Fabrication of a Miniature Paper-Based Electroosmotic Actuator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Patterning
2.3. Electrode Materials
2.4. Plasma Bonding
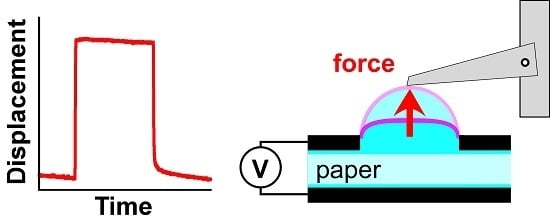
2.5. Actuator Performance Testing
3. Device Design
3.1. Pumping Mechanism
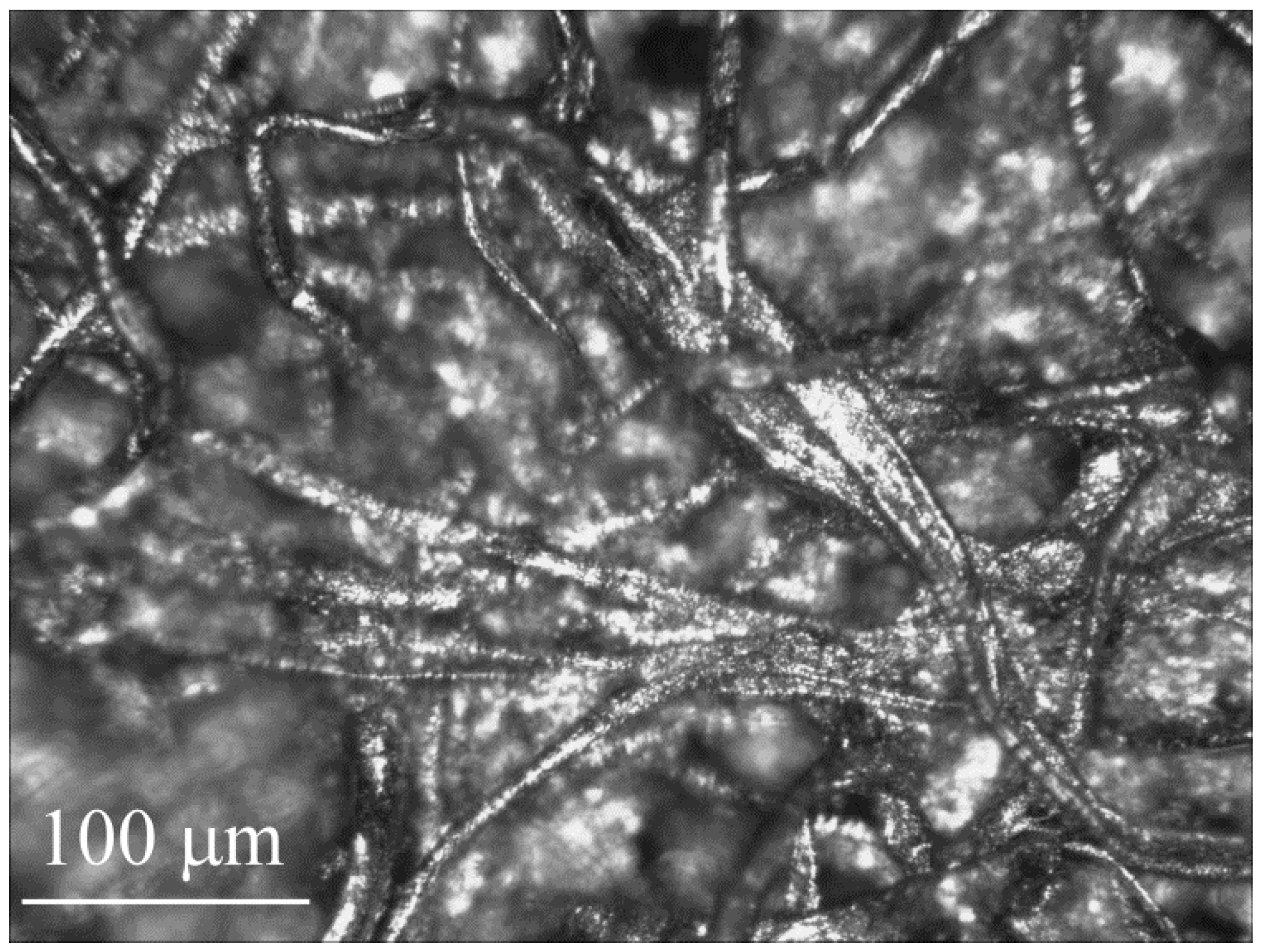
3.2. Paper Microchannels
3.3. Device Design
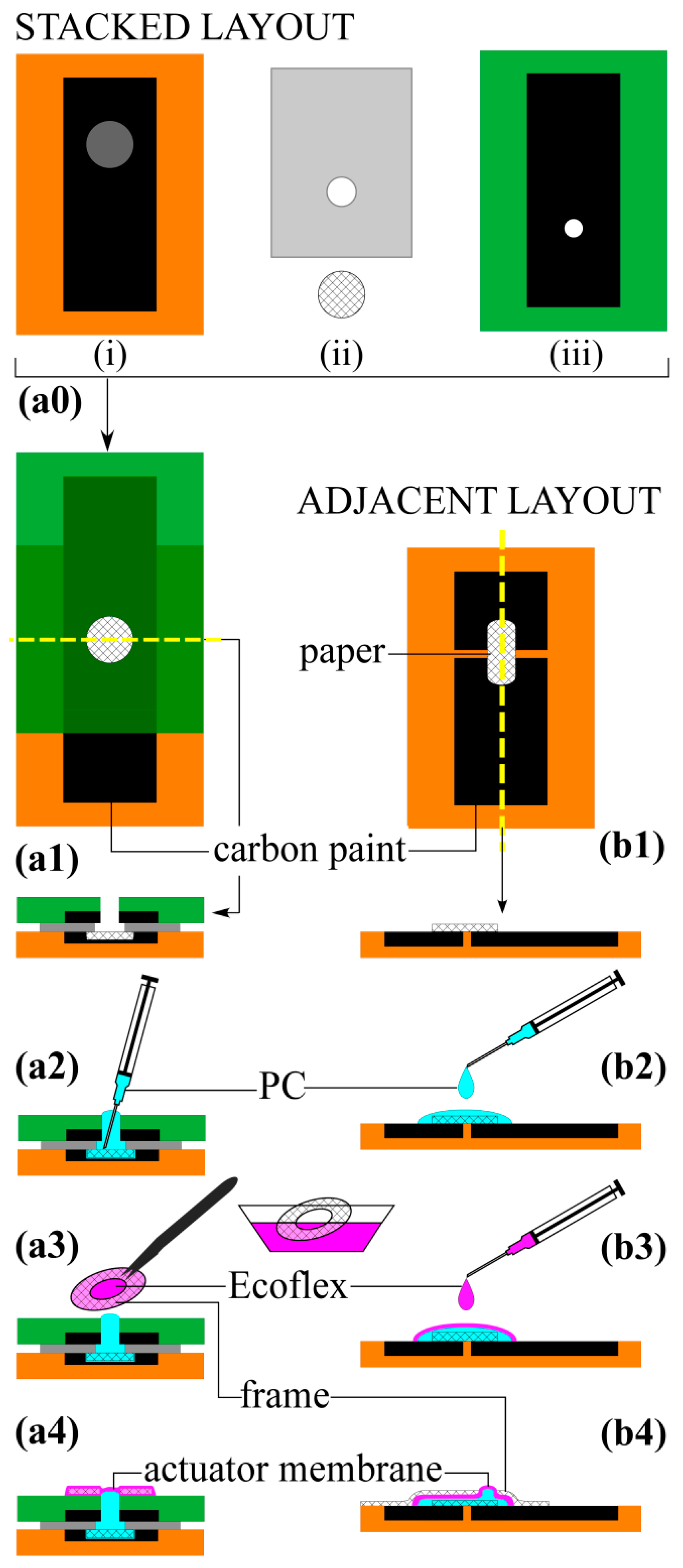
4. Fabrication
4.1. Adjacent Layout
4.2. Stacked Layout
4.3. Observations
5. Results: Actuator Performance
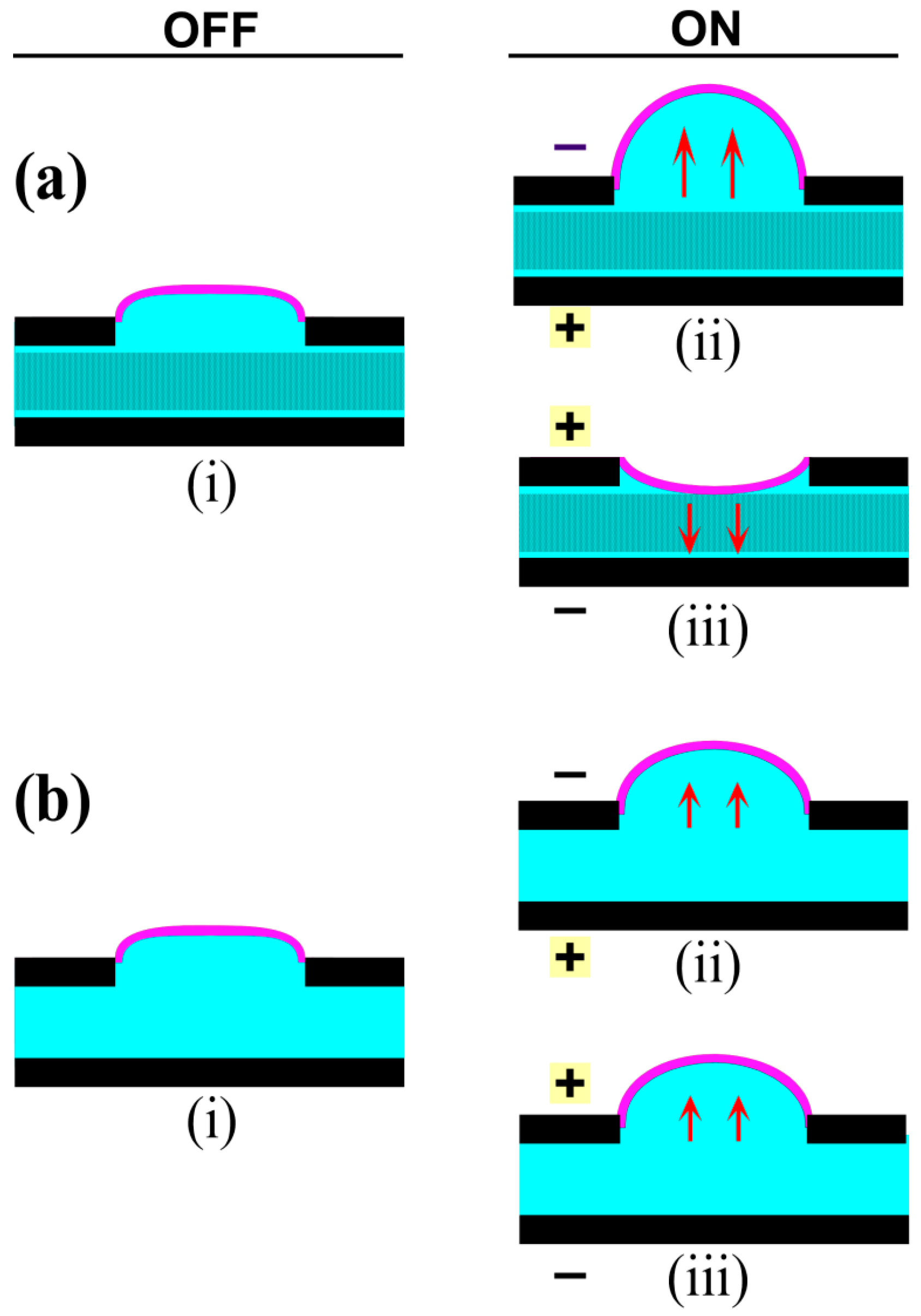
5.1. Electrode Configuration
