Scholar] [CrossRef]
- Pasetto, P.; Blas, H.; Audouin, F.; Boissière, C.; Sanchez, C.; Save, M.; Charleux, B. Mechanistic insight into surface-initiated polymerization of methyl methacrylate and styrene via ATRP from ordered mesoporous silica particles. Macromolecules 2009, 42, 5983–5995. [Google Scholar] [CrossRef]
- Turgman-Cohen, S.; Genzer, J. Simultaneous bulk- and surface-initiated controlled radical polymerization from planar substrates. J. Am. Chem. Soc. 2011, 133, 17567–17569. [Google Scholar] [CrossRef] [PubMed]
- Turgman-Cohen, S.; Genzer, J. Computer simulation of concurrent bulk- and surface-initiated living polymerization. Macromolecules 2012, 45, 2128–2137. [Google Scholar] [CrossRef]
- Patil, R.R.; Turgman-Cohen, S.; Šrogl, J.; Kiserow, D.; Genzer, J. Direct measurement of molecular weight and grafting density by controlled and quantitative degrafting of surface-anchored poly(methyl methacrylate). ACS Macro Lett. 2015, 4, 251–254. [Google Scholar] [CrossRef]
- Morandi, G.; Thielemans, W. Synthesis of cellulose nanocrystals bearing photocleavable grafts by ATRP. Polym. Chem. 2012, 3, 1402–1407. [Google Scholar] [CrossRef]
- Hansson, S.; Antoni, P.; Bergenudd, H.; Malmström, E. Selective cleavage of polymer grafts from solid surfaces: Assessment of initiator content and polymer characteristics. Polym. Chem. 2011, 2, 556–558. [Google Scholar] [CrossRef]
- Von Werne, T.A.; Patten, T.E. Atom transfer radical polymerization from nanoparticles: A tool for the preparation of well-defined hybrid nanostructures and for understanding the chemistry of controlled/“living” radical polymerizations from surfaces. J. Am. Chem. Soc. 2001, 123, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Tsujii, Y.; Fukuda, T. Controlled grafting of a well-defined polymer on a porous glass filter by surface-initiated atom transfer radical polymerization. Polymer 2001, 42, 6811–6815. [Google Scholar] [CrossRef]
- Jones, D.M.; Brown, A.A.; Huck, W.T.S. Surface-initiated polymerizations in aqueous media: Effect of initiator density. Langmuir 2002, 18, 1265–1269. [Google Scholar] [CrossRef]
- Matrab, T.; Chehimi, M.M.; Pinson, J.; Slomkowski, S.; Basinska, T. Growth of polymer brushes by atom transfer radical polymerization on glassy carbon modified by electro-grafted initiators based on aryl diazonium salts. Surf. Interface Anal. 2006, 38, 565–568. [Google Scholar] [CrossRef]
- Lee, S.H.; Dreyer, D.R.; An, J.; Velamakanni, A.; Piner, R.D.; Park, S.; Zhu, Y.; Kim, S.O.; Bielawski, C.W.; Ruoff, R.S. Polymer brushes via controlled, surface-initiated atom transfer radical polymerization (ATRP) from graphene oxide. Macromol. Rapid Commun. 2010, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Andruzzi, L.; Hexemer, A.; Li, X.; Ober, C.K.; Kramer, E.J.; Galli, G.; Chiellini, E.; Fischer, D.A. Control of surface properties using fluorinated polymer brushes produced by surface-initiated controlled radical polymerization. Langmuir 2004, 20, 10498–10506. [Google Scholar] [CrossRef] [PubMed]
- Cedeno, D.; Krawicz, A.; Doak, P.; Yu, M.; Neaton, J.B.; Moore, G.F. Using molecular design to control the performance of hydrogen-producing polymer-brush-modified photocathodes. J. Phys. Chem. Lett. 2014, 5, 3222–3226. [Google Scholar] [CrossRef]
- Desseaux, S.; Klok, H.-A. Fibroblast adhesion on ECM-derived peptide modified poly(2-hydroxyethyl methacrylate) brushes: Ligand co-presentation and 3D-localization. Biomaterials 2015, 44, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wells, M.; Beebe, T.P.; Chilkoti, A. Surface-initiated atom transfer radical polymerization of oligo(ethylene glycol) methyl methacrylate from a mixed self-assembled monolayer on gold. Adv. Func. Mater. 2006, 16, 640–648. [Google Scholar] [CrossRef]
