An Investigation of the High Performance of a Novel Type of Benzobisoxazole Fiber Based on 3,3-Diaminobenzidine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
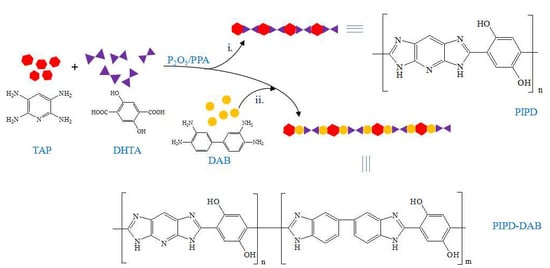
2.2. Copolymerization of PIPD-DAB
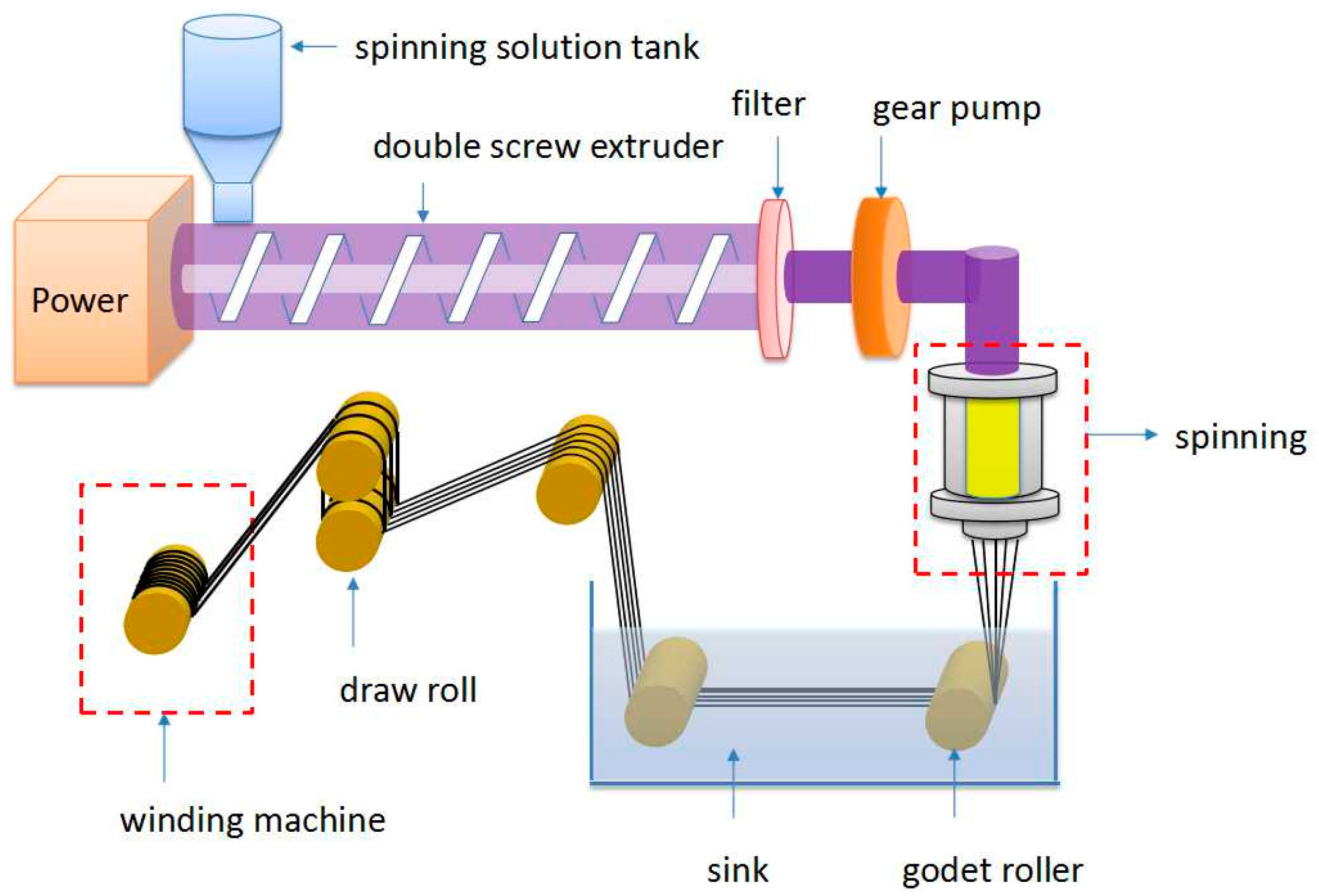
2.3. Spinning Technology
2.4. Characterizations and Measurements
3. Results and Discussion
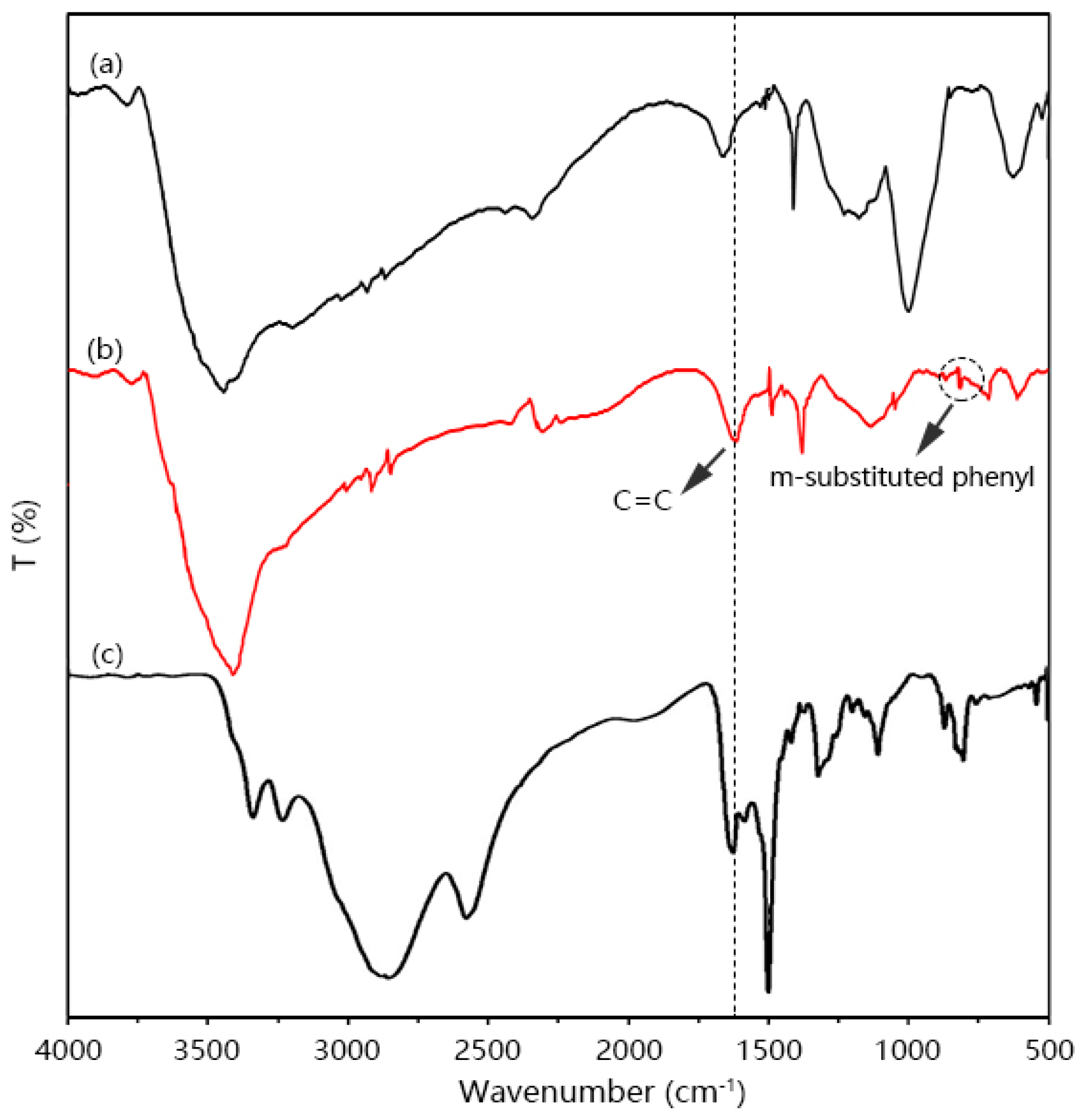
3.1. Synthesis and Characterization of DAB
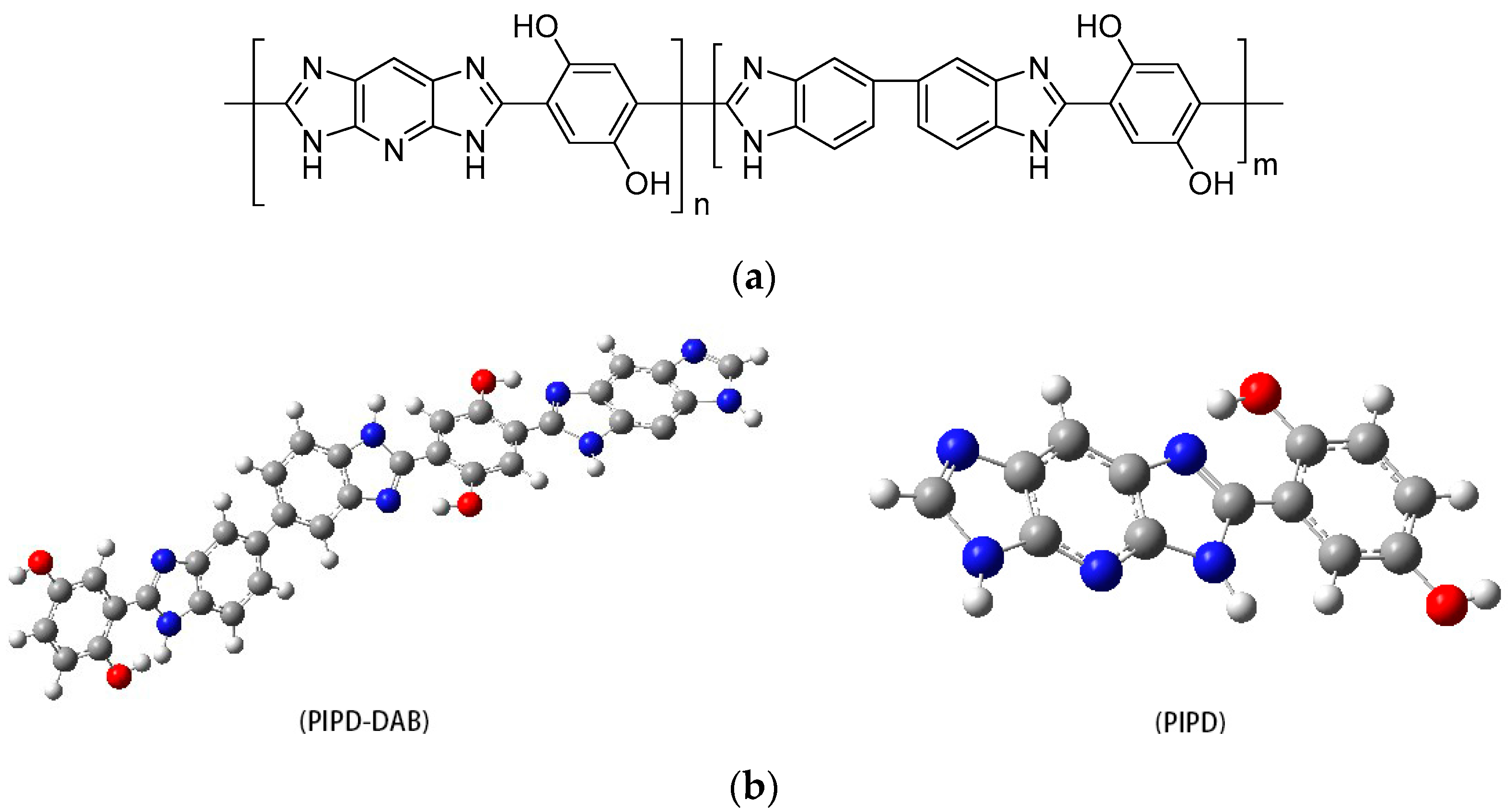
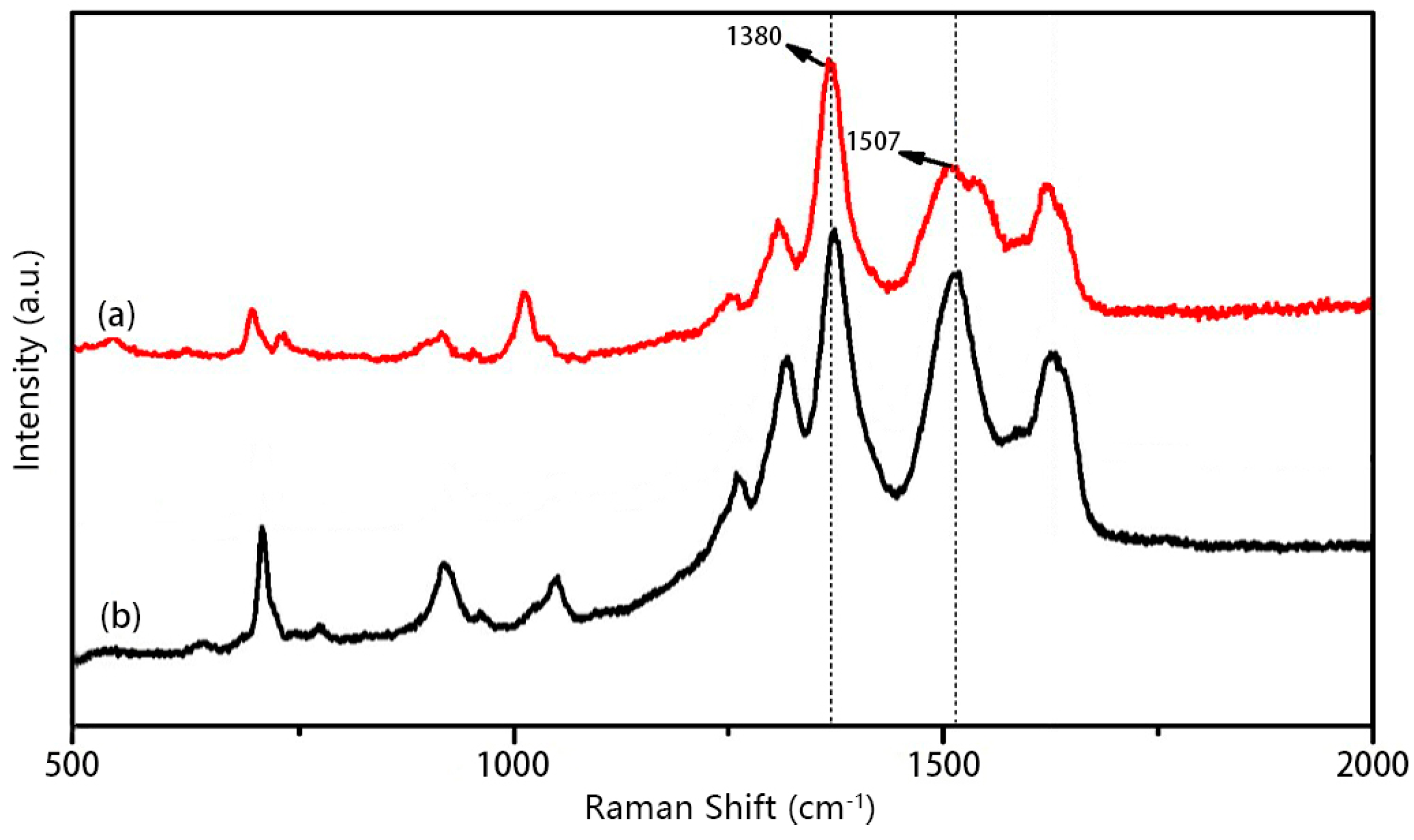
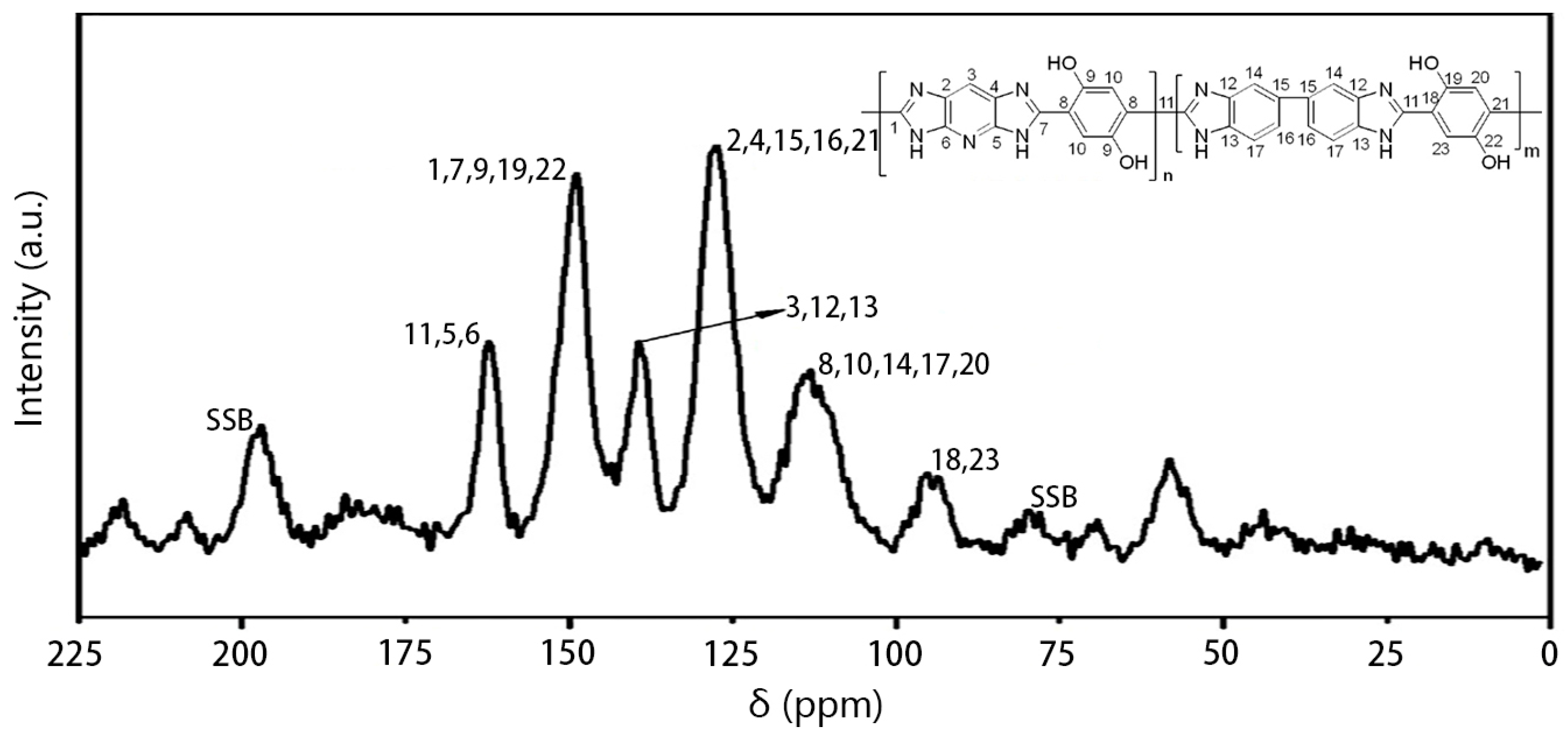
3.2. Structural Characterization of PIPD-DAB Copolymers
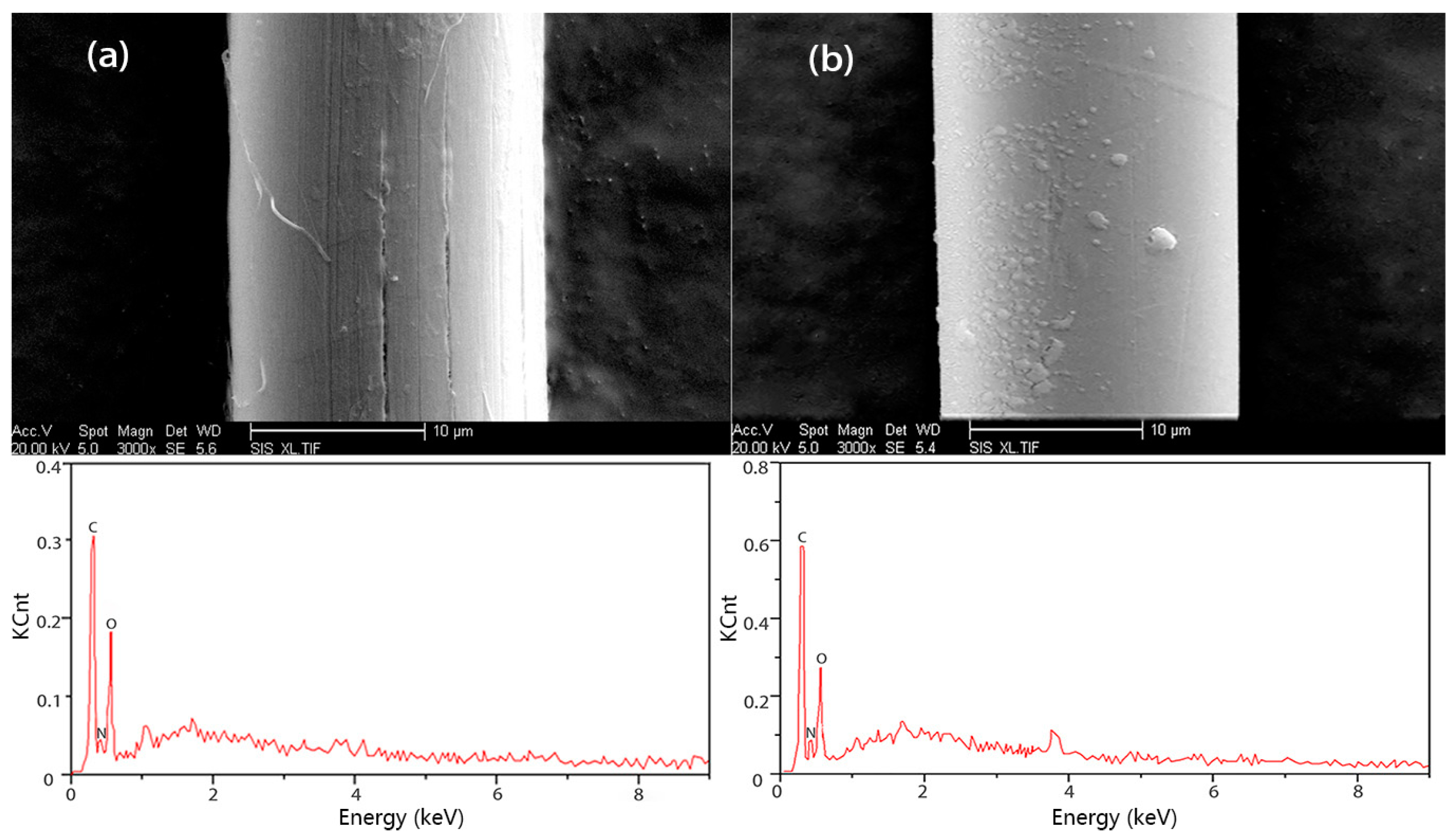
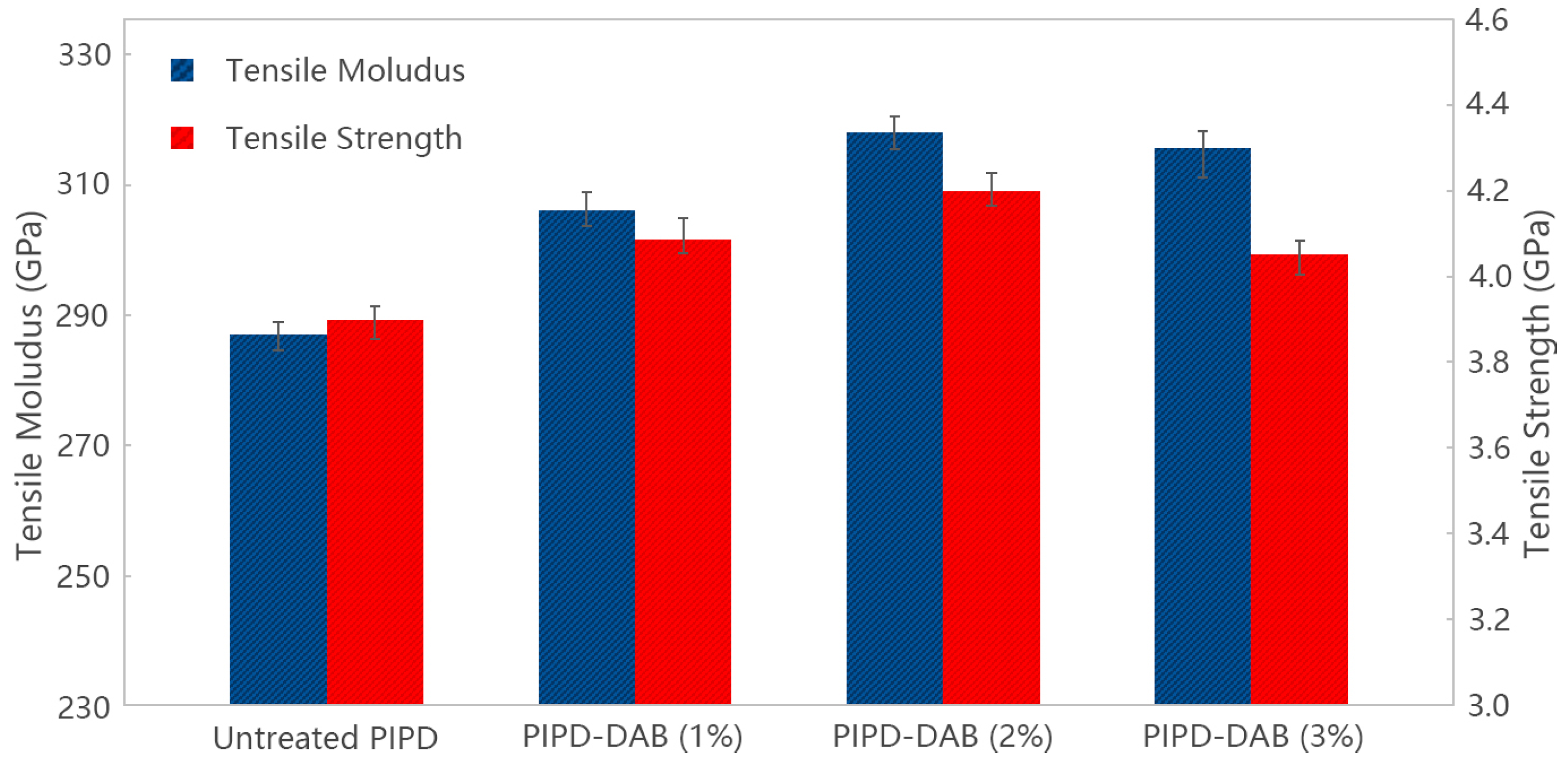
3.3. Characterization and Properties of PIPD-DAB Copolymer Fibers
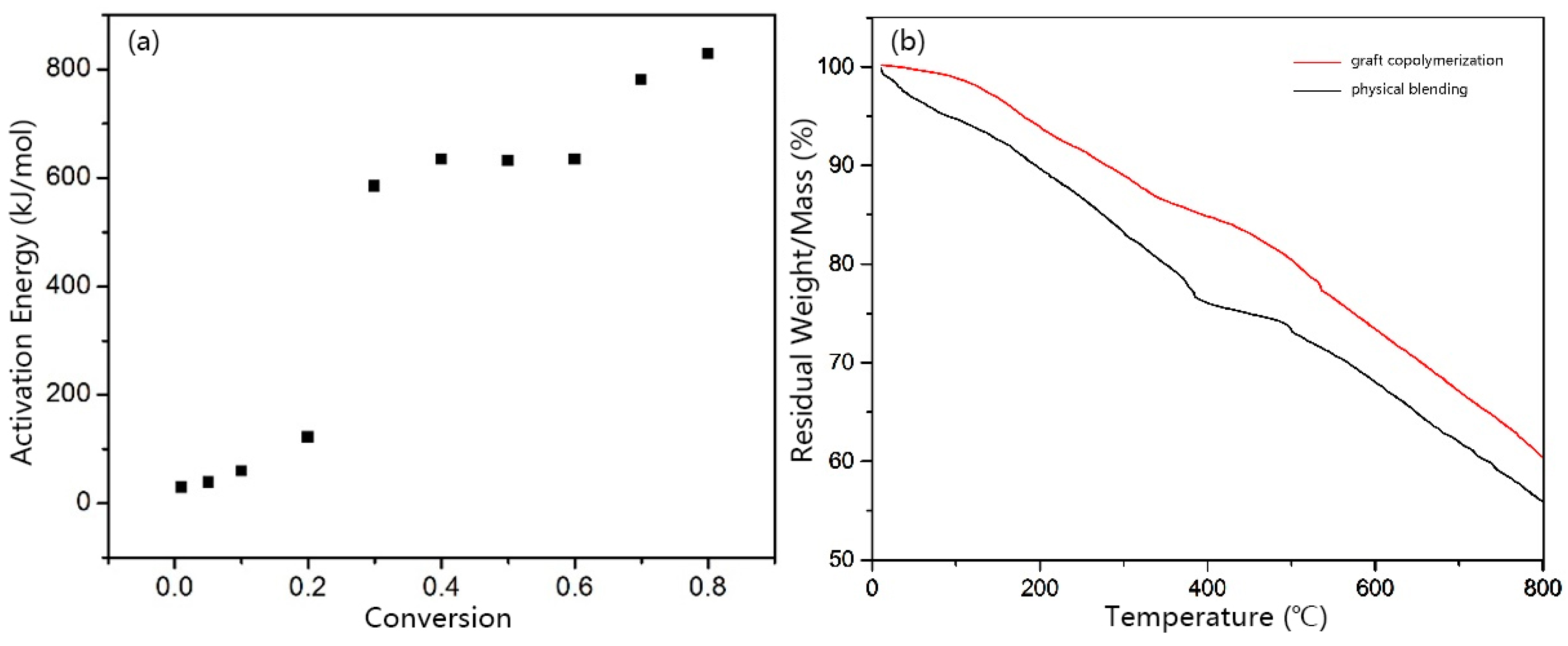
3.4. Thermal Degradation Reaction Kinetics of PIPD Fiber Based on DAB