5.1.1. Adjacent Layout
5.1.2. Stacked Layout
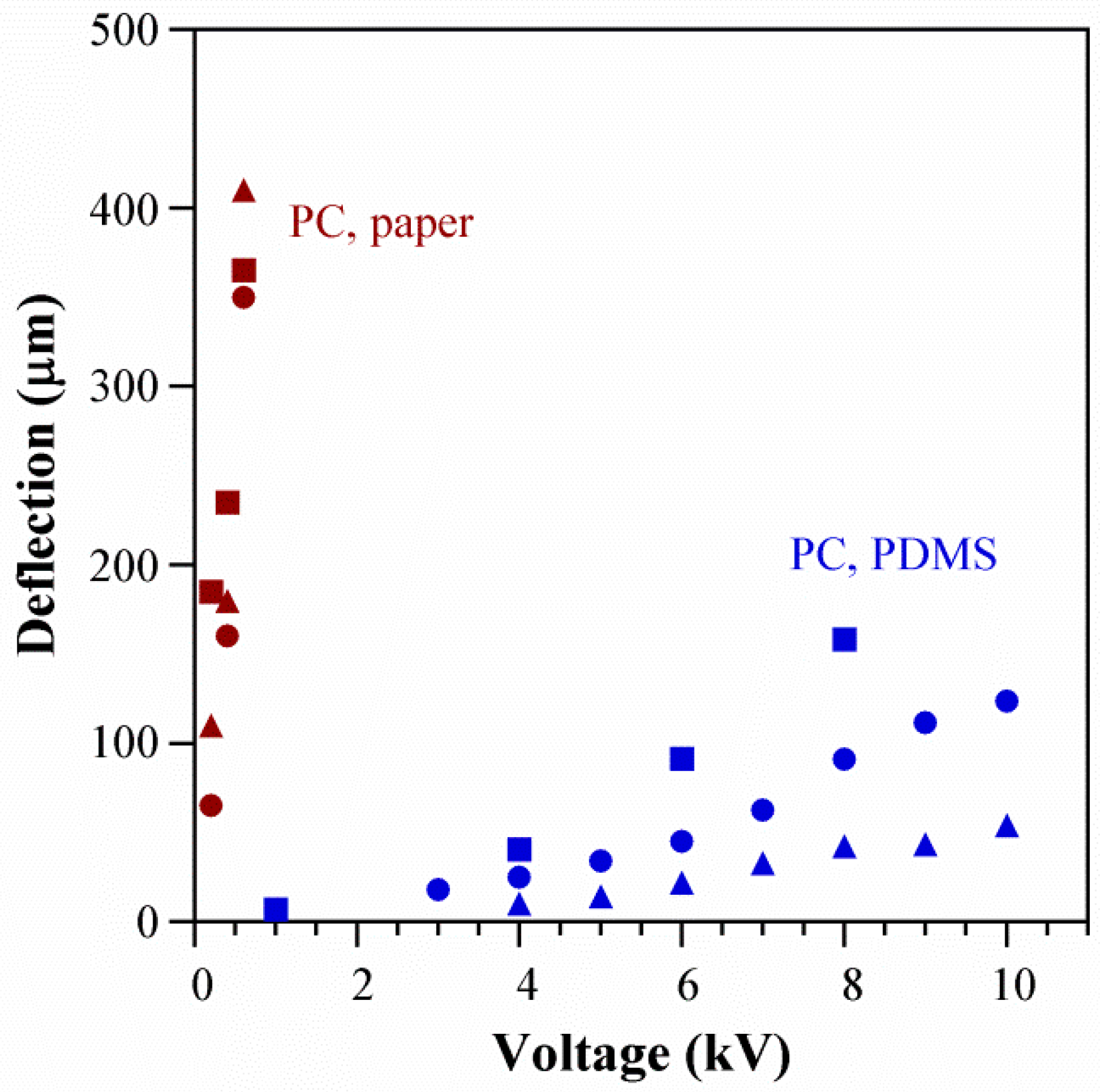
5.2. Actuator Displacement
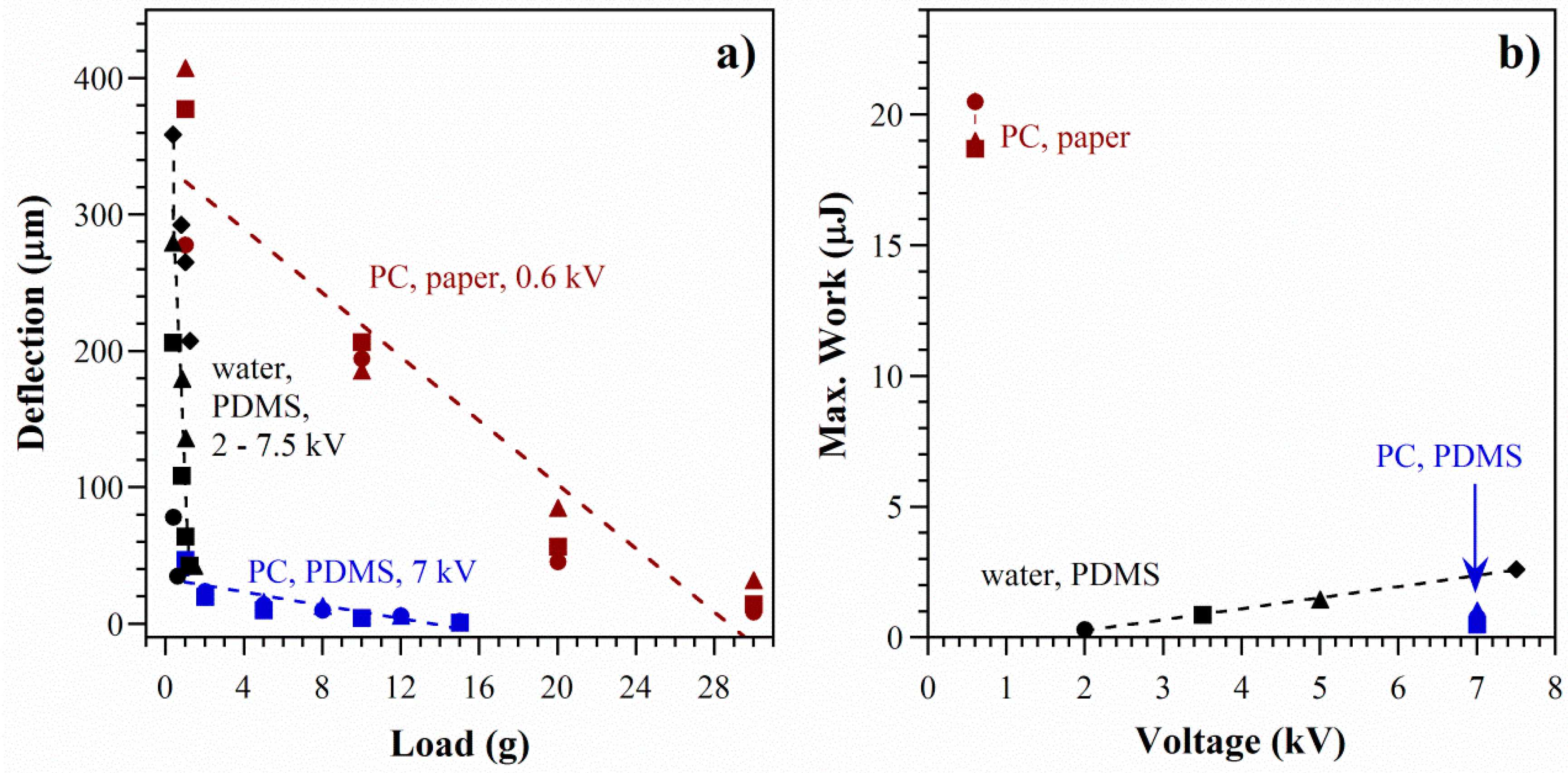
5.3. Actuator Force
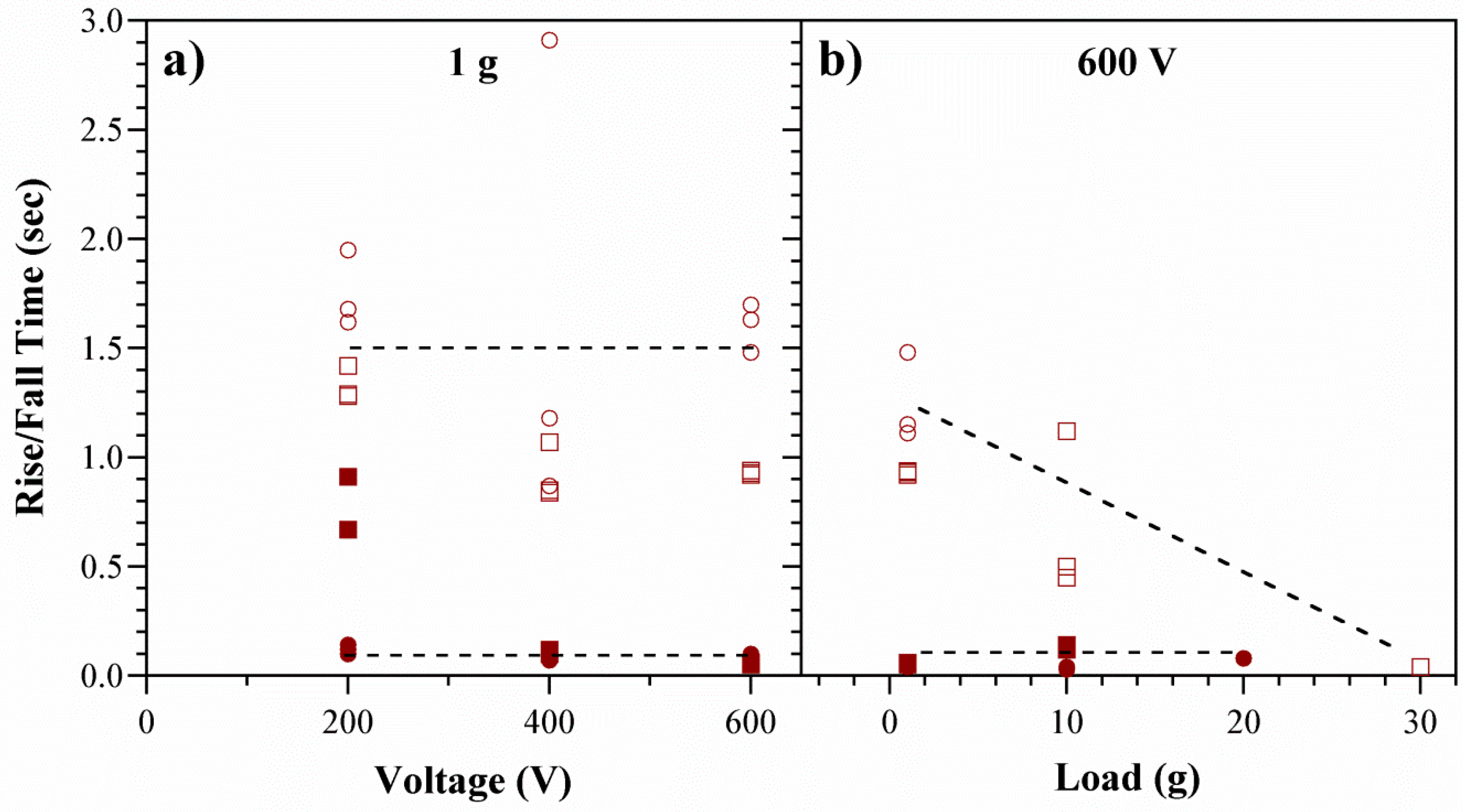
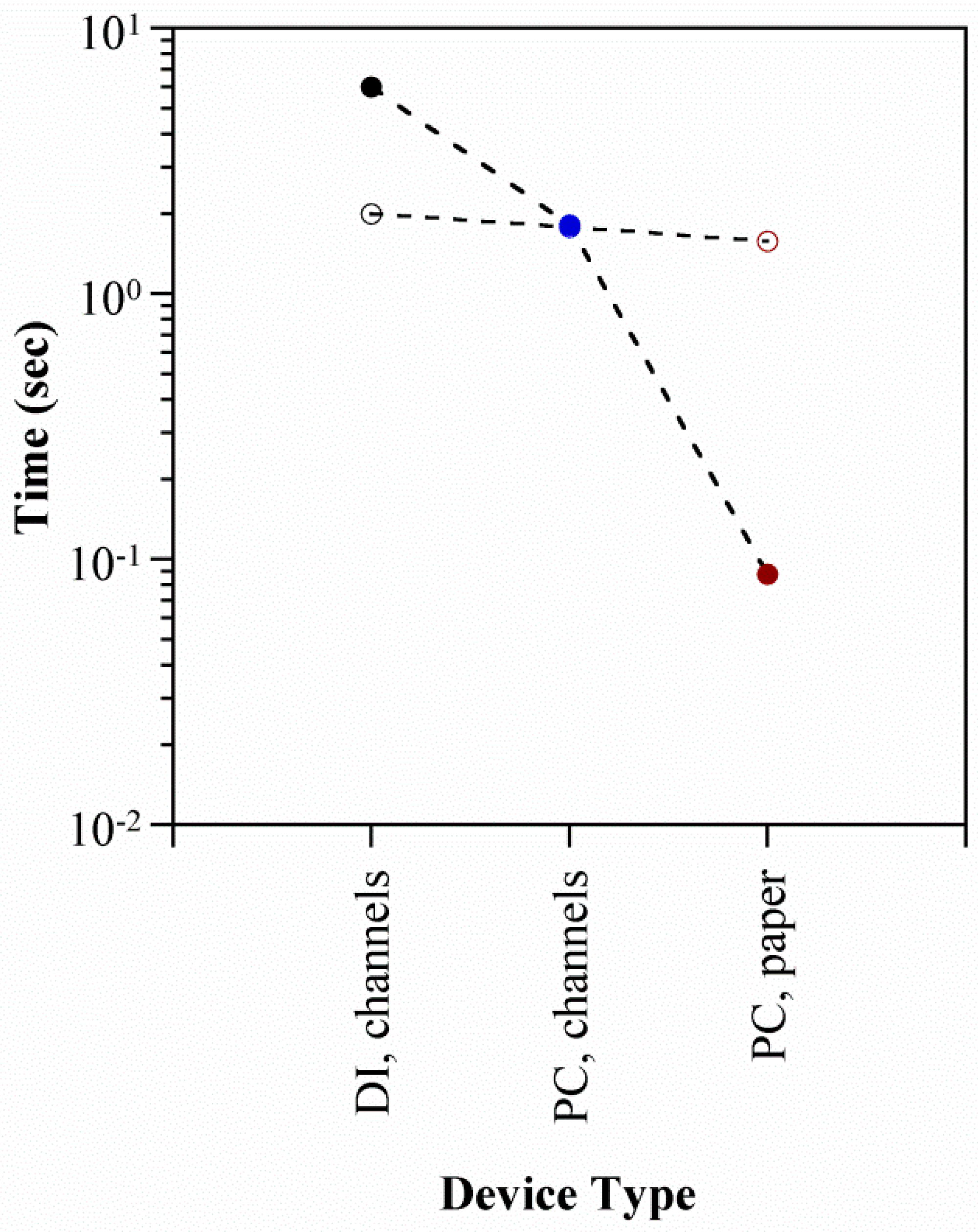
5.4. Actuator Speed
6. Discussion
7. Summary and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trivedi, D.; Rahn, C.D.; Kier, W.M.; Walker, I.D. Soft robotics: Biological inspiration, state of the art, and future research. Appl. Bionics Biomech. 2008, 5, 99–117. