Real-Time Packing Behavior of Core-Shell Silica@Poly(N-isopropylacrylamide) Microspheres as Photonic Crystals for Visualizing in Thermal Sensing
Abstract
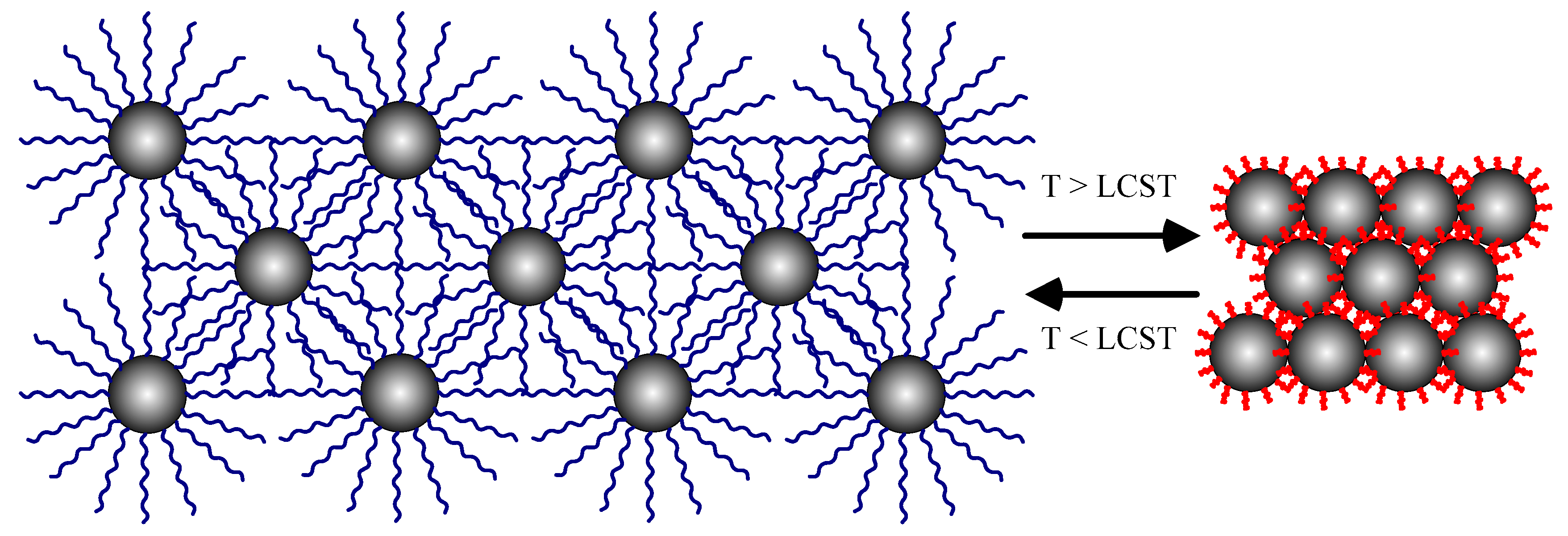
:1. Introduction
2. Experimental Section
2.1. Materials
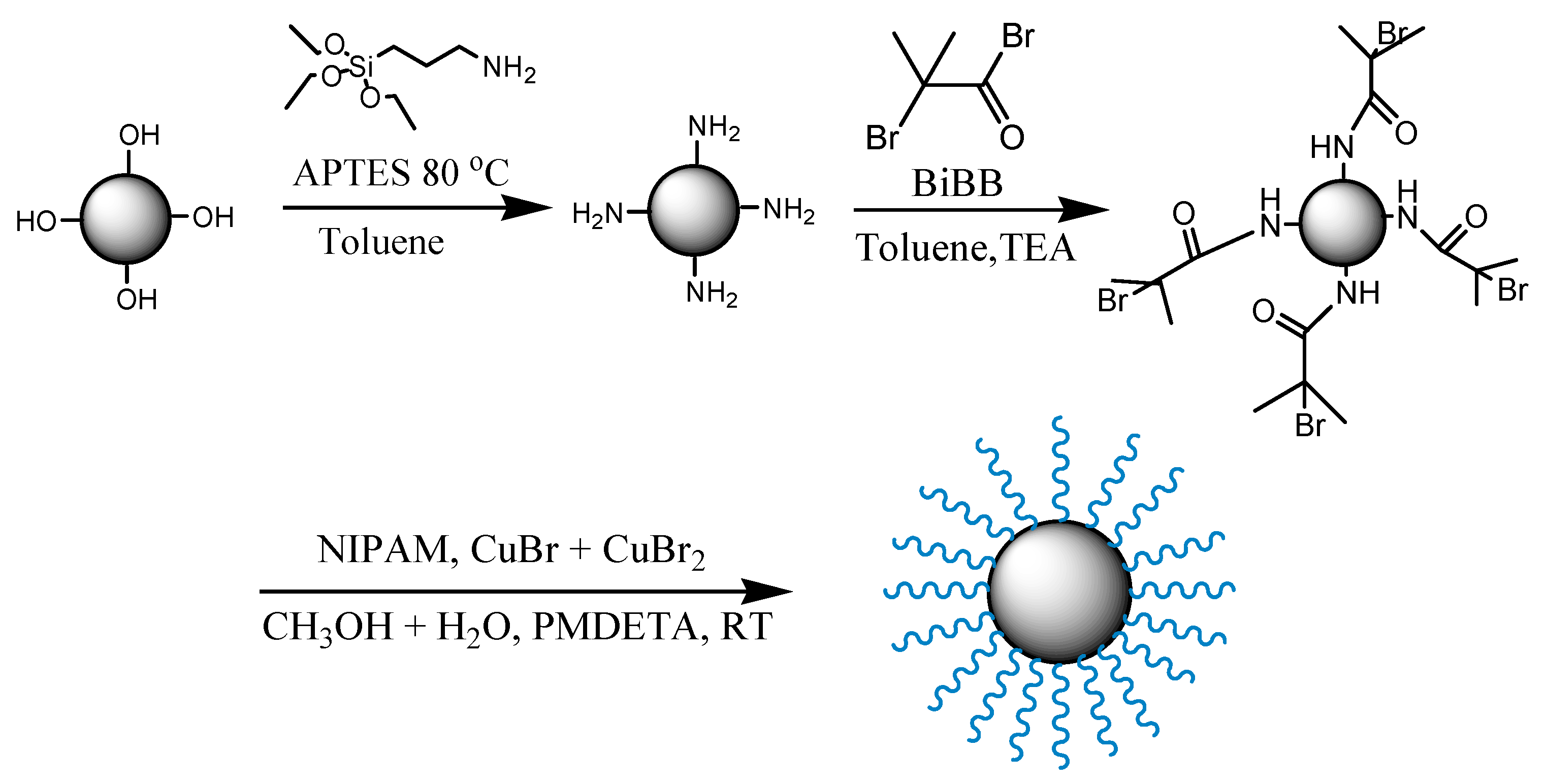
2.2. Synthesis of PNIPAM-Functionalized Silica Microparticle
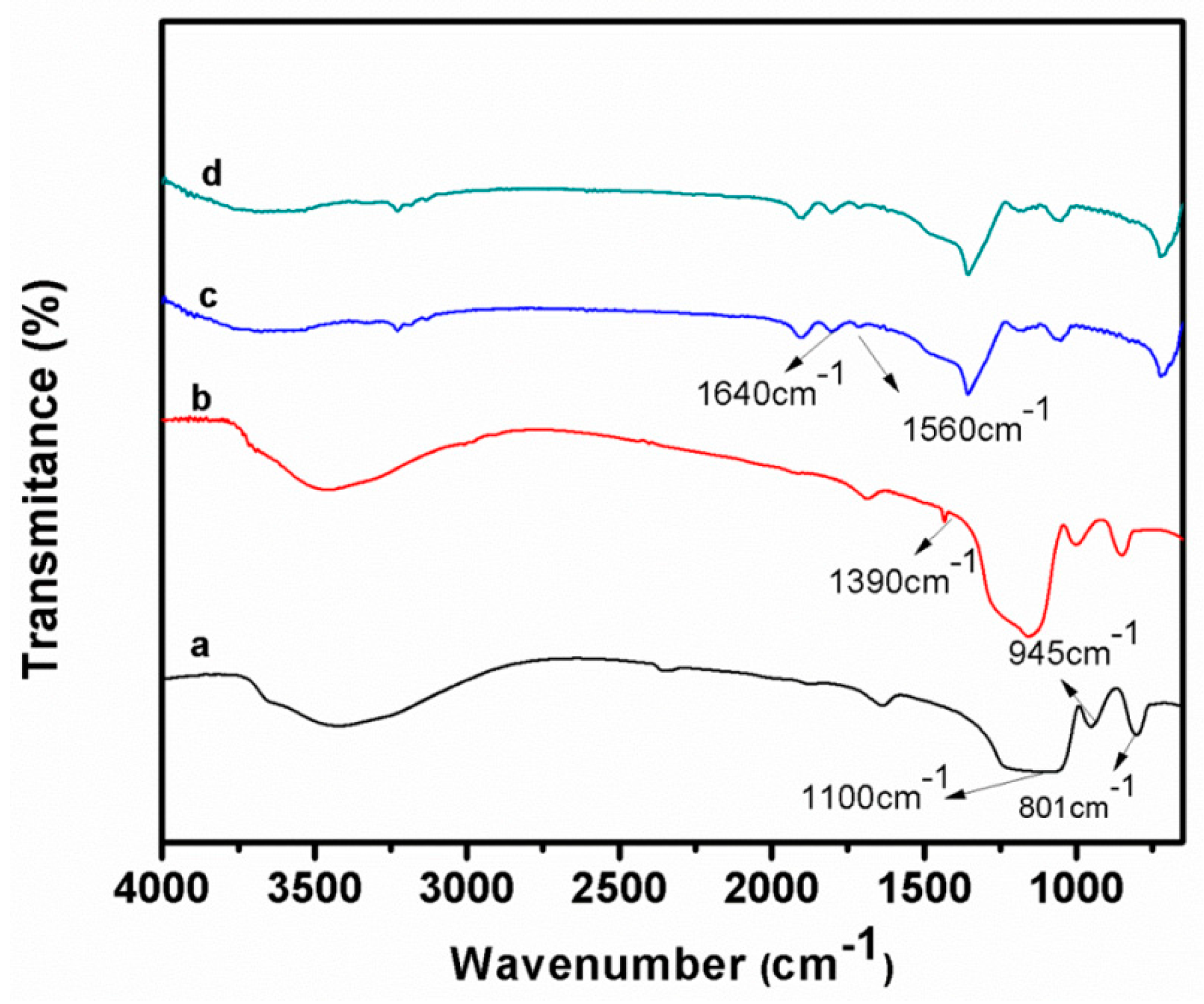
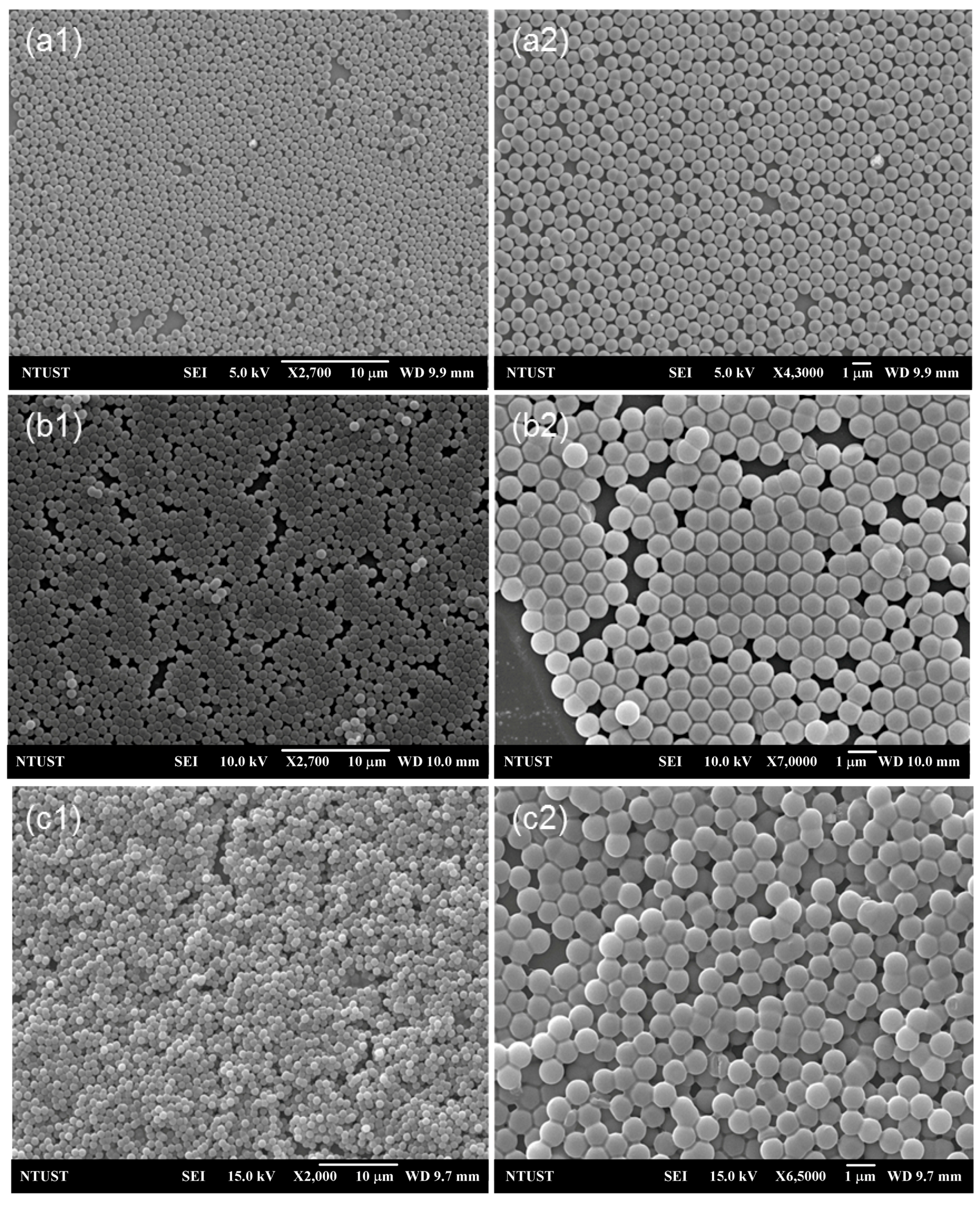
2.3. Characterization
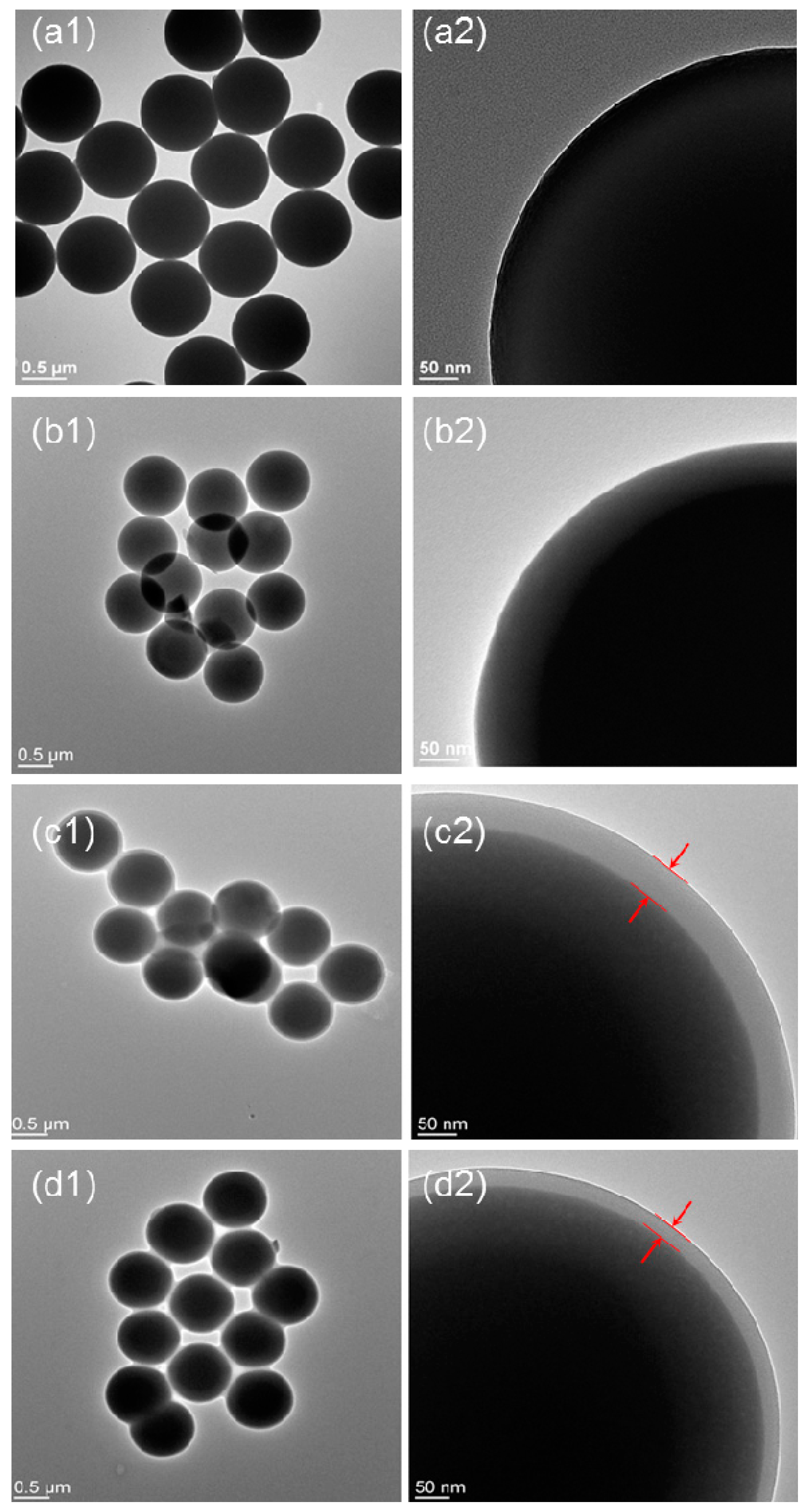
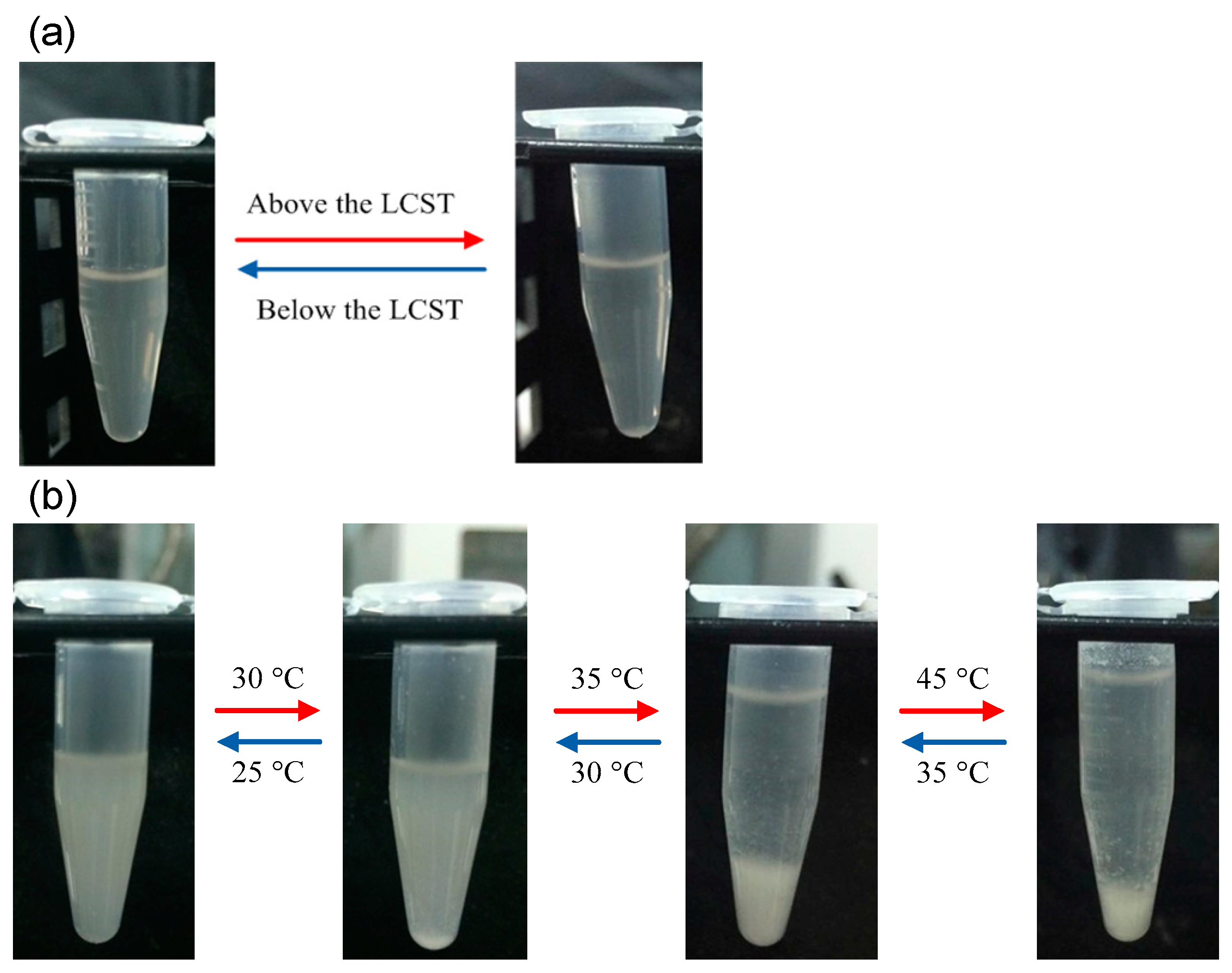
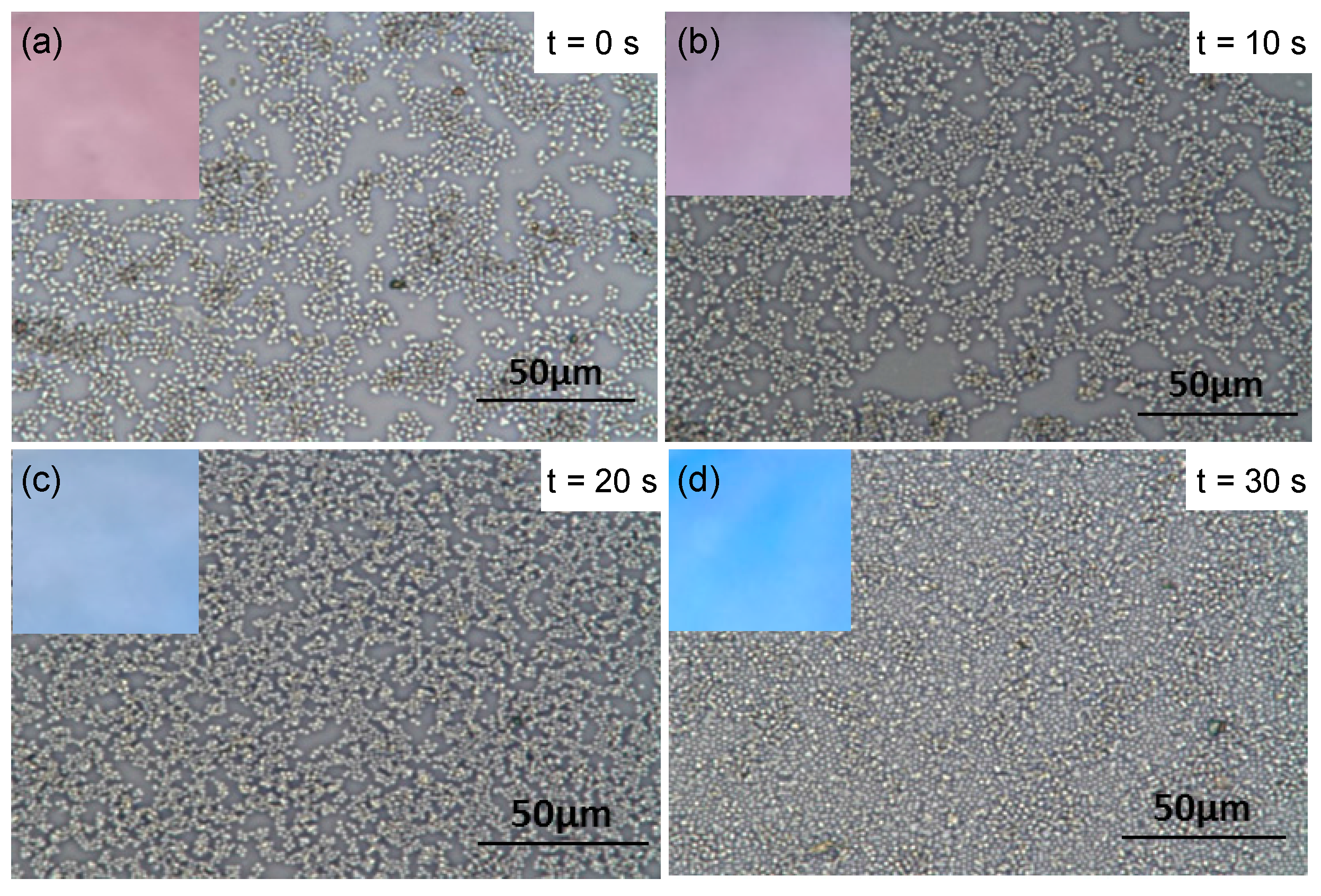
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jang, I.B.; Sung, J.H.; Choi, H.J.; Chin, I. Synthesis and characterization of titania coated polystyrene core–shell spheres for electronic ink. Synth. Met. 2005, 152, 9–12. [Google Scholar] [CrossRef]
- Jiang, H.L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Huang, B.R.; Chang, J.Y.; Chen, J.K. Bifunctional superparamagnetic–luminescent core–shell–satellite structured microspheres: Preparation, characterization, and magnetodisplay application. J. Mater. Chem. C 2015, 3, 4603–4615. [Google Scholar] [CrossRef]
- Li, C.L.; Chang, C.J.; Chen, J.K. Fabrication of sandwich structured devices encapsulating core/shell SiO2/Fe3O4 nanoparticle microspheres as media formagneto-responsive transmittance. Sens. Actuators B 2015, 210, 46–55. [Google Scholar] [CrossRef]
