Hydrogel Layers on the Surface of Polyester-Based Materials for Improvement of Their Biointeractions and Controlled Release of Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Methods
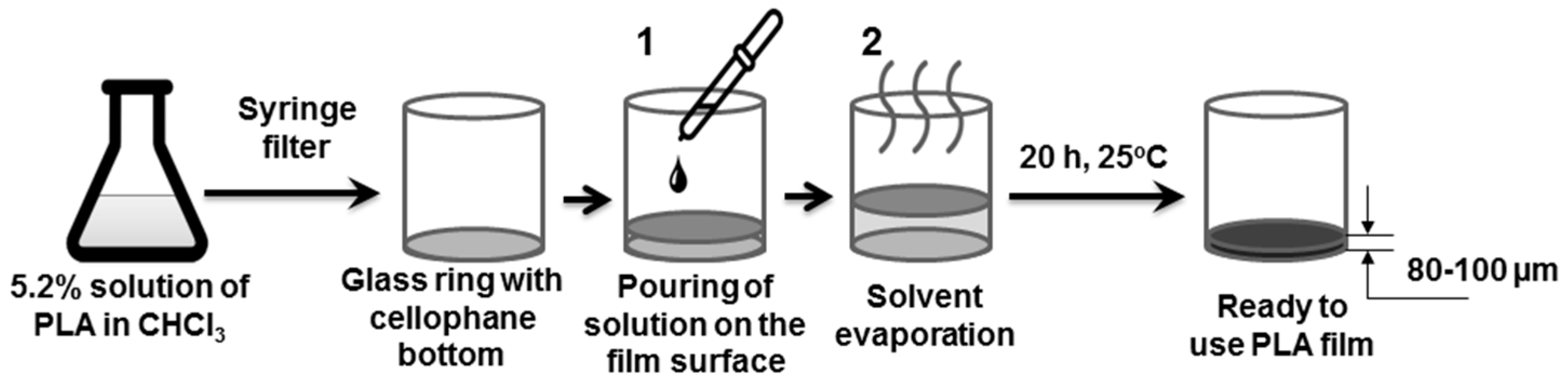
2.2.1. PLA Film Preparation
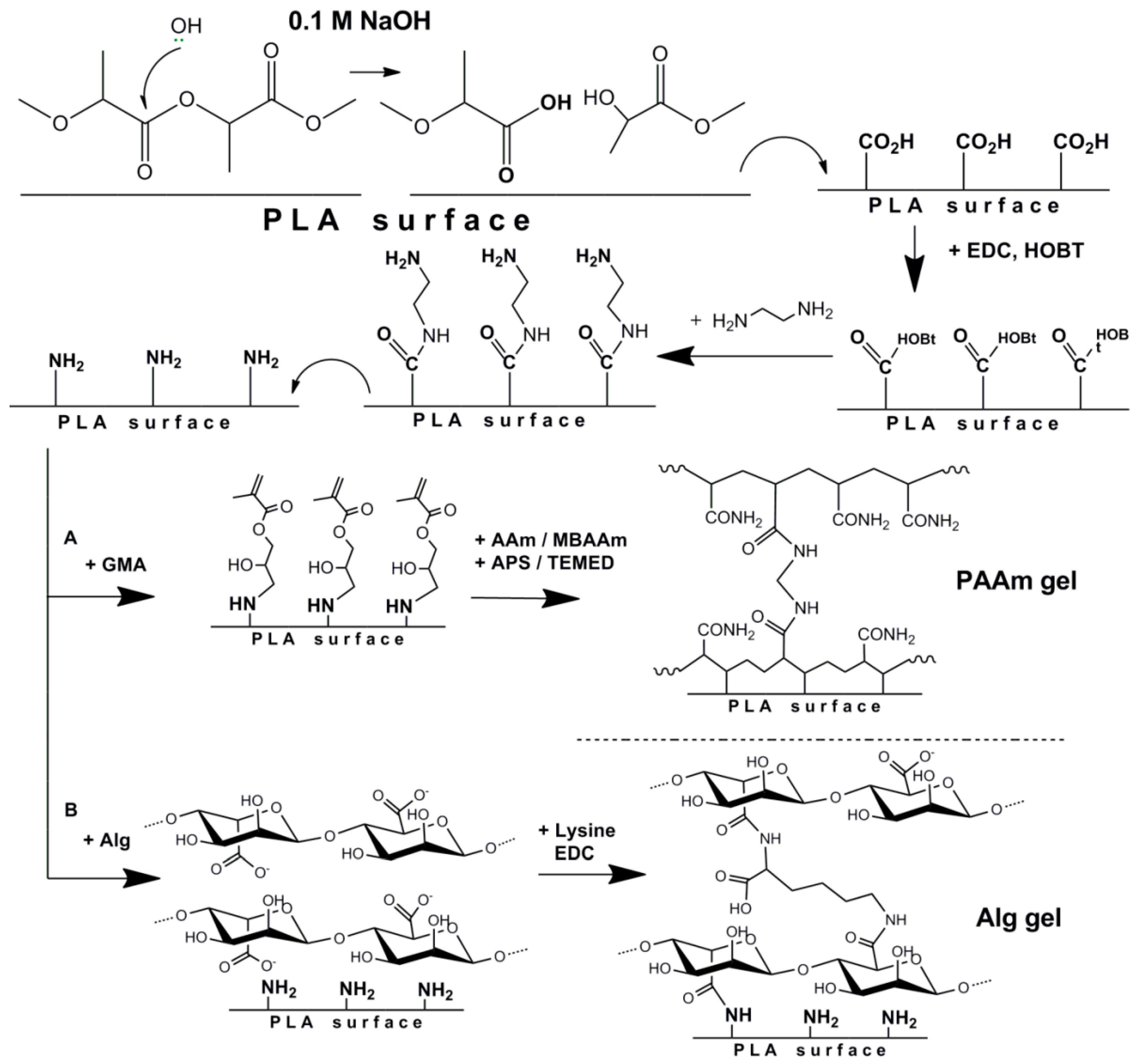
2.2.2. Preparation of Hydrogel Coated PLA-Based Films
Formation of Poly(acrylamide) (PAAm) Gel on the Surface of PLA Films
Formation of Alginate (Alg) Gel on the Surface of PLA Films
2.2.3. Materials Characterization
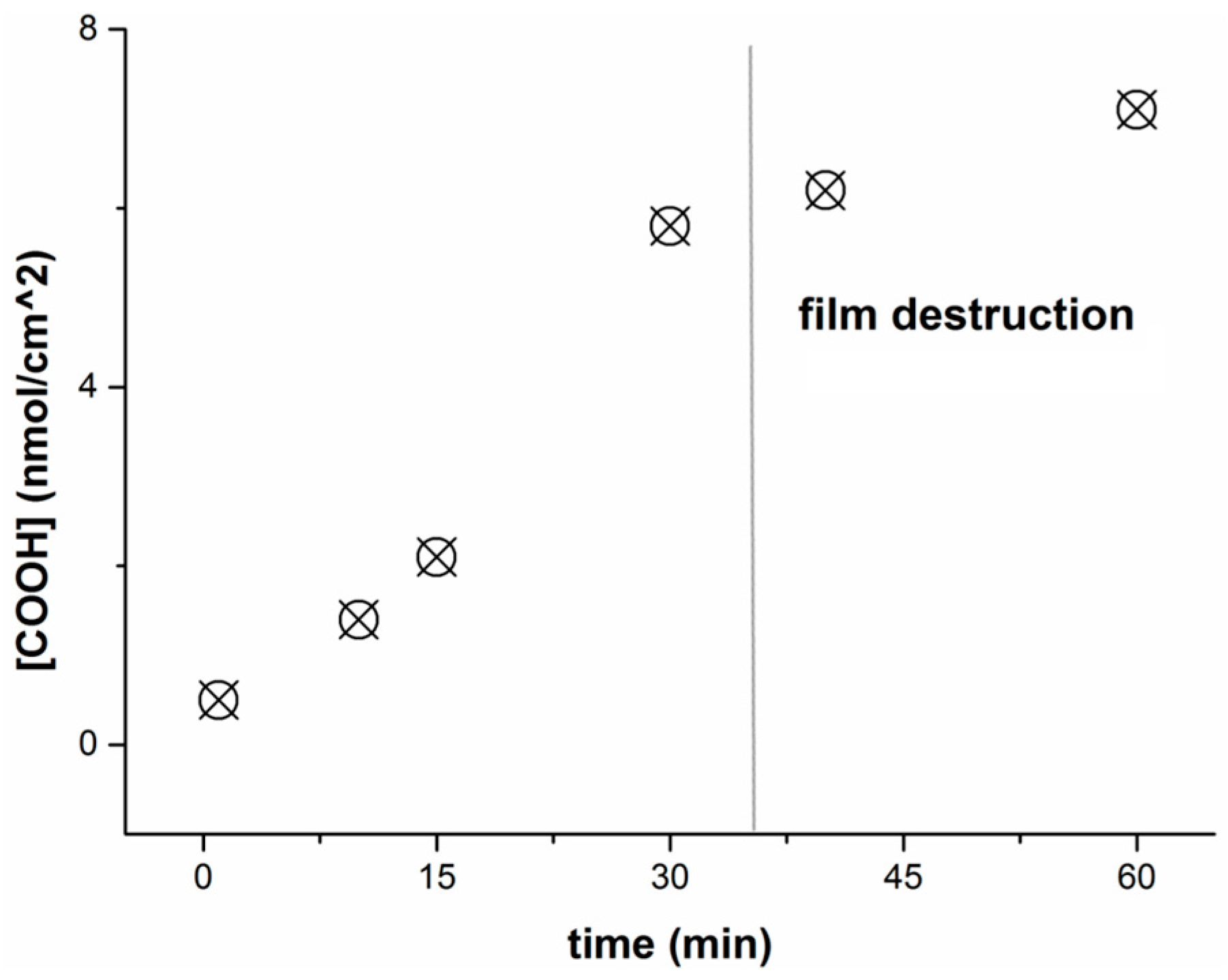
Determination of Carboxylic Group Concentration on the PLA-Based Film Surface
Determination of Amino Groups Concentration on the Surface of PLA-Based Film Surface
Film Thickness
Swelling
Scanning Electron Microscopy (SEM)
Contact Angle
Atomic Force Microscopy (AFM)
2.2.4. Protein Adsorption
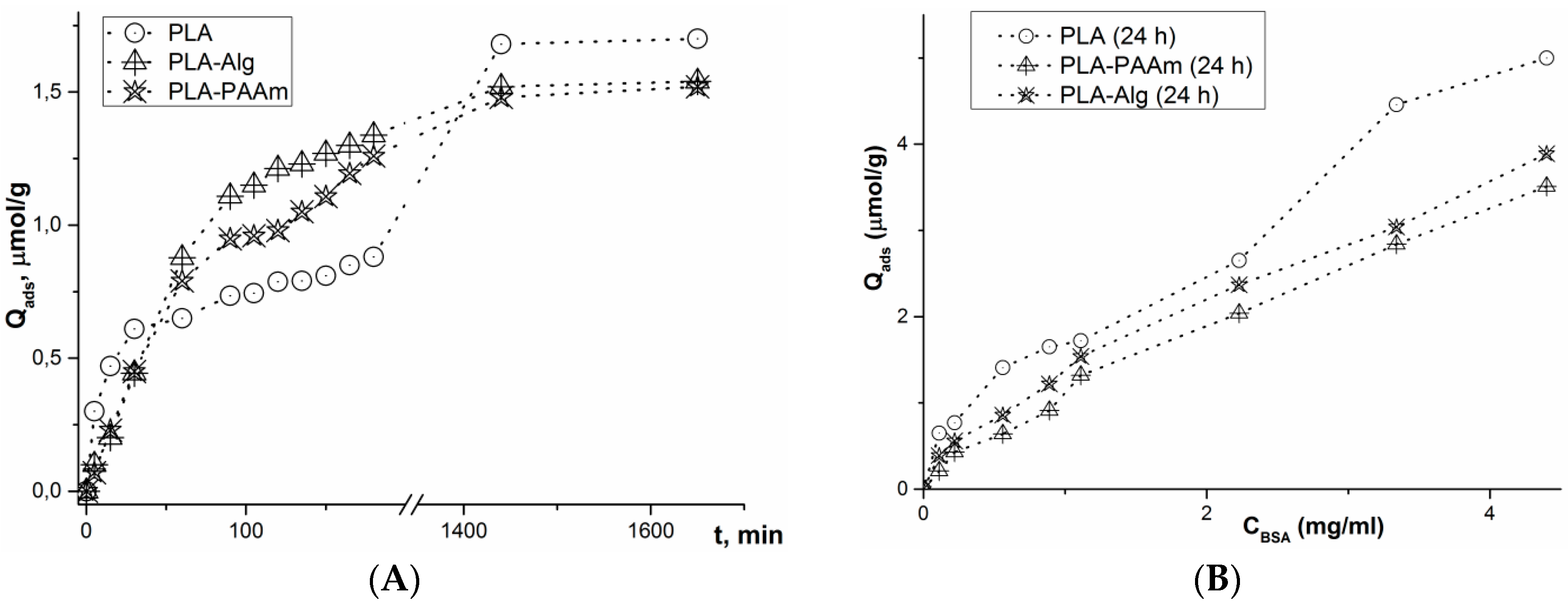
Kinetic Studies
Isotherm Building
2.2.5. Study of Protein Loading and Controlled Release
2.2.6. Hydrolysis of PLA-PAAm
2.2.7. Modification of Hydrogel Surface with Glycine
2.2.8. Cell Culture Experiments
Cells
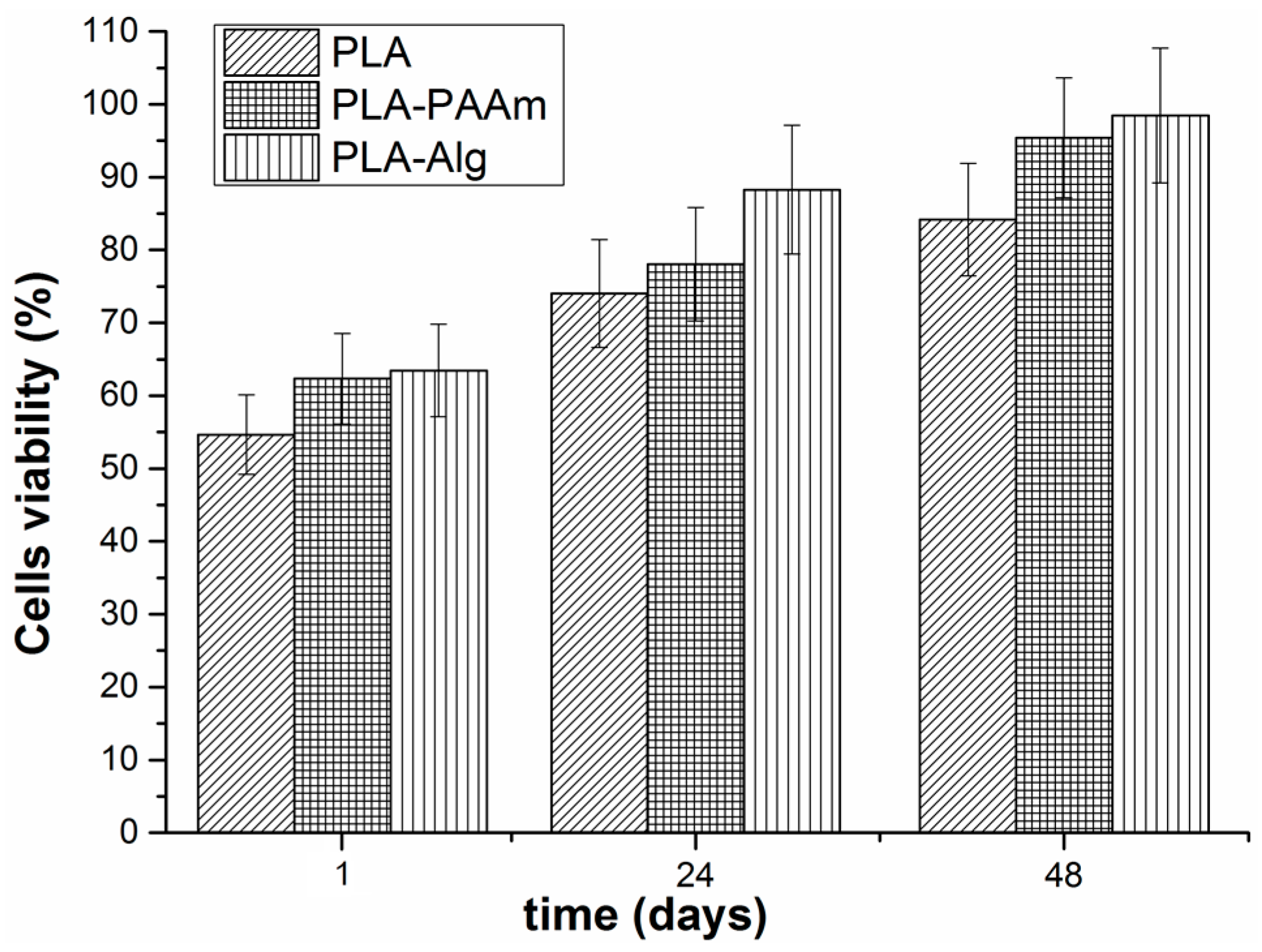
Evaluation of Cell Viability on the Surface of Modified Films
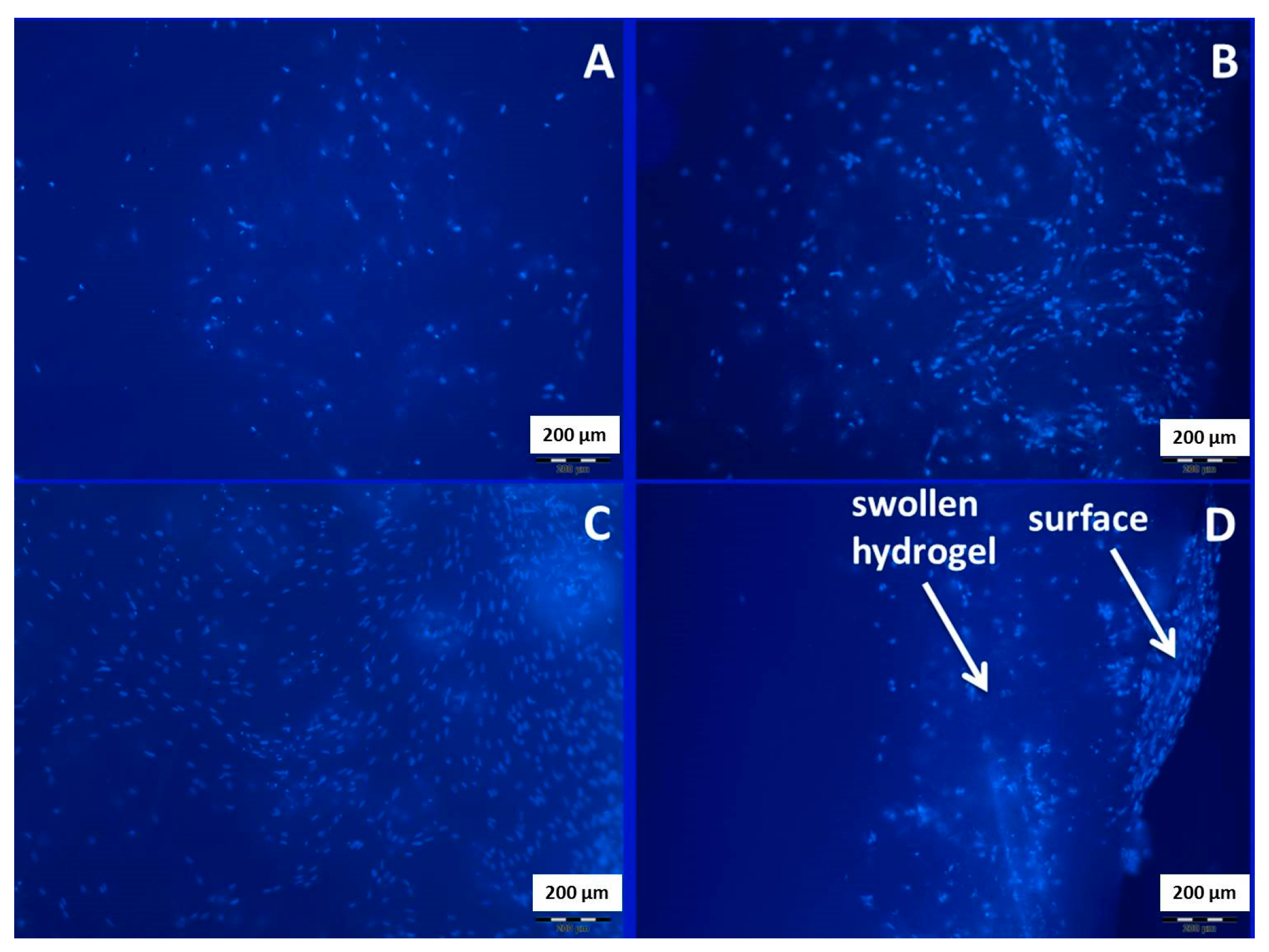
DAPI-Staining
Statistical Analysis
3. Results and Discussion
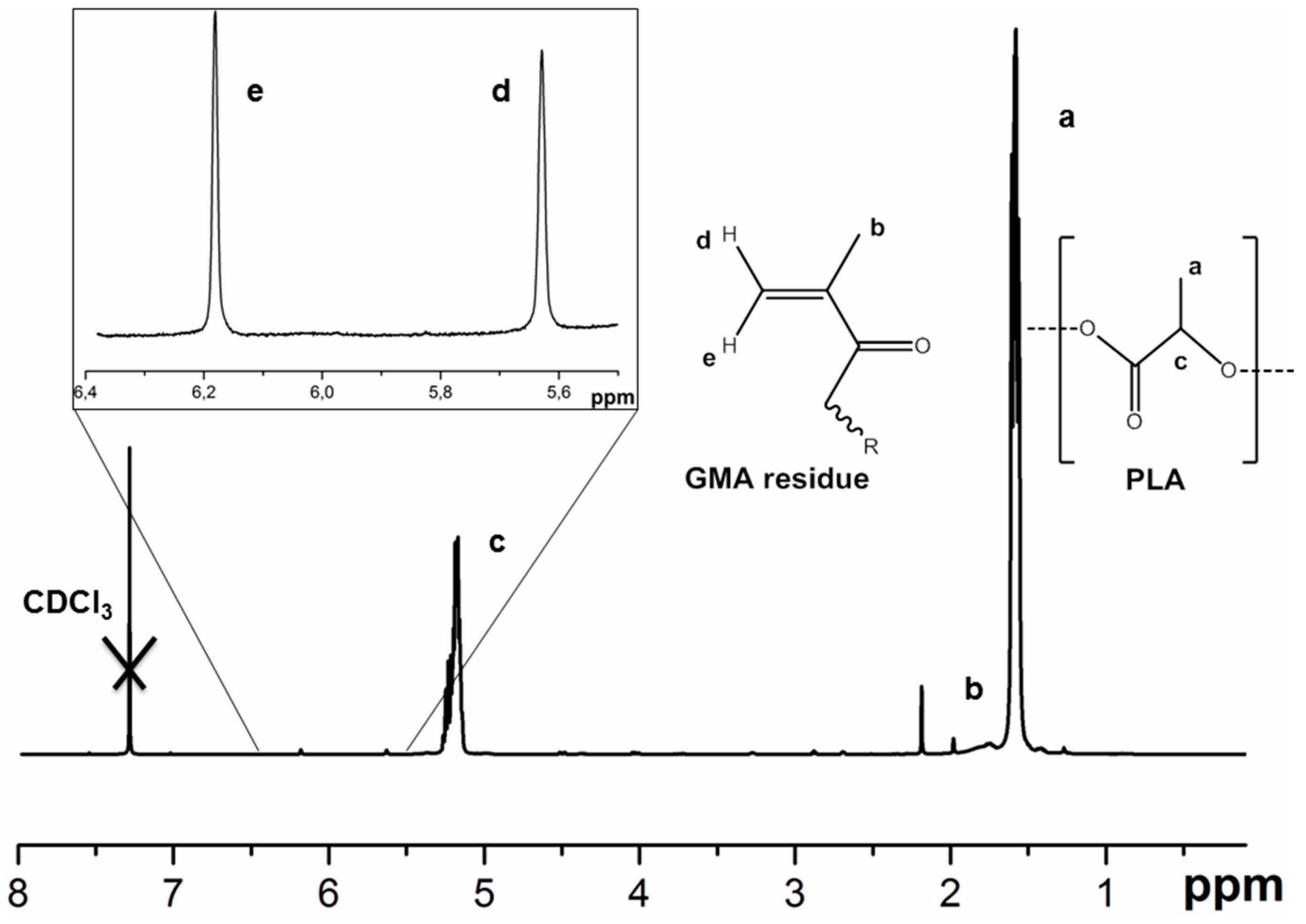
3.1. PLA Film Formation and Hydrogel
3.2. Gel Film Thickness and Swelling
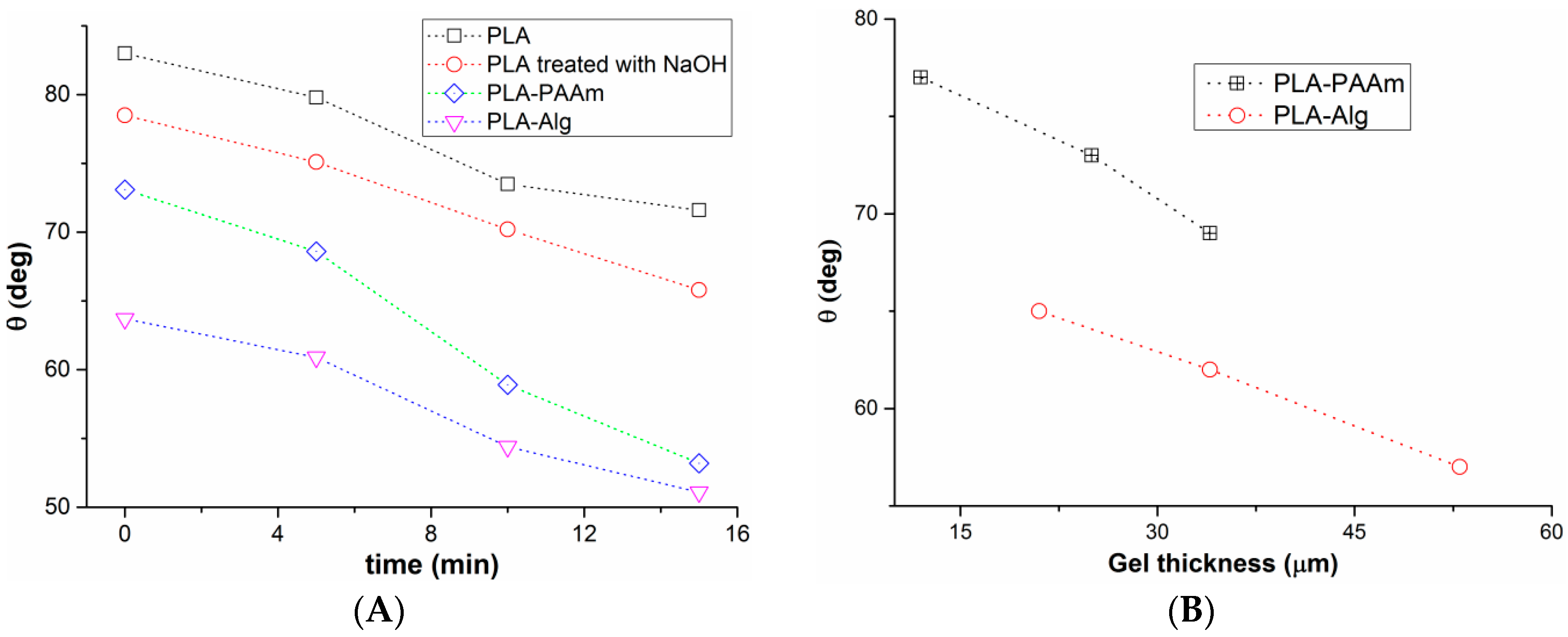
3.3. Film Wettability
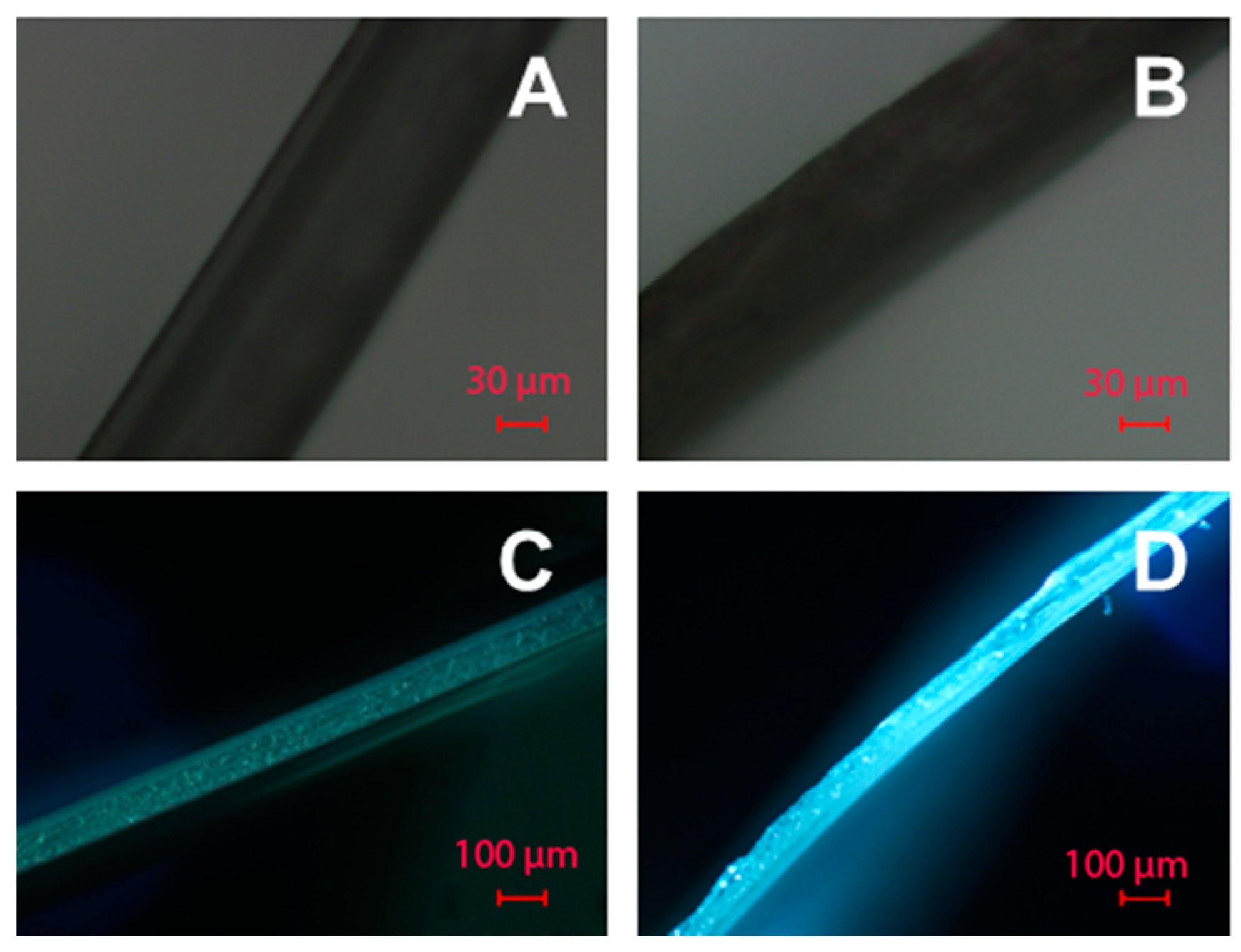
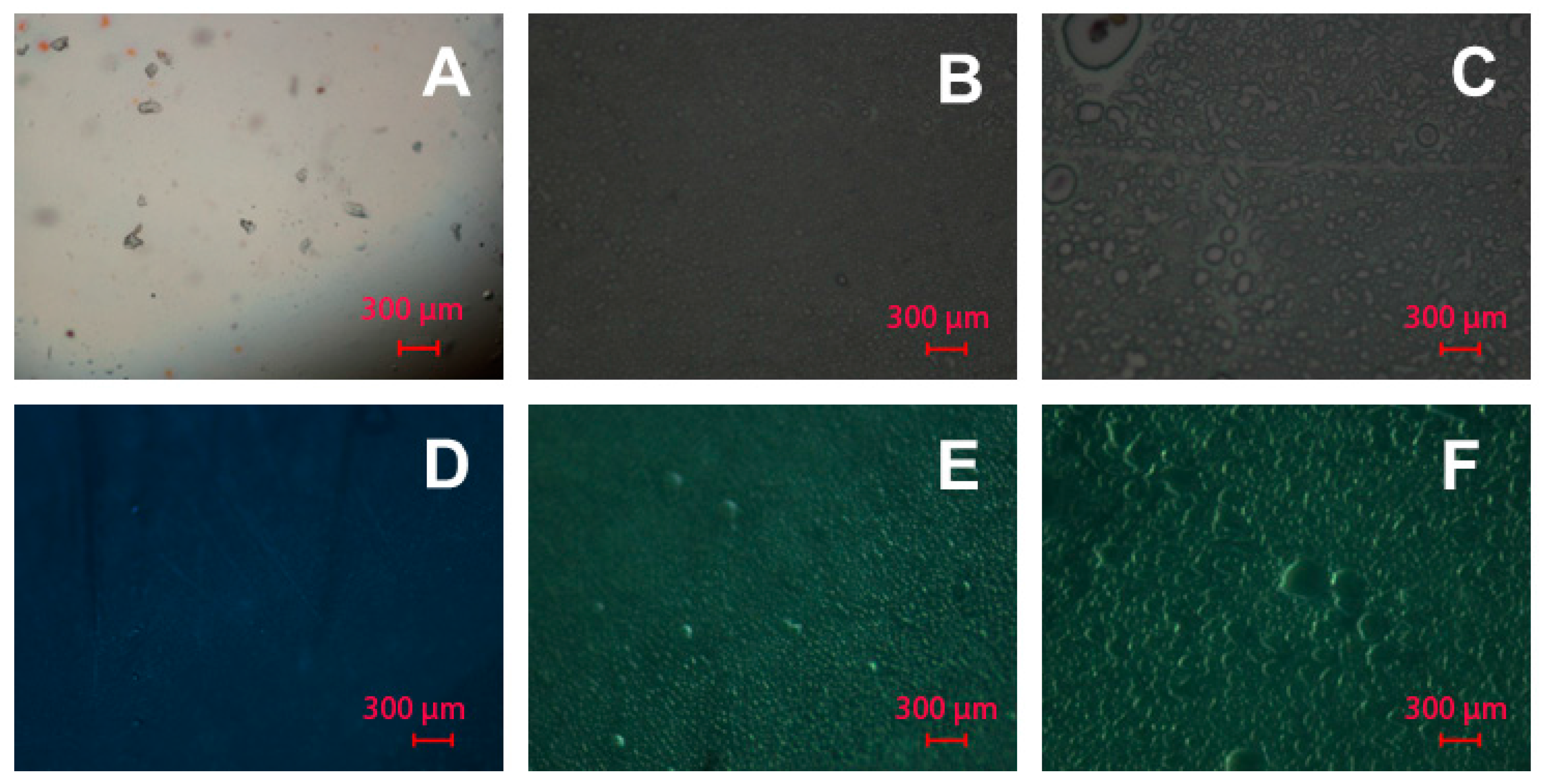
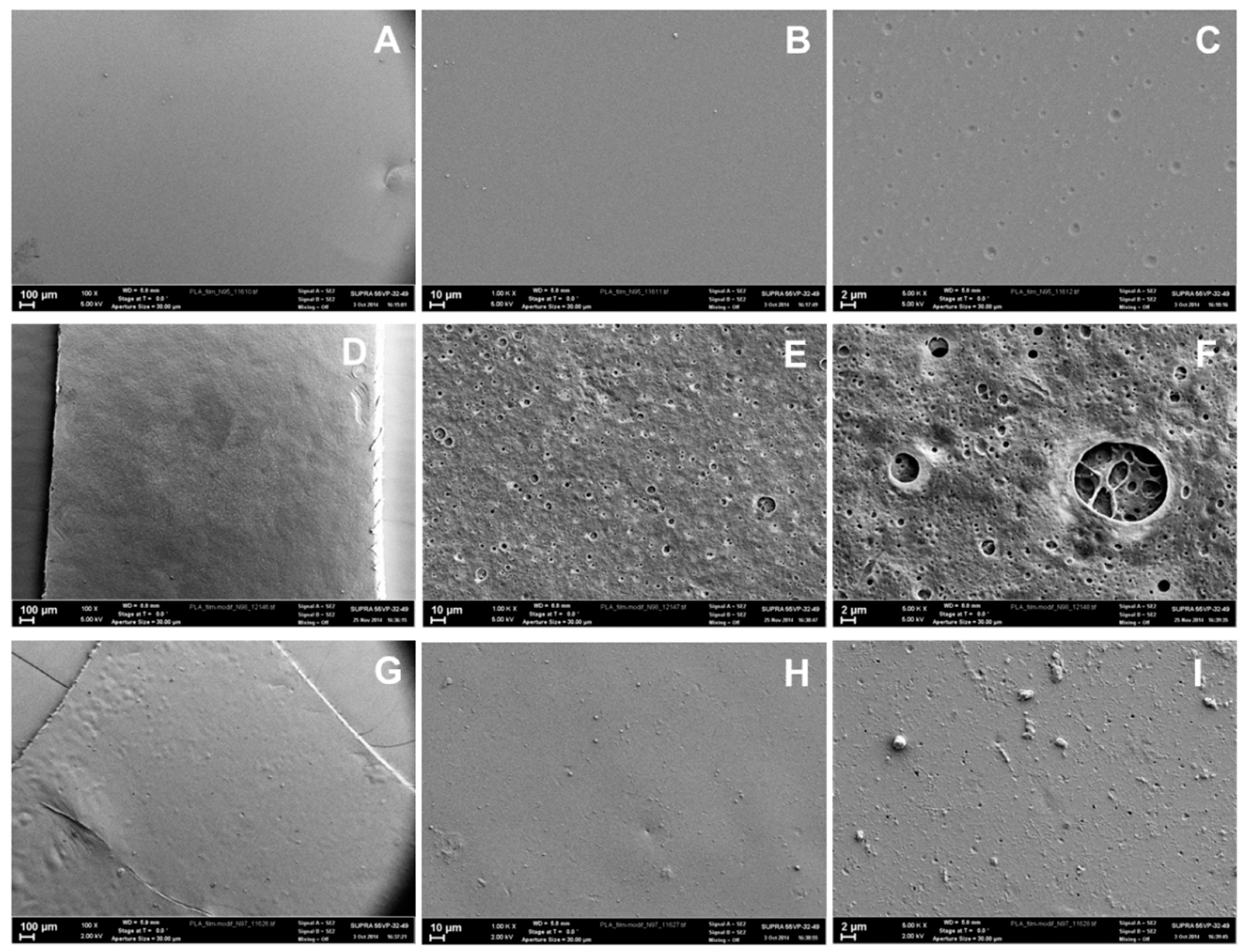
3.4. The Morphology of PAAm and Alg Gels
3.5. Adsorption of Model Protein (BSA)
3.6. Protein Release from Hydrogel Layers