- Tugulu, S.; Barbey, R.; Harms, M.; Fricke, M.; Volkmer, D.; Rossi, A.; Klok, H.-A. Synthesis of poly(methacrylic acid) brushes via surface-initiated atom transfer radical polymerization of sodium methacrylate and their use as substrates for the mineralization of calcium carbonate. Macromolecules 2007, 40, 168–177. [Google Scholar] [CrossRef]
- Xu, D.; Yu, W.H.; Kang, E.T.; Neoh, K.G. Functionalization of hydrogen-terminated silicon via surface-initiated atom-transfer radical polymerization and derivatization of the polymer brushes. J. Colloid Interface Sci. 2004, 279, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wang, H.; Xue, L.; Han, Y. Stimuli-responsive polyelectrolyte block copolymer brushes synthesized from the Si wafer via atom-transfer radical polymerization. Langmuir 2007, 23, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Franking, R.A.; Landis, E.C.; Hamers, R.J. Highly stable molecular layers on nanocrystalline anatase TiO2 through photochemical grafting. Langmuir 2009, 25, 10676–10684. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.J.; Jablonski, A. Evaluation of electron inelastic mean free paths for selected elements and compounds. Surf. Interface Anal. 2000, 29, 108–114. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Bain, C.D.; Whitesides, G.M. Attenuation of photoelectrons in monolayers of normal-alkanethiols adsorbed on copper, silver, and gold. J. Phys. Chem. 1991, 95, 7017–7021. [Google Scholar] [CrossRef]
- Tugulu, S.; Klok, H.-A. Stability and nonfouling properties of poly(poly(ethylene glycol) methacrylate) brushes under cell culture conditions. Biomacromolecules 2008, 9, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takahara, A. Tribological properties of hydrophilic polymer brushes under wet conditions. Chem. Rec. 2010, 10, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lavanant, L.; Pullin, B.; Hubbell, J.A.; Klok, H.-A. A facile strategy for the modification of polyethylene substrates with non-fouling, bioactive poly(poly(ethylene glycol) methacrylate) brushes. Macromol. Biosci. 2010, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Moroni, L.; Gunnewiek, M.K.; Benetti, E.M. Polymer brush coatings regulating cell behavior: Passive interfaces turn into active. Acta Biomater. 2014, 10, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wagner, M.S.; Castner, D.G.; Ratner, B.D.; Horbett, T.A. Multivariate surface analysis of plasma-deposited tetraglyme for reduction of protein adsorption and monocyte adhesion. Langmuir 2003, 19, 1692–1699. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that determine the protein resistance of oligoether self-assembled monolayers—Internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J. Am. Chem. Soc. 2003, 125, 9395–9366. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Aizawa, H.; Talib, Z.A.; Atthoff, B.; Hilborn, J. Synthesis of tethered-polymer brush by atom transfer radical polymerization from a plasma-polymerized-film-coated quartz crystal microbalance and its application for immunosensors. Biosens. Bioelectron. 2004, 20, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.F.; Demoustier-Champagne, S.; Dupont-Gillain, C.C. Quartz crystal microbalance study of ionic strength and pH-dependent polymer conformation and protein adsorption/desorption on PAA, PEO, and mixed PEO/PAA brushes. Langmuir 2014, 30, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “zero” protein adsorption of poly(carboxybetaine) from undiluted blood serum and plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, D.K.; Jakob, T.A.M.; Robertson, J.W.; Cai, M.; Pemberton, J.E.; Knoll, W. Novel silicon dioxide sol–gel films for potential sensor applications: A surface plasmon resonance study. Langmuir 2001, 17, 1169–1175. [Google Scholar] [CrossRef]