- Lee, J.H.; Koh, C.Y.; Singer, J.P.; Jeon, S.J.; Maldovan, M.; Stein, O.; Thomas, E.L. 25th Anniversary Article: Ordered polymer structures for the engineering of photons and phonons. Adv. Mater. 2014, 26, 532–569. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.L.; Li, M.; Li, W.J.; Mai, J.D. Gold nano-particle-based thermal sensors fabricated using microspotting and DEP techniques. Sens. Actuators A 2012, 178, 32–39. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, J.; Tan, C.; Lin, X.; Hu, S.; Chen, J.; You, X.; Li, S. Designed self-assembled hybrid Au@CdS core-shell nanoparticles with negative charge and their application as highly selective biosensors. J. Mater. Chem. B 2015, 3, 217–224. [Google Scholar] [CrossRef]
- Kalele, S.A.; Ashtaputre, S.S.; Hebalkar, N.Y.; Gosavi, S.W.; Deobagkar, D.N.; Deobagkar, D.D.; Kulkarni, S.K. Optical detection of antibody using silica–silver corecshell particles. Chem. Phys. Lett. 2005, 404, 136–141. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Mpoukouvalas, K.; Lienkamp, K.; Lieberwirth, I.; Fassbender, B.; Bonaccurso, E.; Brunklaus, G.; Muehlebach, A.; Beierlein, T.; et al. Construction of redispersible polypyrrole core-shell nanoparticles for application in polymer electronics. Adv. Mater. 