3.7. Gel Modification with Model Bioligand
3.8. Cell Culture Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations:
| AAm | acrylamide |
| Alg | sodium alginate |
| APS | ammonium peroxodisulfate |
| BBS | borate buffered solution |
| BSA | bovine serum albumin |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EDA | ethylene diamine |
| GMA | glycidyl methacrylate |
| HOBT | 1-hydroxybenzotriazol |
| HELF | Human embryonic lung fibroblasts |
| HMSCs | Human mesenchymal stem cells |
| MBAAm | N,N′-methylene-bis-acrylamide |
| PLA | poly(lactic acid) |
| PLLA | poly(l-lactic acid) |
| PDLLA | poly(d,l-lactic acid) |
| PLA-PAAm | PLA-based film the surface of which is modified by PAAm |
| PLA-Alg | PLA-based film the surface of which is modified by Alg |
| SDS | sodium dodecyl sulfate |
| TNBS | 2,4,6-trinitrobenzenesulfonic acid |
| PBS | phosphate buffered solution |
References
- Chen, V.J.; Ma, P.X. Scaffolding in Tissue Engineering; Taylor & Francis: Milton, UK, 2006; pp. 125–138. [Google Scholar]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(lactic acid)-based biomaterials: Synthesis, modification and applications. In Biomedical Science, Engineering and Technology; InTechOpen: Rijeka, Croatia, 2006; pp. 247–282. [Google Scholar]
- Davachi, S.M.; Kaffashi, B. Polymer-plastics technology and engineering polylactic acid in medicine polylactic acid in medicine. Polym. Plast. Technol. Eng. 2015, 549, 944–967. [Google Scholar] [CrossRef]
- Gou, M.; Zheng, L.; Peng, X.; Men, K.; Zheng, X.; Zeng, S.; Guo, G.; Luo, F.; Zhao, X.; Chen, L.; et al. Poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) (PCL-PEG-PCL) nanoparticles for honokiol delivery in vitro. Int. J. Pharm. 2009, 375, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M. Nanoparticles based on PLGA and its co-polymer: An overview. Asian J. Pharm. 2009, 3, 266–273. [Google Scholar] [CrossRef]
