All-Inorganic Intumescent Nanocoating Containing Montmorillonite Nanoplatelets in Ammonium Polyphosphate Matrix Capable of Preventing Cotton Ignition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Deposition
2.3. Characterization
3. Results
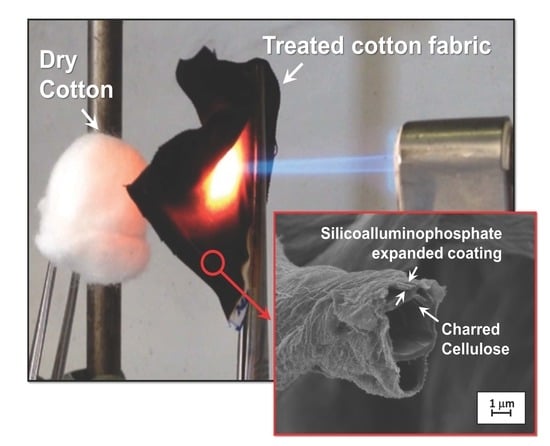
3.1. Coating Morphology on Cotton
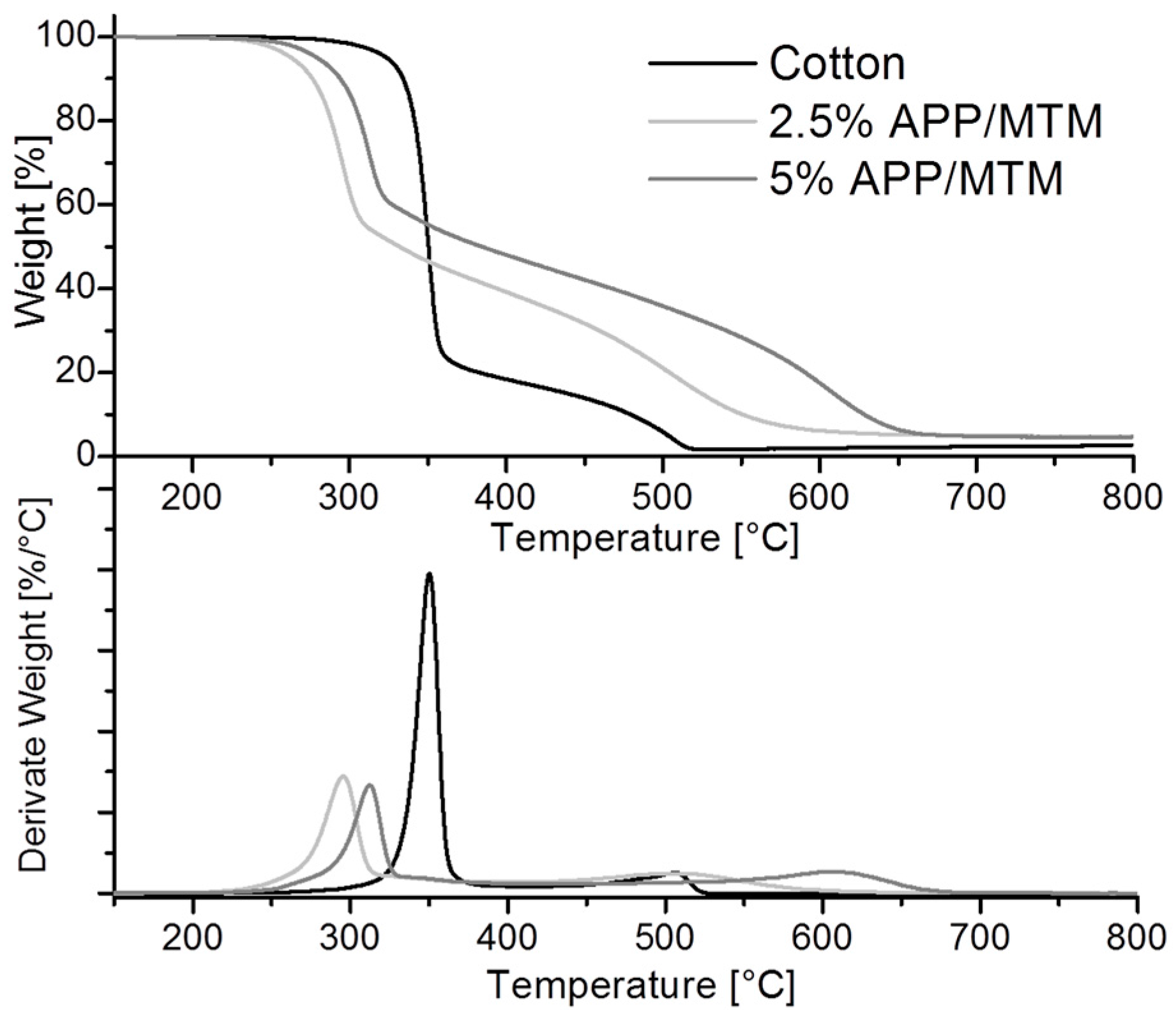
3.2. Thermal and Thermo-Oxidative Stability
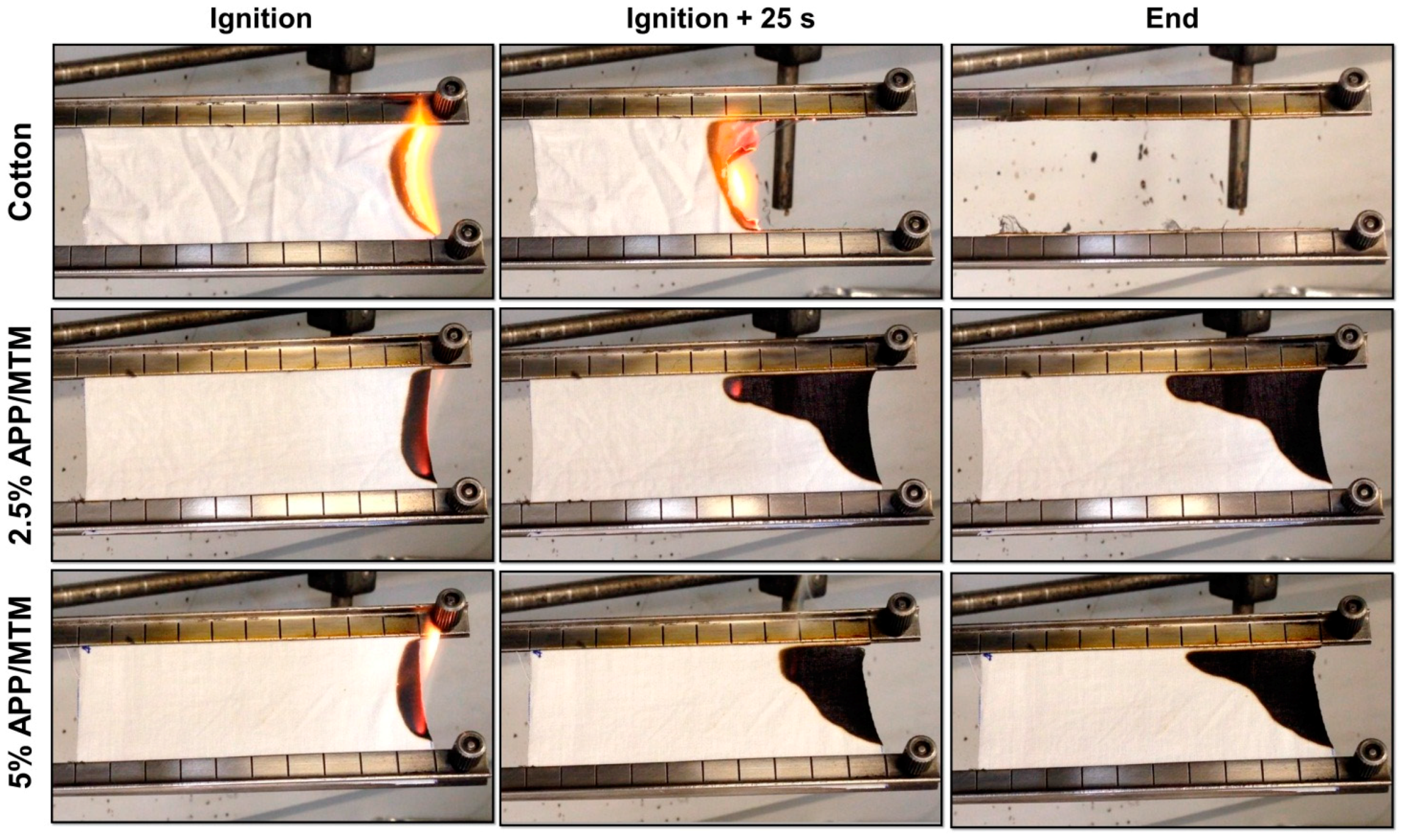
3.3. Horizontal Flame Spread Tests
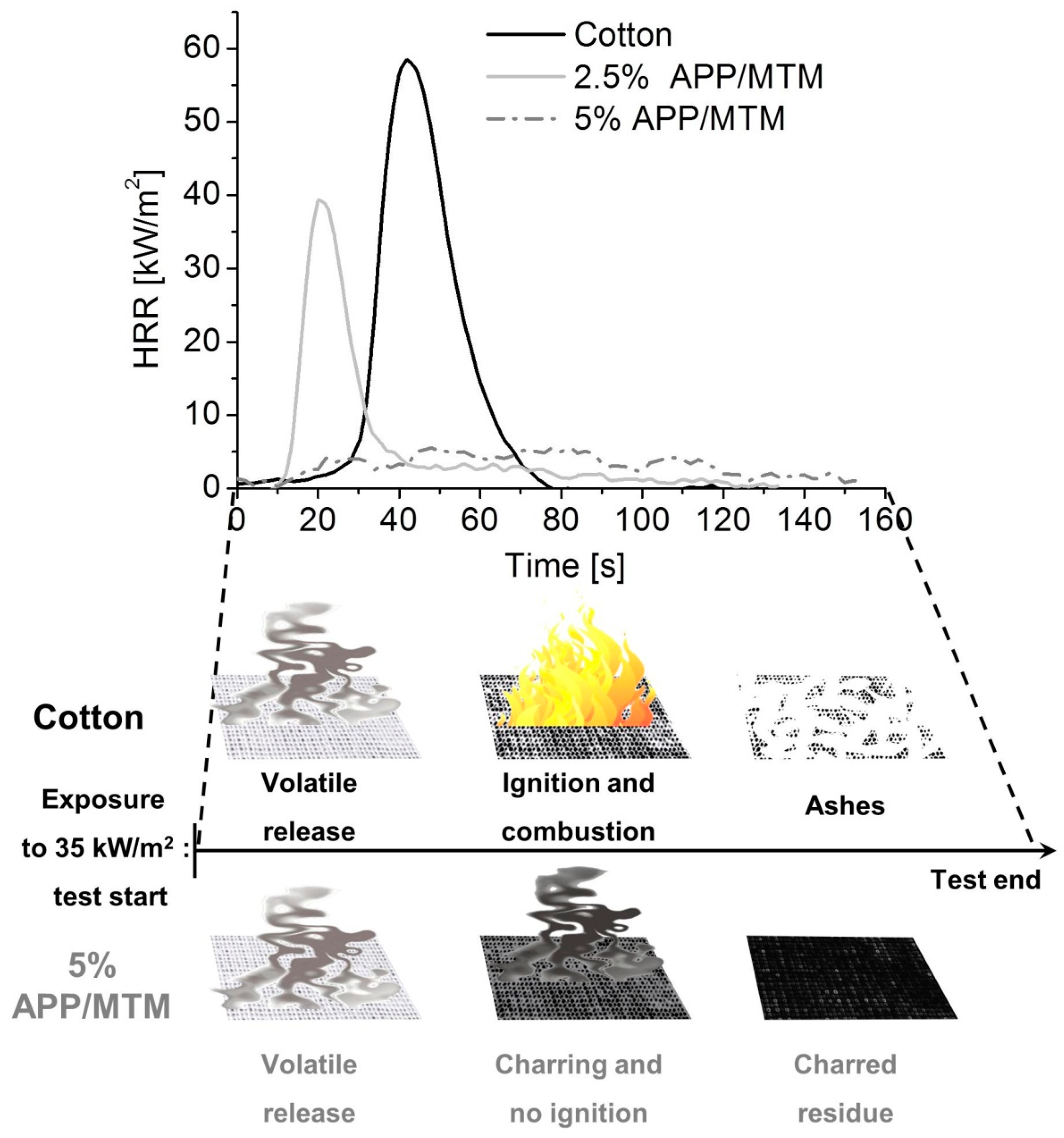
3.4. Cone Calorimetry
3.5. Residue Analysis and Coating Mechanism
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2003, 112, 9–17. [Google Scholar] [CrossRef]
- Xiao, H.; Shen, L.; Su, Y.; Barresi, E.; Dejong, M.; Hung, H.; Lei, Y.D.; Wania, F.; Reiner, E.J.; Sverko, E.; et al. Atmospheric concentrations of halogenated flame retardants at two remote locations: The Canadian high arctic and the Tibetan plateau. Environ. Pollut. 2012, 161, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, D.; Kostianoy, A.G. Handbook environmental chemistry. In Brominated Flame Retardants; Heidelberg, S.-V.B., Ed.; Springer: New York, NY, USA, 2011; Volume 16. [Google Scholar]
- Stieger, G.; Scheringer, M.; Ng, C.A.; Hungerbuhler, K. Assessing the persistence, bioaccumulation potential and toxicity of brominated flame retardants: Data availability and quality for 36 alternative brominated flame retardants. Chemosphere 2014, 116, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Fernandes, N.J.; Akbarzadeh, J.; Peterlik, H.; Giannelis, E.P. Synthesis and properties of highly dispersed ionic silica-poly(ethylene oxide) nanohybrids. ACS Nano 2013, 7, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Priolo, M.A.; Holder, K.M.; Guin, T.; Grunlan, J.C. Recent advances in gas barrier thin films via layer-by-layer assembly of polymers and platelets. Macromol. Rapid. Commun. 2015, 36, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, G.; Carosio, F.; Alongi, J.; Fina, A.; Frache, A.; Camino, G. Materials engineering for surface-confined flame retardancy. Mat. Sci. Eng. R 2014, 84, 1–20. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay—Chitosan nanobrick walls: Completely renewable gas barrier and flame-retardant nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Colonna, S.; Fina, A.; Rydzek, G.; Hemmerle, J.; Jierry, L.; Schaaf, P.; Boulmedais, F. Efficient gas and water vapor barrier properties of thin poly(lactic acid) packaging films: Functionalization with moisture resistant nafion and clay multilayers. Chem. Mater. 2014, 26, 5459–5466. [Google Scholar] [CrossRef]
- Alongi, J.; Tata, J.; Carosio, F.; Rosace, G.; Frache, A.; Camino, G. A comparative analysis of nanoparticle adsorption as fire-protection approach for fabrics. Polymers 2015, 7, 47–68. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Kiekens, P. Recent advances in the design of water based-flame retardant coatings for polyester and polyester-cotton blends. Polymers 2016, 8, 357–380. [Google Scholar] [CrossRef] [Green Version]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Laufer, G.; Carosio, F.; Martinez, R.; Camino, G.; Grunlan, J.C. Growth and fire resistance of colloidal silica-polyelectrolyte thin film assemblies. J. Colloid. Interface Sci. 2011, 356, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Cuttica, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Layer by layer assembly of flame retardant thin films on closed cell pet foams: Efficiency of ammonium polyphosphate versus DNA. Polym. Degrad. Stab. 2015, 113, 189–196. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Layer by layer nanoarchitectures for the surface protection of polycarbonate. Eur. Polym. J. 2013, 49, 397–404. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine-montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stab. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Fina, A.; Cuttica, F.; Camino, G. Ignition of polypropylene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2012, 97, 2619–2626. [Google Scholar] [CrossRef]
- Cain, A.A.; Nolen, C.R.; Li, Y.C.; Davis, R.; Grunlan, J.C. Phosphorous-filled nanobrick wall multilayer thin film eliminates polyurethane melt dripping and reduces heat release associated with fire. Polym. Degrad. Stab. 2013, 98, 2645–2652. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: Flammability and combustion behaviour. Cellulose 2012, 19, 1041–1050. [Google Scholar] [CrossRef]