2009, 21, 1137–1141. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Walt, D.R. Very high density sensing arrays. Chem. Rev. 2008, 108, 614–637. [Google Scholar] [CrossRef] [PubMed]
- Bings, N.H.; Bogaerts, A.; Broekaert, J.A. Atomic spectroscopy: A review. Anal. Chem. 2010, 82, 4653–4681. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Aoki, K.; Yano, T.; Yamane, M. Preparation of silica microspheres containing Ag nanoparticles. J. Sol Gel Sci. Technol. 1998, 11, 279–287. [Google Scholar] [CrossRef]
- Russell, P. Photonic crystal fibers. Science 2003, 299, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yu, H.; Xu, Q.; Xu, G.; Wang, K. Thermoresponsive photonic crystal: Synergistic effect of poly(N-isopropylacrylamide)-co-acrylic acid and morpho butterfly wing. ACS Appl. Mater. Interfaces 2015, 7, 8750–8756. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.R.; Vogel, N.; Hu, Y.; Kolle, M.; Perry, C.C.; Aizenberg, J. Tunable anisotropy in inverse opals and emerging optical properties. Chem. Mater. 2014, 26, 1622–1628. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Xie, J.; Li, C.; Li, Y.; Liang, S.; Tian, Z.; Wang, T.; Zhang, H.; Li, H.; et al. Bioinspired water-vapor-responsive organic/inorganic hybrid one-dimensional photonic crystals with tunable full-color stop band. Adv. Funct. Mater. 2010, 20, 3784–3790. [Google Scholar] [CrossRef]
- Yan, X.; Yao, J.; Lu, G.; Li, X.; Zhang, J.; Han, K.; Yang, B. Fabrication of non-close-packed arrays of colloidal spheres by soft lithography. J. Am. Chem. Soc. 2005, 127, 7688–7689. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y. Stimuli-responsive opals: Colloidal crystals and colloidal amorphous arrays for use in functional structurally colored materials. J. Mater. Chem. C 2013, 1, 6059–6074. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, X.; Yang, B. Colloidal self-assembly meets nanofabrication: From two-dimensional colloidal crystals to nanostructure arrays. Adv. Mater. 2010, 22, 4249–4269. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Asher, S.A. Fabrication of silica shell photonic crystals through flexible core templates. Chem. Mater. 2009, 21, 4608–4613. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, R.; Jiang, P.; Colvin, V.; Mittleman, D. Optical properties of a photonic crystal of hollow spherical shells. Appl. Phys. Lett. 2000, 77, 3517–3519. [Google Scholar] [CrossRef]