- Wang, J.; Han, H.; Jiang, X.; Huang, L.; Chen, L.; Li, N. Quantum dot-based near-infrared electrochemiluminescent immunosensor with gold nanoparticle-graphene nanosheet hybrids and silica nanospheres double-assisted signal amplification. Anal. Chem. 2012, 84, 4893–4899. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Anraku, Y.; Shinohara, H. Sensing capabilities of colloidal gold monolayer modified with a phenylboronic acid-carrying polymer brush. Biomacromolecules 2006, 7, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Surman, F.; Riedel, T.; Bruns, M.; Kostina, N.Y.; Sedláková, Z.; Rodriguez-Emmenegger, C. Polymer brushes interfacing blood as a route toward high performance blood contacting devices. Macromol. Biosci. 2015, 15, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Emmenegger, C.; Brynda, E.; Riedel, T.; Houska, M.; Šubr, V.; Alles, A.B.; Hasan, E.; Gautrot, J.E.; Huck, W.T.S. Polymer brushes showing non-fouling in blood plasma challenge the currently accepted design of protein resistant surfaces. Macromol. Rapid Commun. 2011, 32, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.L.S.; Rodriguez-Emmenegger, C.; Surman, F.; Riedel, T.; Alles, A.B.; Brynda, E. Use of pooled blood plasmas in the assessment of fouling resistance. RSC Adv. 2014, 4, 2318–2321. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.F.L.; Creagh, A.L.; Janzen, J.; Haynes, C.A.; Brooks, D.E.; Kizhakkedathu, J.N. The induction of thrombus generation on nanostructured neutral polymer brush surfaces. Biomaterials 2010, 31, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, L.; Zheng, J. Achieving highly effective nonfouling performance for surface-grafted poly(HPMA) via atom-transfer radical polymerization. Langmuir 2010, 26, 17375–17382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, L.; Wang, Q.; Yu, Q.; Zheng, J. Effect of film thickness on the antifouling performance of poly(hydroxy-functional methacrylates) grafted surfaces. Langmuir 2011, 27, 4906–4913. [Google Scholar] [CrossRef] [PubMed]
- Barbey, R.; Laporte, V.; Alnabulsi, S.; Klok, H.-A. Postpolymerization modification of poly(glycidyl methacrylate) brushes: An XPS depth-profiling study. Macromolecules 2013, 46, 6151–6158. [Google Scholar] [CrossRef]
- Cullen, S.P.; Mandel, I.C.; Gopalan, P. Surface-anchored poly(2-vinyl-4,4-dimethyl azlactone) brushes as templates for enzyme immobilization. Langmuir 2008, 24, 13701–13709. [Google Scholar] [CrossRef] [PubMed]
- Takasu, K.; Kushiro, K.; Hayashi, K.; Iwasaki, Y.; Inoue, S.; Tamechika, E.; Takai, M. Polymer brush biointerfaces for highly sensitive biosensors that preserve the structure and function of immobilized proteins. Sens. Actuator B 2015, 216, 428–433. [Google Scholar] [CrossRef]
- De Vos, K.; Girones, J.; Popelka, S.; Schacht, E.; Baets, R.; Bienstman, P. SOI optical microring resonator with poly(ethylene glycol) polymer brush for label-free biosensor applications. Biosens. Bioelectron. 2009, 24, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.E.; Ritzert, N.L.; Chen, H.; Smith, N.L.; Tague, M.E.; Xu, Y.; Baird, B.A.; Abruña, H.D.; Ober, C.K. Generalized platform for antibody detection using the antibody catalyzed water oxidation pathway. J. Am. Chem. Soc. 2014, 136, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Boujakhrout, A.; Sanchez, A.; Diez, P.; Jimenez-Falcao, S.; Martinez-Ruiz, P.; Pena-Alvarez, M.; Pingarron, J.M.; Villalonga, R. Decorating graphene oxide/nanogold with dextran-based polymer brushes for the construction of ultrasensitive electrochemical enzyme biosensors. J. Mater. Chem. B 2015, 3, 3518–3524. [Google Scholar] [CrossRef]