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Hilber, W. Stimulus-active polymer actuators for next-generation microfluidic devices. Appl. Phys. A 2016, 122, 751. [Google Scholar] [CrossRef]
- Borgmann, H. (Ed.) Proceedings of the 15th International Conference on New Actuators, Bremen, Germany, 13–15 June 2016; Messe Bremen: Bremen, Germany, 2016.
- Special Issue “Selected papers from the 7th International Conference on Biomimetics, Artificial Muscles and Nano-bio (BAMN2013)”. In Smart Materials and Structures; 2014; Volume 23, Available online: http://iopscience.iop.org/issue/0964-1726/23/7 (accessed on 26 October 2016).
- Bar-Cohen, Y.; Vidal, F. (Eds.) Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2016, Las Vegas, NV, USA, 20–24 March 2016; SPIE: Bellingham, DC, USA, 2016; Volume 9798.
- Piyasena, M.E.; Newby, R.; Miller, T.J.; Shapiro, B.; Smela, E. Electroosmotically driven microfluidic actuators. Sens. Actuators B 2009, 141, 263–269. [Google Scholar] [CrossRef]
- Sritharan, D.; Chen, A.S.; Aluthgama, P.; Naved, B.; Smela, E. Bubble-free electrokinetic flow with propylene carbonate. Electrophoresis 2015, 36, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.; Reynaerts, D. Pneumatic and hydraulic microactuators: A review. J. Micromech. Microeng. 2010, 20, 1–19. [Google Scholar] [CrossRef]
- Daerden, F.; Lefeber, D. Pneumatic artificial muscles: Actuators for robotics and automation. EJMEE 2002, 47, 11–21. [Google Scholar]
- Chou, C.-P.; Hannaford, B. Measurement and modeling of McKibben pneumatic artificial muscles. IEEE Trans. Robot. Autom. 1996, 12, 90–102. [Google Scholar] [CrossRef]
- Pritts, M.B.; Rahn, C.D. Design of an artificial muscle continuum robot. In Proceedings of the 2004 IEEE International Conference on Robotics and Automation (ICRA), New Orleans, LA, USA, 26 April–1 May 2004; Volume 5, pp. 4742–4746.