- Busch, K.; John, S. Photonic band gap formation in certain self-organizing systems. Phys. Rev. E 1998, 58, 3896–3908. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Huang, Y.T. Inorganic-organic hybrid nanomaterials for therapeutic and diagnostic imaging applications. Int. J. Mol. Sci. 2011, 12, 3888–3927. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and multifuctional application in biomedical science. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Brunella, V.; Jadhav, S.A.; Miletto, I.; Berlier, G.; Ugazio, E.; Sapino, S.; Scalarone, D. Hybrid drug carriers with temperature-controlled on-off release: A simple and reliable synthesis of PNIPAM-functionalized mesoporous silica nanoparticles. React. Funct. Polym. 2016, 98, 31–37. [Google Scholar] [CrossRef]
- Burdukova, E.; Li, H.; Ishida, N.; O’Shea, J.P.; Franks, G.V. Temperature controlled surface hydrophobicity and interaction forces induced by poly(N-isopropylacrylamide). J. Colloid Interface Sci. 2010, 342, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Schafer, C.G.; Winter, T.; Heidt, S.; Dietz, C.; Ding, T.; Baumberg, J.J.; Gallei, M. Smart polymer inverse-opal photonic crystal films by melt-shear organization for hybrid core–shell architectures. J. Mater. Chem. C 2015, 3, 2204–2214. [Google Scholar] [CrossRef]