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jozsef, K.K.; Haroon, M.; Alessandro, P. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [Green Version]
- Hu, Z.; Li, J.; Tang, P.; Li, D.; Song, Y.; Li, Y.; Zhao, L.; Li, C.; Huang, Y. One-pot preparation and continuous spinning of carbon nanotube/poly(p-phenylene benzobisoxazole) copolymer fibers. J. Mater. Chem. 2012, 22, 19863–19871. [Google Scholar] [CrossRef]
- Klairutsamee, W.; Supaphol, P.; Jangchud, I. Electrospinnability of poly(butylene succinate): Effects of solvents and organic salt on the fiber size and morphology. J. Appl. Polym. Sci. 2015, 132, 42716–42726. [Google Scholar] [CrossRef]
- Garcia-Avila, M.; Portanova, M.; Rabiei, A. Ballistic Performance of Composite Metal Foams. Compos. Struct. 2014, 4, 151–156. [Google Scholar] [CrossRef]
- Leal, A.A.; Deitzel, J.M.; Mcknight, S.H.; Gillespie, J.W. Spectroscopic analysis and kinetics of intermolecular hydrogen bond formation in poly-pyridobisimidazole (M5) fiber. J. Polym. Sci. Part B 2009, 47, 1809–1824. [Google Scholar] [CrossRef]