- Marchese, A.D.; Katzschmann, R.K.; Rus, D. A recipe for soft fluidic elastomer robots. Soft Robot. 2015, 2, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M.; Truby, R.L.; Fitzgerald, D.J.; Mosadegh, B.; Whitesides, G.M.; Lewis, J.A.; Wood, R.J. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 2016, 536, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.F.; Ilievski, F.; Choi, W.; Morin, S.A.; Stokes, A.A.; Mazzeo, A.D.; Chen, X.; Wang, M.; Whitesides, G.M. Multigait soft robot. Proc. Natl. Acad. Sci. USA 2011, 108, 20400–20403. [Google Scholar] [CrossRef] [PubMed]
- Ilievski, F.; Mazzeo, A.D.; Shepherd, R.F.; Chen, X.; Whitesides, G.M. Soft robotics for chemists. Angew. Chem. Int. Ed. 2011, 50, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Suzumori, K.; Endo, S.; Kanda, T.; Kato, N.; Suzuki, H. A Bending Pneumatic Rubber Actuator Realizing Soft-bodied Manta Swimming Robot. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation (ICRA), Roma, Italy, 10–14 April 2007; pp. 4975–4980.
- Martinez, R.V.; Branch, J.L.; Fish, C.R.; Jin, L.; Shepherd, R.F.; Nunes, R.M.D.; Suo, Z.; Whitesides, G.M. Robotic Tentacles with Three-Dimensional Mobility Based on Flexible Elastomers. Adv. Mater. 2013, 25, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.F.; Stokes, A.A.; Nunes, R.M.D.; Whitesides, G.M. Soft Machines That Are Resistant to Puncture and That Self Seal. Adv. Mater. 2013, 25, 6709–6713. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.A.; Shepherd, R.F.; Kwok, S.W.; Stokes, A.A.; Nemiroski, A.; Whitesides, G.M. Camouflage and Display for Soft Machines. Science 2012, 337, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, B.; Polygerinos, P.; Keplinger, C.; Wennstedt, S.; Shepherd, R.F.; Gupta, U.; Shim, J.; Bertoldi, K.; Walsh, C.J.; Whitesides, G.M. Pneumatic Networks for Soft Robotics that Actuate Rapidly. Adv. Funct. Mater. 2014, 24, 2163–2170. [Google Scholar] [CrossRef]
- De Volder, M. Capillary Based Sealing. In Surface Tension in Microsystems: Engineering Below the Capillary Length; Lambert, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 211–226. [Google Scholar]
- Onal, C.D.; Chen, X.; Whitesides, G.M.; Rus, D. Soft mobile robots with on-board chemical pressure generation. In Proceedings of the International Symposium on Robotics Research (ISRR), Flagstaff, AZ, USA, 28 August–1 September 2011; pp. 1–16.
- De Koninck, D.A.; Lopez, F.M.; Briand, D.; Rooij, N.F.D. Foil-level fabrication of inkjet-printed pyroMEMS balloon actuators. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 64–67.
- Liwei, L.; Pisano, A.P.; Lee, A.P. Microbubble powered actuator. In Proceedings of the International Conference on Solid-State Sensors and Actuators (TRANSDUCERS), San Francisco, CA, USA, 24–27 June 1991; pp. 1041–1044.
- Bergstrom, P.L.; Jin, J.; Yu-Ning, L.; Kaviany, M.; Wise, K.D. Thermally driven phase-change microactuation. J. Microelectromech. Syst. 1995, 4, 10–17. [Google Scholar] [CrossRef]
- Ateya, D.A.; Shah, A.A.; Hua, S.Z. An electrolytically actuated micropump. Rev. Sci. Instrum. 2004, 75, 915–920. [Google Scholar] [CrossRef]
- Hsu, L.; Ramunas, J.; Gonzalez, J.; Santiago, J.; Strickland, D.G. Toward an Electrolytic Micropump Actuator Design with Controlled Cyclic Bubble Growth and Recombination. ECS Trans. 2011, 35, 3–11. [Google Scholar]
- Castellanos, A. Electrohydrodynamics; Springer: New York, NY, USA, 1998; pp. 22–24. [Google Scholar]
- Dow Corning. Sylgard 184 Silicone Elastomer: Product Information; Dow Corning: Midland, MI, USA, 2014; pp. 1–4. [Google Scholar]
- Smooth-On. Ecoflex Series: Product Overview; Smooth-On: Macungie, PA, USA, 2014. [Google Scholar]
- Daikhin, L.I.; Kornyshev, A.A.; Urbakh, M. Double layer capacitance on a rough metal surface: Surface roughness measured by “Debye ruler”. Electrochim. Acta 1997, 42, 2853–2860. [Google Scholar] [CrossRef]
- De Levie, R. The influence of surface roughness of solid electrodes on electrochemical measurements. Electrochim. Acta 1965, 10, 113–130. [Google Scholar] [CrossRef]
- Messinger, R.J.; Squires, T.M. Suppression of Electro-Osmotic Flow by Surface Roughness. Phys. Rev. Lett. 2010, 105, 144503. [Google Scholar] [CrossRef] [PubMed]