- Bae, B.C.; Na, K. Development of polymeric cargo for delivery of photosensitizer in photodynamic therapy. Int. J. Photoenergy 2012, 2012, 431975. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Burg, K.J.; Holder, W.D.; Culberson, C.R.; Beiler, R.J.; Greene, K.G.; Loebsack, A.B.; Roland, W.D.; Mooney, D.J.; Halberstadt, C.R. Parameters affecting cellular adhesion to polylactide films. J. Biomater. Sci. Polym. Ed. 1999, 10, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.; Gander, B.; Blarer, N.; Merkle, H.P.; Subirá, M.L.; Irache, J.M.; Gamazo, C. In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. Eur. J. Pharm. Sci. 2002, 15, 197–207. [Google Scholar] [CrossRef]
- Palacio, J.; Agudelo, N.A.; Lopez, B.L. PEGylation of PLA nanoparticles to improve mucus-penetration and colloidal stability for oral delivery systems. Curr. Opin. Chem. Eng. 2016, 11, 14–19. [Google Scholar] [CrossRef]
- Ahsan, F. Targeting to macrophages: Role of physicochemical properties of particulate carriers—Liposomes and microspheres—On the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Mosqueira, V.C.F.; Legrand, P.; Gulik, A.; Bourdon, O.; Gref, R.; Labarre, D.; Barratt, G. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials 2001, 22, 2967–2979. [Google Scholar] [CrossRef]
- Ilhm, L.; Jacobsen, O.; Miiller, R.H.; Mak, E.; Davis, S.S. Surface characteristics and and the interaction of colloidal particles with mouse peritoneal macrophages. Biomaterials 1986, 8, 113–117. [Google