- Northolt, M.G.; Sikkema, D.J.; Zegers, H.C.; Klop, E.A. PIPD, a new high-modulus and high-strength polymer fibre with exceptional fireprotection properties. Fire Mater. 2002, 26, 169–172. [Google Scholar] [CrossRef]
- Richard, K. Recent Advances in Polymer Fibers. Polym. Rev. 2008, 48, 221–229. [Google Scholar]
- Davies, R.J.; Eichhorn, S.J.; Riekel, C.; Young, R.J. Crystal lattice deformation in single poly(p-phenylene benzobisoxazole) fibres. Polymer 2004, 45, 7693–7704. [Google Scholar] [CrossRef]
- Hageman, J.C.L.; van der Horst, J.W.; Groot, R.A.D. An ab initio study of the structural and physical properties of a novel rigid-rod polymer: PIPD. Polymer 1999, 40, 1313–1323. [Google Scholar] [CrossRef]
- Chae, H.G.; Kumar, S. Materials science. Making strong fibers. Science 2008, 319, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Perepelkin, K.E. High-strength, high-modulus fibres based on linear polymers: Principles of production, structure, properties, use. Fibre Chem. 2010, 42, 79–87. [Google Scholar] [CrossRef]
- Volokhina, A.V.; Kiya-Oglu, V.N.; Lukasheva, N.V.; Sokira, A.N.; Pedchenko, N.V.; Poleeva, I.V. Synthesis of new aramid fiber. Polym. Sci. Ser. A 2010, 52, 1239–1243. [Google Scholar] [CrossRef]
- Leal, A.A.; Deitzel, J.M.; Mcknight, S.H.; Gillespie, J.W. Interfacial behavior of high performance organic fibers. Polymer 2009, 50, 1228–1235. [Google Scholar] [CrossRef]
- Hageman, J.; Wijs, G.A.D.; Groot, R.A.D.; Klop, E.A. The role of the hydrogen bonding network for the shear modulus of PIPD. Polymer 2005, 46, 9144–9154. [Google Scholar] [CrossRef]
- Zeng, S.Z.; Jin, N.Z.; Zhang, H.L.; Hai, B.; Chen, X.H. High-modulus all-carbon ladder polymer of hydroquinone and formaldehyde that bridges the gap between single-strand polymers and graphene nanoribbons. RSC Adv. 2014, 4, 18676–18682. [Google Scholar] [CrossRef]