- Rafique, S.; Bin, W.; Bhatti, A.S. Electrochemical immunosensor for prostate-specific antigens using a label-free second antibody based on silica nanoparticles and polymer brush. Bioelectrochemistry 2015, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Crulhas, B.P.; Sempionatto, J.R.; Cabral, M.F.; Minko, S.; Pedrosa, V.A. Stimuli-responsive biointerface based on polymer brushes for glucose detection. Electroanalysis 2014, 26, 815–822. [Google Scholar] [CrossRef]
- Piliarik, M.; Sandoghdar, V. Direct optical sensing of single unlabelled proteins and super-resolution imaging of their binding sites. Nat. Commun. 2014, 5, 4495. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Nascetti, A.; Scipinotti, R.; Domenici, F.; Sennato, S.; Gazza, L.; Bordi, F.; Pogna, N.; Manetti, C.; Caputo, D.; et al. On-chip detection of multiple serum antibodies against epitopes of celiac disease by an array of amorphous silicon sensors. RSC Adv. 2014, 4, 2073–2080. [Google Scholar] [CrossRef]
- Barbey, R.; Kauffmann, E.; Ehrat, M.; Klok, H.-A. Protein microarrays based on polymer brushes prepared via surface-initiated atom transfer radical polymerization. Biomacromolecules 2010, 11, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Aied, A.; Zheng, Y.; Pandit, A.; Wang, W. DNA Immobilization and detection on cellulose paper using a surface grown cationic polymer via ATRP. ACS Appl. Mater. Interfaces 2012, 4, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Nandivada, H.; Ding, J.; Nogueira-de-Souza, N.C.; Krebsbach, P.H.; O’Shea, K.S.; Lahann, J.; Smith, G.D. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotech. 2010, 28, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Villa-Diaz, L.G.; Kumar, R.; Lahann, J.; Krebsbach, P.H. Enhancement of the propagation of human embryonic stem cells by modifications in the gel architecture of PMEDSAH polymer coatings. Biomaterials 2014, 35, 9581–9590. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Zhao, X.; Li, Q.; Ye, Z.; Li, Z.; Liu, Y.; Zhou, Y.; Ma, H.; Pan, G.; et al. Long-term self-renewal of human pluripotent stem cells on peptide-decorated poly(OEGMA-co-HEMA) brushes under fully defined conditions. Acta Biomater. 2013, 9, 8840–8850. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Raynor, J.E.; Reyes, C.D.; Burns, K.L.; Collard, D.M.; García, A.J. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials 2008, 29, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.A.; Raynor, J.E.; Dumbauld, D.W.; Lee, T.T.; Jagtap, S.; Templeman, K.L.; Collard, D.M.; García, A.J. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci. Transl. Med. 2010, 2, 45ra60. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wu, Y.; Cheng, Y.; Ma, H.; Wei, S. Fibronectin and bone morphogenetic protein-2-decorated poly(OEGMA-r-HEMA) brushes promote osseointegration of titanium surfaces. Langmuir 2011, 27, 12069–12073. [Google Scholar] [CrossRef] [PubMed]
- Christman, K.L.; Vázquez-Dorbatt, V.; Schopf, E.; Kolodziej, C.M.; Li, R.C.; Broyer, R.M.; Chen, Y.; Maynard, H.D. Nanoscale growth factor patterns by immobilization on a heparin-mimicking polymer. J. Am. Chem. Soc. 2008, 130, 16585–16591. [Google Scholar] [CrossRef] [PubMed]