Scholar]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Griffith, L.G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Legrand, P.; Gref, R.; Heurtault, B.; Appel, M.; Barratt, G. Interactions between a macrophage cell line (J774A1) and surface-modified Poly(d,l-lactide) nanocapsules bearing poly(ethylene glycol). J. Drug Target. 1999, 7, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Atthoff, B.; Hilborn, J. Protein adsorption onto polyester surfaces: Is there a need for surface activation? J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Quirk, R.A.; Chan, W.C.; Davies, M.C.; Tendler, S.J.B.; Shakesheff, K.M. Poly(l-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid). Biomaterials 2001, 22, 865–872. [Google Scholar] [CrossRef]
- Lin, C.-C.; Fu, S.-J. Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. Self-assembly of poly(ethylene glycol)-based block copolymers for biomedical applications. Curr. Opin. Colloid Interface Sci. 2001, 6, 3–10. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, A.; Lin, R.; Gao, C.; Feng, L.; Shen, J. Surface engineering of poly(d,l-lactic acid) by entrapment of chitosan-based derivatives for the promotion of chondrogenesis. J. Biomed. Mater. Res. 2002, 62, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Quirk, R.A.; Davies, M.C.; Tendler, S.J.B.; Shakesheff, K.M. Surface engineering of poly(lactic acid) by entrapment of modifying species. Macromolecules 2000, 33, 258–260. [Google Scholar] [CrossRef]
- Quirk, R.A.; Davies, M.C.; Tendler, S.J.B.; Chan, W.C.; Shakesheff, K.M. Controlling biological interactions with poly(lactic acid) by surface entrapment modification. Langmuir 2001, 17, 2817–2820. [Google Scholar] [CrossRef]