- Yu, K.J.; Du, Z.J.; Li, H.Q.; Zhang, C. Preparation of rigid-rod poly{2,6-diimidazo[4,5-b:4′5′-e]pyridinylene-1,4(2,5-dihydroxy) phenylene} (PIPD) templated material. Mater. Lett. 2009, 63, 664–666. [Google Scholar] [CrossRef]
- Driscoll, C.P.; Helminiak, T.E. Process for the Production of Particulate Polymeric Material Having an Unusually High Surface Area. U.S. Patent US3987015, 19 October 1976. [Google Scholar]
- Lammers, M.; Klop, E.A.; Northolt, M.G.; Sikkema, D.J. Mechanical properties and structural transitions in the new rigid-rod polymer fibre PIPD (‘M5’) during the manufacturing process. Polymer 1998, 39, 5999–6005. [Google Scholar] [CrossRef]
- Takahashi, Y. Neutron structure analysis of poly(p-phenylenebenzobisthiazole). Int. J. Biol. Macromol. 2001, 34, 2012–2014. [Google Scholar] [CrossRef]
- Sirichaisit, J.; Young, R.J. Tensile and compressive deformation of polypyridobisimidazole (PIPD)-based ‘M5’ rigid-rod polymer fibres. Polymer 1999, 40, 3421–3431. [Google Scholar] [CrossRef]
- Farkas, E.; Csóka, H.; Gama, S.; Santos, M.A. Dihydroxamate based siderophore model, piperazine-1,4-bis-(N-methyl-acetohydroxamic acid (PIPDMAHA), as a chelating agent of molybdenum(VI). Talanta 2002, 57, 935–943. [Google Scholar] [CrossRef]
- Bourbigot, S.; Flambard, X.; Ferreira, M.; Devaux, E.; Poutch, F. Characterisation and reaction to fire of “M5” rigid rod polymer fibres. J. Mater. Sci. 2003, 38, 2187–2194. [Google Scholar] [CrossRef]
- Tan, L.S.; Arnold, F.E.; Dang, T.D.; Chuah, H.H.; Wei, K.H. Pseudo-ladder rigid-rod polymers: Dihydroxy pendent benzothiazole aromatic heterocyclic polymer and copolymers. Polymer 1994, 35, 3091–3101. [Google Scholar] [CrossRef]
- Druin, M.L.; Oringer, K. Synthesis of Pure 3,3′-Diaminobenzidine. U.S. Patent US3943175, 9 March 1976. [Google Scholar]