- Wang, D.; Rogach, A.L.; Caruso, F. Composite photonic crystals from semiconductor nanocrystal/polyelectrolyte-coated colloidal spheres. Chem. Mater. 2003, 15, 2724–2729. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wang, L.; Chao, X.; Asher, S.A. Periodicity-controlled two-dimensional crystalline colloidal arrays. Langmuir 2011, 27, 15230–15235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Wang, L.; Lamont, D.N.; Velankar, S.S.; Asher, S.A. Fabrication of large-area two-dimensional colloidal crystals. Angew. Chem. Int. Ed. 2012, 51, 6117–6120. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Hellweg, T. Smart inorganic/organic hybrid microgels: Synthesis and characterisation. J. Mater. Chem. 2009, 19, 8714–8727. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Zhang, H.; Zhao, S.; Wang, D.; Wang, J. Surface-initiated atom transfer radical polymerization on spherical silicon gel in water-borne system at ambient temperature. Mater. Chem. Phys. 2006, 96, 477–482. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Wang, X.; Liu, S. Fabrication of hybrid silica nanoparticles densely grafted with thermoresponsive poly(N-isopropylacrylamide) brushes of controlled thickness via surface-initiated atom transfer radical polymerization. Chem. Mater. 2008, 20, 101–109. [Google Scholar] [CrossRef]