- Hudalla, G.A.; Koepsel, J.T.; Murphy, W.L. Surfaces that sequester serum-borne heparin amplify growth factor activity. Adv. Mater. 2011, 23, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.M.; Tang, S.; Bao, C.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Truncated wedge-shaped nanostructures formed from lateral microphase separation of mixed homopolymer brushes grafted on 67 nm silica nanoparticles: Evidence of the effect of substrate curvature. ACS Macro Lett. 2012, 1, 1061–1065. [Google Scholar] [CrossRef]
- Price, A.D.; Hur, S.-M.; Fredrickson, G.H.; Frischknecht, A.L.; Huber, D.L. Exploring lateral microphase separation in mixed polymer brushes by experiment and self-consistent field theory simulations. Macromolecules 2012, 45, 510–524. [Google Scholar] [CrossRef]
- Li, W.; Bao, C.; Wright, R.A.E.; Zhao, B. Synthesis of mixed poly(ε-caprolactone)/polystyrene brushes from Y-initiator-functionalized silica particles by surface-initiated ring-opening polymerization and nitroxide-mediated radical polymerization. RSC Adv. 2014, 4, 18772–18781. [Google Scholar] [CrossRef]
- Hur, S.-M.; Frischknecht, A.L.; Huber, D.L.; Fredrickson, G.H. Self-assembly in a mixed polymer brush with inhomogeneous grafting density composition. Soft Matter 2013, 9, 5341–5354. [Google Scholar] [CrossRef]
- Calabrese, D.R.; Ditter, D.; Liedel, C.; Blumfield, A.; Zentel, R.; Ober, C.K. Design, synthesis, and use of Y-Shaped ATRP/NMP surface tethered initiator. ACS Macro Lett. 2015, 4, 606–610. [Google Scholar] [CrossRef]
- Kim, J.-B.; Huang, W.; Bruening, M.L.; Baker, G.L. Synthesis of triblock copolymer brushes by surface-initiated atom transfer radical polymerization. Macromolecules 2002, 35, 5410–5416. [Google Scholar] [CrossRef]
- Poelma, J.E.; Fors, B.P.; Meyers, G.F.; Kramer, J.W.; Hawker, C.J. Fabrication of complex three-dimensional polymer brush nanostructures through light-mediated living radical polymerization. Angew. Chem. Int. Ed. 2013, 52, 6844–6848. [Google Scholar] [CrossRef] [PubMed]
- Coad, B.R.; Bilgic, T.; Klok, H.-A. Polymer brush gradients grafted from plasma-polymerized surfaces. Langmuir 2014, 30, 8357–8365. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xiao, P.; Gu, J.; Chen, J.; Cai, Z.; Zhang, J.; Wang, W.; Chen, T. Polymer brush functionalized janus graphene oxide/chitosan hybrid membranes. RSC Adv. 2014, 4, 22759–22762. [Google Scholar] [CrossRef]
- Kelby, T.S.; Wang, M.; Huck, W.T.S. Controlled folding of 2D Au-polymer brush composites into 3D microstructures. Adv. Func. Mater. 2011, 21, 652–657. [Google Scholar] [CrossRef]
- Kohri, M.; Shinoda, Y.; Kohma, H.; Nannichi, Y.; Yamauchi, M.; Yagai, S.; Kojima, T.; Taniguchi, T.; Kishikawa, K. Facile synthesis of free-standing polymer brush films based on a colorless polydopamine thin layer. Macromol. Rapid Commun. 2013, 34, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Schmitt, S.K.; Choi, J.W.; Krutty, J.D.; Gopalan, P. From Self-Assembled Monolayers to Coatings: Advances in the Synthesis and Nanobio Applications of Polymer Brushes. Polymers 2015, 7, 1346-1378. https://doi.org/10.3390/polym7071346
Kim M, Schmitt SK, Choi JW, Krutty JD, Gopalan P. From Self-Assembled Monolayers to Coatings: Advances in the Synthesis and Nanobio Applications of Polymer Brushes. Polymers. 2015; 7(7):1346-1378. https://doi.org/10.3390/polym7071346
Chicago/Turabian StyleKim, Myungwoong, Samantha K. Schmitt, Jonathan W. Choi, John D. Krutty, and Padma Gopalan. 2015. "From Self-Assembled Monolayers to Coatings: Advances in the Synthesis and Nanobio Applications of Polymer Brushes" Polymers 7, no. 7: 1346-1378. https://doi.org/10.3390/polym7071346