- Shukla, R.K.; Emmanuvel, L.; Rameshkumar, C. Reusable Transition Metal Complex Catalyst Useful for the Preparation of High Pure Quality 3,3′-Diaminobenzidine and Its Analogues and a Process Thereof. U.S. Patent US7999112, 16 August 2011. [Google Scholar]
- Wang, Y.H.; Hu, Z.; Meng, X.L.; Jing, J.H.; Song, Y.J.; Zhang, C.H.; Huang, Y.D. A simple and efficient route for preparing 2,3,5,6-tetraaminopyridine hydrochloride salt. Molecules 2009, 14, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Niu, H.Q.; Zhang, M.Y.; Ge, Q.Y.; Li, Y.; Wu, D.Z. Structures and properties of polyimide fibers containing ether units. J. Mater. Sci. 2015, 50, 4104–4114. [Google Scholar] [CrossRef]
- White, J.L.; Spruiell, J.E. The specification of orientation and its development in polymer processing. Polym. Eng. Sci. 1983, 23, 247–256. [Google Scholar] [CrossRef]
- Johnson, A.C.; Hayes, S.A.; Jones, F.R. The role of matrix cracks and fibre/matrix debonding on the stress transfer between fibre and matrix in a single fibre fragmentation test. Compos. Part A Appl. Sci. Manuf. 2012, 43, 65–72. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Song, Y.; Zhao, L.; Rahoui, N.; Jiang, B.; Huang, Y. An Investigation of the High Performance of a Novel Type of Benzobisoxazole Fiber Based on 3,3-Diaminobenzidine. Polymers 2016, 8, 420. https://doi.org/10.3390/polym8120420
Wang Y, Song Y, Zhao L, Rahoui N, Jiang B, Huang Y. An Investigation of the High Performance of a Novel Type of Benzobisoxazole Fiber Based on 3,3-Diaminobenzidine. Polymers. 2016; 8(12):420. https://doi.org/10.3390/polym8120420
Chicago/Turabian StyleWang, Yang, Yuanjun Song, Lei Zhao, Nahla Rahoui, Bo Jiang, and Yudong Huang. 2016. "An Investigation of the High Performance of a Novel Type of Benzobisoxazole Fiber Based on 3,3-Diaminobenzidine" Polymers 8, no. 12: 420. https://doi.org/10.3390/polym8120420