- Martone, P.T.; Boller, M.; Burgert, I.; Dumais, J.; Edwards, J.; Mach, K.; Rowe, N.; Rueggeberg, M.; Seidel, R.; Speck, T. Mechanics without muscle: Biomechanical inspiration from the plant world. Integr. Comp. Biol. 2010, 50, 888–907. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Yamamoto, H.; Takemura, K.; Yokota, S.; Edamura, K. Dominant factors inducing electro-conjugate fluid flow. Sens. Actuators A 2011, 167, 84–90. [Google Scholar] [CrossRef]
- Kim, J.-W.; Suzuki, T.; Yokota, S.; Edamura, K. Tube-type micropump by using electro-conjugated fluid (ECF). Sens. Actuators A 2012, 174, 155–161. [Google Scholar] [CrossRef]
- Yokota, S.; Yajima, F.; Takemura, K.; Edamura, K. Electro-Conjugate Fluid Jet-Driven Micro Artificial Antagonistic Muscle Actuators and their Integration. Adv. Robot. 2010, 24, 1929–1943. [Google Scholar] [CrossRef]
- Yokota, S.; Sadamoto, A.; Kondoh, Y.; Otsubo, Y.; Edamura, K. A micro motor using electroconjugate fluids (ECFs). JSME Int. J. Ser. C Mech. Syst. Mach. Elem. Manuf. 2001, 44, 756–762. [Google Scholar] [CrossRef]
- Han, D.; Kim, J.W.; Yokota, S.; Edamura, K. ECF micropump fabricated by electroforming with novel self-aligned micro-molding technology. J. Phys. Conf. Ser. 2015, 660, 012029. [Google Scholar] [CrossRef]
- Kedzierski, J.; Meng, K.; Thorsen, T.; Cabrera, R.; Berry, S. Microhydraulic Electrowetting Actuators. J. Micromech. Microeng. 2016, 25, 394–400. [Google Scholar] [CrossRef]
- Butt, H.-J.; Kappl, M. Surface and Interfacial Forces; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Seibel, K.; Schoeler, L.; Schaefer, H.; Boehm, M. A programmable planar electroosmotic micropump for lab-on-a-chip applications. J. Micromech. Microeng. 2008, 18, 025008. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, C.; Wang, S.; Liu, S. Electroosmotic pumps and their applications in microfluidic systems. Microfluid. Nanofluid. 2009, 6, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Jia, Z.; Zhu, Z.; He, C.; Wang, W.; Morgan, A.; Lu, J.J.; Liu, S. Miniaturized electroosmotic pump capable of generating pressures of more than 1200 Bar. Anal. Chem. 2012, 84, 9609–9614. [Google Scholar] [CrossRef] [PubMed]
- McKnight, T.E.; Culbertson, C.T.; Jacobson, S.C.; Ramsey, M.J. Electroosmotically induced hydraulic pumping with integrated electrodes on microfluidic devices. Anal. Chem. 2001, 73, 4045–4049. [Google Scholar] [CrossRef] [PubMed]
- Tripp, J.A.; Svec, F.; Fréchet, J.M.J.; Zeng, S.; Mikkelsen, J.C.; Santiago, J.G. High-pressure electroosmotic pumps based on porous polymer monoliths. Sens. Actuators B 2004, 99, 66–73. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, C.; Mikkelsen, J.C.J.; Santiago, J.G. Fabrication and characterization of electroosmotic micro pumps. Sens. Actuators B 2001, 79, 107–114. [Google Scholar] [CrossRef]
- Schroeder, M.A.; Salloum, K.S.; Perbost, M.; Lebl, M.; Posner, J.D. Radial flow electroosmotic pump. Sens. Actuators A 2011, 169, 250–255. [Google Scholar] [CrossRef]
- Santiago, J.; Posner, J.; Prinz, F.B.; Fabian, T.; Eaton, J.; Cha, S.-W.; Buie, C.; Kim, D.; Tsuru, H.; Sasahara, J.; et al. Fuel Cell with Electroosmotic Pump. U.S. Patent 7799453 B2, 21 September 2010. [Google Scholar]
- Luque, A.; Soto, J.M.; Perdigones, F.; Aracil, C.; Quero, J.M. Electroosmotic impulsion device for integration in PCB-MEMS. In Proceedings of the 2013 IEEE Spanish Conference on Electron Devices (CDE), Valladolid, Spain, 12–14 February 2013; pp. 119–122.
- Chen, C.-H.; Santiago, J.G. A Planar Electroosmotic Micropump. J. Micromech. Microeng. 2002, 11, 672–683. [Google Scholar] [CrossRef]
- Chen, R.-L.; Chen, C.-H.; Chen, B.-C.; Chen, C.-L. Electroosmotically actuated hydrogel for nastic actuation. In Plants and Mechanical Motion: A Synthetic Approach to Nastic Materials and Structures; Wereley, N.M., Sater, J.M., Eds.; DEStech Publications, Inc.: Lancaster, PA, USA, 2012; pp. 87–97. [Google Scholar]
- Chen, L.; Ma, J.; Guan, Y. Study of an electroosmotic pump for liquid delivery and its application in capillary column liquid chromatography. J. Chromatogr. A 2004, 1028, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.P.; Mogensen, K.B.; Klank, H.; Geschke, O. Microfluidics-Components. In Microsystem Engineering of Lab-on-a-Chip Devices; Klank, H., Geschke, O., Telleman, P., Eds.; Wiley-VCH: Weinheim, Germany, 2004; pp. 39–77. [Google Scholar]
- Sritharan, D.; Motsebo, M.; Tumbic, J.; Smela, E. Stable electroosmotically driven actuators. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2013, San Diego, CA, USA, 11–14 March 2013; Volume 8687.