- Irvine, D.J.; Ruzette, A.V.G.; Mayes, A.M.; Griffith, L.G. Nanoscale clustering of RGD peptides at surfaces using comb polymers. 2. Surface segregation of comb polymers in polylactide. Biomacromolecules 2001, 2, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Bertóti, I.; Vargha-Butler, E.I. XPS and wettability characterization of modified poly(lactic acid) and poly(lactic/glycolic acid) films. J. Colloid Interface Sci. 2002, 245, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, T.; Nakayama, K.; Tsujisaka, T.; Mas, A.; Schue, F. Plasma surface treatments of melt-extruded sheets of poly(l-lactic acid). Polym. Eng. Sci. 2002, 42, 299–306. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (d,l-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.L.; Chapman-Sheath, P.J.; Walsh, W.R. Surface modification of poly (d,l-lactic acid) with chitosan and its effects on the culture of osteoblasts in vitro. J. Biomed. Mater. Res. 2002, 60, 398–404. [Google Scholar]
- Janorkar, A.V.; Proulx, S.E.; Metters, A.T.; Hirt, D.E. Surface-confined photopolymerization of single- and mixed-monomer systems to tailor the wettability of poly(l-lactide) film. J. Polym. Sci. A Polym. Chem. 2006, 44, 6534–6543. [Google Scholar] [CrossRef]
- Källrot, M.; Edlund, U.; Albertsson, A.C. Covalent grafting of poly(l-lactide) to tune the in vitro degradation rate. Biomacromolecules 2007, 8, 2492–2496. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Jeevitha, K.; Nivedhitha Sundaram, M.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid hydrogel coated poly(caprolactone) multiscale bilayer scaffold for ligament regeneration. Chem. Eng. J. 2015, 260, 478–485. [Google Scholar] [CrossRef]