- Wu, T.; Ge, Z.; Liu, S. Fabrication of thermoresponsive cross-linked poly(N-isopropylacrylamide) nanocapsules and silver nanoparticle-embedded hybrid capsules with controlled shell thickness. Chem. Mater. 2011, 23, 2370–2380. [Google Scholar] [CrossRef]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma polymerized N-isopropylacrylamide: Synthesis and characterization of a smart thermally responsive coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ward Muscatello, M.M.; Stunja, L.E.; Asher, S.A. Polymerized crystalline colloidal array sensing of high glucose concentrations. Anal. Chem. 2009, 81, 4978–4986. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.K.; Pai, P.C.; Chang, J.Y.; Fan, S.K. pH-Responsive one-dimensional periodic relief grating of polymer brush–gold nanoassemblies on silicon surface. ACS Appl. Mater. Interfaces 2012, 4, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F. Surface-initiated atom transfer radical polymerization for applications in sensors, non-biofouling surfaces and adsorbents. Polym. J. 2016, 48, 341–350. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Su, B.; Shi, L.; Wang, J.; Chen, S.; Wang, L.; Zi, J.; Song, Y.; Jiang, L. Colloidal photonic crystals with narrow stopbands assembled from low-adhesive superhydrophobic substrates. J. Am. Chem. Soc. 2012, 134, 17053–17058. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, F.; Meng, R.; Xu, J.; Zuo, C.; Ge, B. Anomalous change of airy disk with changing size of spherical particles. J. Quant. Spectrosc. Radiat. Transf. 2016, 170, 83–89. [Google Scholar] [CrossRef]



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manivannan, K.; Huang, Y.-S.; Huang, B.-R.; Huang, C.-F.; Chen, J.-K. Real-Time Packing Behavior of Core-Shell Silica@Poly(N-isopropylacrylamide) Microspheres as Photonic Crystals for Visualizing in Thermal Sensing. Polymers 2016, 8, 428. https://doi.org/10.3390/polym8120428
Manivannan K, Huang Y-S, Huang B-R, Huang C-F, Chen J-K. Real-Time Packing Behavior of Core-Shell Silica@Poly(N-isopropylacrylamide) Microspheres as Photonic Crystals for Visualizing in Thermal Sensing. Polymers. 2016; 8(12):428. https://doi.org/10.3390/polym8120428
Chicago/Turabian StyleManivannan, Karthikeyan, Yi-Shen Huang, Bohr-Ran Huang, Chih-Feng Huang, and Jem-Kun Chen. 2016. "Real-Time Packing Behavior of Core-Shell Silica@Poly(N-isopropylacrylamide) Microspheres as Photonic Crystals for Visualizing in Thermal Sensing" Polymers 8, no. 12: 428. https://doi.org/10.3390/polym8120428