- Kim, S.E.; List, R.S.; Maveety, J.G.; Myers, A.M.; Vu, Q.T.; Prasher, R.; Mahajan, R.; Vandentop, G. Using External Radiators with Electroosmotic Pumps for Cooling. U.S. Patent 6992381 B2, 31 January 2006. [Google Scholar]
- Chen, L.; Ma, J.; Tan, F.; Guan, Y. Generating high-pressure sub-microliter flow rate in packed microchannel by electroosmotic force: Potential application in microfluidic systems. Sens. Actuators B 2003, 88, 260–265. [Google Scholar] [CrossRef]
- Bellan, L.M.; Pearsall, M.; Cropek, D.M.; Langer, R. A 3D Interconnected Microchannel Network Formed in Gelatin by Sacrificial Shellac Microfibers. Adv. Mater. 2012, 24, 5187–5191. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Smela, E. A novel surface modification technique for forming porous polymer monoliths in poly(dimethylsiloxane). Biomicrofluidics 2012, 6, 016506. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2009, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 011301. [Google Scholar] [CrossRef] [PubMed]
- Engelke, J.L.; Strain, H.H.; Wood, S.E. Electroosmosis in Paper Electrochromatography with Electrode Vessels. Anal. Chem. 1954, 26, 1864–1868. [Google Scholar] [CrossRef]
- Balakrisnan, B.; Nacev, A.; Burke, J.M.; Dasgupta, A.; Smela, E. Design of compliant meanders for applications in MEMS, actuators, and flexible electronics. Smart Mater. Struct. 2012, 21, 075033. [Google Scholar] [CrossRef]
- Gerratt, A.P.; Balakrisnan, B.; Penskiy, I.; Bergbreiter, S. Dielectric elastomer actuators fabricated using a micro-molding process. Smart Mater. Struct. 2014, 23, 055004. [Google Scholar] [CrossRef]
- Brun, M.; Chateaux, J.-F.; Deman, A.-L.; Pittet, P.; Ferrigno, R. Nanocomposite Carbon-PDMS Material for Chip-Based Electrochemical Detection. Electroanalysis 2010, 23, 321–324. [Google Scholar]
- Kujawski, M.; Pearse, J.; Smela, E. PDMS/graphite stretchable electrodes for dielectric elastomer actuators. In Proceedings of the Electroactive Polymer Actuators and Devices (EAPAD) 2010, San Diego, CA, USA, 8–11 March 2010; Volume 7642, p. 76420R.
- Yamada, H.; Manas-Zloczower, I.; Feke, D.L. Influence of matrix infiltration on the dispersion kinetics of carbon black agglomerates. Powder Technol. 1997, 92, 163–169. [Google Scholar] [CrossRef]
- Van Nierop, E.A.; Scheid, B.; Stone, H.A. On the thickness of soap films: An alternative to Frankel’s law. J. Fluid Mech. 2008, 602, 119–127. [Google Scholar] [CrossRef]
- Digital Materials Data Sheet; Stratasys: Eden Prairie, MN, USA, 2015.
- Binh-Khiem, N.; Matsumoto, K.; Shimoyama, I. Tensile Film Stress of Parylene Deposited on Liquid. Langmuir 2010, 26, 18771–18775. [Google Scholar] [CrossRef] [PubMed]
- Miki, N. (Ed.) Liquid Encapsulation Technology for Microelectromechanical Systems; InTech: Rijeka, Croatia, 2013.
- Marín, Á.G.; Lohse, D. Building water bridges in air: Electrohydrodynamics of the floating water bridge. Phys. Fluids 2010, 22, 122104. [Google Scholar] [CrossRef]
- Wexler, A.D.; Sáenz, M.L.; Schreer, O.; Woisetschläger, J.; Fuchs, E.C. The Preparation of Electrohydrodynamic Bridges from Polar Dielectric Liquids. J. Vis. Exp. 2014, 91, 51819. [Google Scholar] [CrossRef] [PubMed]
- Pickard, W.F. Experimental investigation of the sumoto effect. J. Appl. Phys. 1961, 32, 1888–1893. [Google Scholar] [CrossRef]
- Fuchs, E.C.; Woisetschlaeger, J.; Gatterer, K.; Maier, E.; Pecnik, R.; Holler, G.; Eisenkoelbl, H. The floating water bridge. J. Phys. D Appl. Phys. 2007, 40, 6112–6114. [Google Scholar] [CrossRef]
- Duan, H.; Hu, Z.W.; Fang, Z.C. Study on deformation characters of a large rubber circular plate. Model. Simul. Mater. Sci. Eng. 2004, 12, 245. [Google Scholar] [CrossRef]
- Srivastava, A.; Hui, C.-Y. Large deformation contact mechanics of a pressurized long rectangular membrane. II. Adhesive contact. Proc. R. Soc. A 2013, 469. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Kolb, J.F.; Malik, M.A.; Lu, X.; Laroussi, M.; Joshi, R.P.; Schamiloglu, E.; Schoenbach, K.H. Electrical Breakdown and Dielectric Recovery of Propylene Carbonate. IEEE Trans. Plasma Sci. 2006, 34, 1653–1661. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Wågberg, L.; Hägglund, R. Kinetics of polyelectrolyte adsorption on cellulosic fibers. Langmuir 2001, 17, 1096–1103. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Duarte, A.P.; Salah, A.B.; Gandini, A. Modification of cellulosic fibres with functionalised silanes: Development of surface properties. Int. J. Adhes. Adhes. 2004, 24, 43–54. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Bowden, N.; Brittain, S.; Evans, A.G.; Hutchinson, J.W.; Whitesides, G.M. Spontaneous formation of ordered structures in thin films of metals supported on an elastomeric polymer. Nature 1998, 393, 146–149. [Google Scholar]
- Material Safety Data Sheet (MSDS) for Anhydrous Propylene Carbonate, 99.7%. Available online: www.sigmaaldrich.com/catalog/product/sial/310328?lang=en®ion=US (accessed on 26 October 2016).
- Gnanaraj, J.S.; Thompson, R.W.; DiCarlo, J.F.; Abraham, K.M. The Role of Carbonate Solvents on Lithium Intercalation into Graphite. J. Electrochem. Soc. 2007, 154, A185–A191. [Google Scholar] [CrossRef]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sritharan, D.; Smela, E. Fabrication of a Miniature Paper-Based Electroosmotic Actuator. Polymers 2016, 8, 400. https://doi.org/10.3390/polym8110400
Sritharan D, Smela E. Fabrication of a Miniature Paper-Based Electroosmotic Actuator. Polymers. 2016; 8(11):400. https://doi.org/10.3390/polym8110400
Chicago/Turabian StyleSritharan, Deepa, and Elisabeth Smela. 2016. "Fabrication of a Miniature Paper-Based Electroosmotic Actuator" Polymers 8, no. 11: 400. https://doi.org/10.3390/polym8110400