- Arnal-Pastor, M.; Pérez-Garnes, M.; Monleón Pradas, M.; Vallés Lluch, A. Topologically controlled hyaluronan-based gel coatings of hydrophobic grid-like scaffolds to modulate drug delivery. Colloids Surf. B Biointerfaces 2016, 140, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; León, I.E.; Gonzalez, J.S.; Porto, L.M.; Alvarez, V.A.; Castro, G.R. Modified bacterial cellulose scaffolds for localized doxorubicin release in human colorectal HT-29 cells. Colloids Surf. B Biointerfaces 2016, 140, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Lee, H.; Yang, G.H.; Choi, C.H.; Lee, D.; Hwang, H.; Jung, W.K.; Yoon, H.; Kim, G.H. Mechanically reinforced cell-laden scaffolds formed using alginate-based bioink printed onto the surface of a PCL/alginate mesh structure for regeneration of hard tissue. J. Colloid Interface Sci. 2016, 461, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Goujon, L.J.; Hariharan, S.; Sayyar, B.; Burke, N.A.D.; Cranston, E.D.; Andrews, D.W.; Stöver, H.D.H. Tunable hydrogel thin films from reactive synthetic polymers as potential two-dimensional cell scaffolds. Langmuir 2015, 31, 5623–5632. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.A.; Collins, M.N. Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym. Compos. 2016. [Google Scholar] [CrossRef]
- Korzhikov, V.A.; Gusevskaya, K.V.; Litvinchuk, E.N.; Vlakh, E.G.; Tennikova, T.B. Enzyme-mediated ring-opening polymerization of pentadecalactone to obtain biodegradable polymer for fabrication of scaffolds for bone tissue engineering. Int. J. Polym. Sci. 2013, 2013, 476748. [Google Scholar] [CrossRef]
- Korzhikov, V.; Averianov, I.; Litvinchuk, E.; Tennikova, T.B. Polyester-based microparticles of different hydrophobicity: The patterns of lipophilic drug entrapment and release. J. Microencapsul. 2016, 33, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Korzhikov, V.A.; Tennikova, T.B. Synthesis of poly(lactic acid) and the formation of poly(lactic acid)-based supraporous biofunctional materials for tissue engineering. Polym. Sci. Ser. B 2015, 57, 336–348. [Google Scholar] [CrossRef]
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.J.S.C.; Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Protein Sci. 2007. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.J.; Jones, R.A.L.; Sferrazza, M. Adsorption and displacement of a globular protein on hydrophilic and hydrophobic surfaces. Colloids Surf. B Biointerfaces 2002, 23, 31–42. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ishizuya, T.; Kintou, N.; Wada, Y.; Katagiri, T.; Wozney, J.M.; Rosen, V.; Yoshiki, S. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem. Biophys. Res. Commun. 1996, 220, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chayosumrit, M.; Tuch, B.; Sidhu, K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials 2010, 31, 505–514. [Google Scholar] [CrossRef] [PubMed]
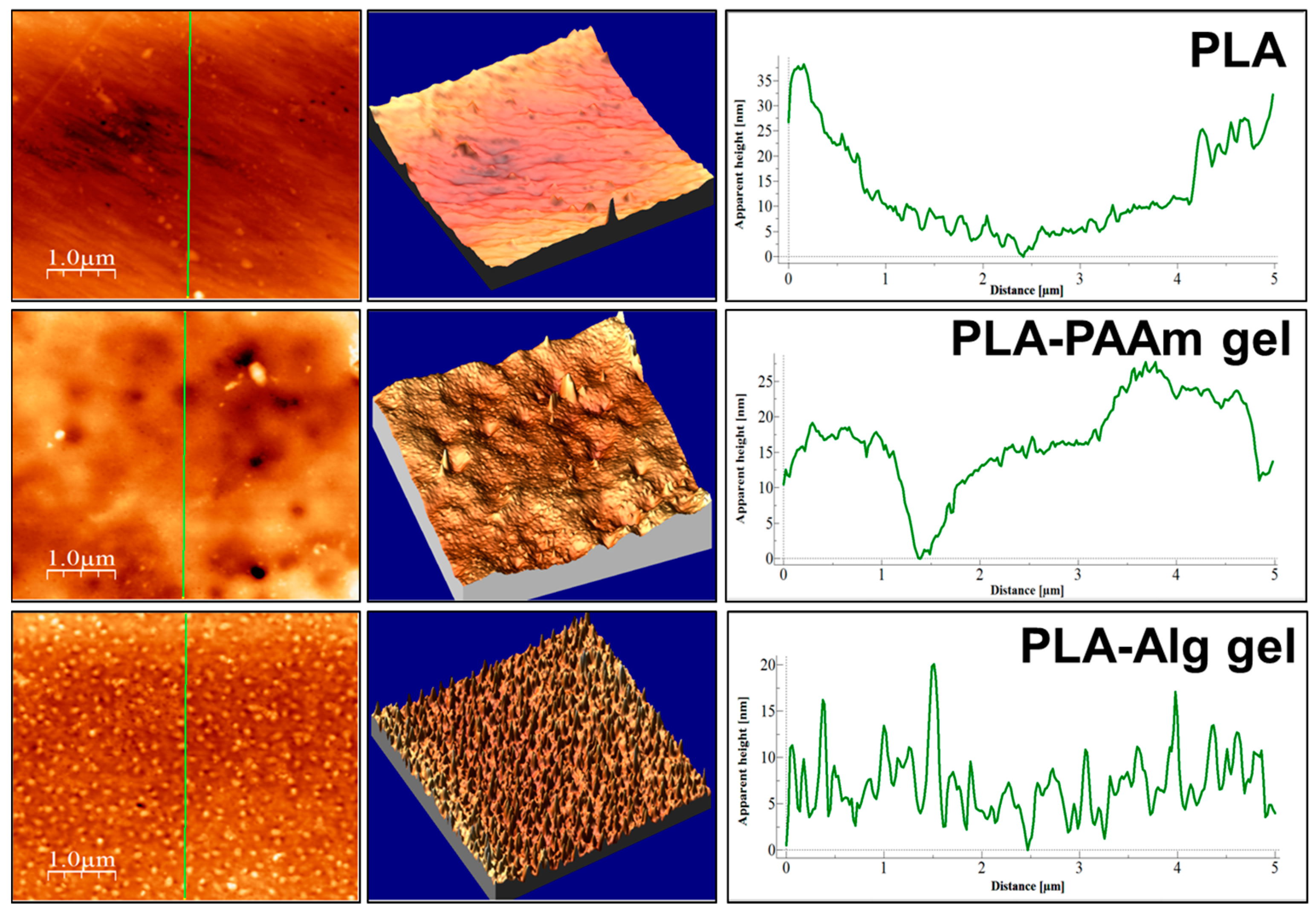

| Initial PLA film | |||||
| Sample | Polymer concentration, wt % | Initial film thickness, µm | Swelling, % | ||
| 1 | 5.0 | 108 ± 32 | 4 ± 0.6 | ||
| PLA film with PAAm gel | |||||
| Sample | Monomers concentration, wt % | [AAm]/[MBAAm], mol/mol | Entire film thickness, µm | Gel layer thickness, µm | Swelling, % |
| 2 | 1.0 | 6 | 122 ± 37 | 12 ± 4 | 28 ± 4.2 |
| 3 | 3.0 | 6 | 135 ± 41 | 25 ± 8 | 30 ± 4.5 |
| 4 | 5.0 | 6 | 147 ± 44 | 34 ± 10 | 33 ± 5.0 |
| 5 | 3.0 | 4 | 141 ± 42 | 29 ± 9 | 35 ± 5.3 |
| 6 | 3.0 | 2 | 129 ± 32 | 20 ± 6 | 27 ± 4.1 |
| PLA film with Alg gel | |||||
| Sample | Alginate concentration, wt % | Cross-linker concentration (lysine), wt % | Entire film thickness, µm | Gel layer thickness, µm | Swelling, % |
| 7 | 0.10 | 0.10 | 132 ± 40 | 21 ± 6 | 12 ± 1.8 |
| 8 | 0.25 | 0.10 | 143 ± 43 | 34 ± 10 | 26 ± 3.9 |
| 9 | 0.50 | 0.10 | 162 ± 49 | 53 ± 16 | 38 ± 5.7 |
| 10 | 0.25 | 0.05 | 138 ± 41 | 26 ± 8 | 32 ± 4.8 |
| 11 | 0.25 | 2.00 | 149 ± 45 | 38 ± 11 | 41 ± 6.2 |
| Calculated parameter | Pure PLA | PLA-PAAm | PLA-Alg |
|---|---|---|---|
| Sr, µm2 | 1550 | 2134 | 3475 |
| Sr/S0 | 62 | 85 | 139 |
| Sample | qinit(Gly), (mg) | qfin(Gly), (mg) | qcoupled(Gly), (mg) | q(–COOH), (μmol) |
|---|---|---|---|---|
| PLA | 0.33 | 0.27 | 0.06 | 0.80 |
| PLA-PAAm | 0.33 | 0.18 | 0.15 | 2.00 |
| PLA-Alg | 0.33 | 0.09 | 0.24 | 3.24 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzhikov-Vlakh, V.; Krylova, M.; Sinitsyna, E.; Ivankova, E.; Averianov, I.; Tennikova, T.B. Hydrogel Layers on the Surface of Polyester-Based Materials for Improvement of Their Biointeractions and Controlled Release of Proteins. Polymers 2016, 8, 418. https://doi.org/10.3390/polym8120418
Korzhikov-Vlakh V, Krylova M, Sinitsyna E, Ivankova E, Averianov I, Tennikova TB. Hydrogel Layers on the Surface of Polyester-Based Materials for Improvement of Their Biointeractions and Controlled Release of Proteins. Polymers. 2016; 8(12):418. https://doi.org/10.3390/polym8120418
Chicago/Turabian StyleKorzhikov-Vlakh, Viktor, Maria Krylova, Ekaterina Sinitsyna, Elena Ivankova, Ilia Averianov, and Tatiana B. Tennikova. 2016. "Hydrogel Layers on the Surface of Polyester-Based Materials for Improvement of Their Biointeractions and Controlled Release of Proteins" Polymers 8, no. 12: 418. https://doi.org/10.3390/polym8120418