- Holder, K.M.; Huff, M.E.; Cosio, M.N.; Grunlan, J.C. Intumescing multilayer thin film deposited on clay-based nanobrick wall to produce self-extinguishing flame retardant polyurethane. J. Mater. Sci. 2015, 50, 2451–2458. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Ultra-fast layer-by-layer approach for depositing flame retardant coatings on flexible pu foams within seconds. ACS Appl. Mater. Interfaces 2016, 8, 6315–6319. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Alongi, J.; Malucelli, G. Flammability and combustion properties of ammonium polyphosphate-/poly(acrylic acid)-based layer by layer architectures deposited on cotton, polyester and their blends. Polym. Degrad. Stab. 2013, 98, 1626–1637. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by layer ammonium polyphosphate-based coatings for flame retardancy of polyester-cotton blends. Carbohydr. Polym. 2012, 88, 1460–1469. [Google Scholar] [CrossRef]
- Li, Y.C.; Mannen, S.; Morgan, A.B.; Chang, S.C.; Yang, Y.H.; Condon, B.; Grunlan, J.C. Intumescent all-polymer multilayer nanocoating capable of extinguishing flame on fabric. Adv. Mater. 2011, 23, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Fontaine, G.; Alongi, J.; Bourbigot, S. Starch-based layer by layer assembly: Efficient and sustainable approach to cotton fire protection. ACS Appl. Mater. Interfaces 2015, 7, 12158–12167. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Yang, R. Ammonium polyphosphate/montmorillonite nanocompounds in polypropylene. J. Appl. Polym. Sci. 2010, 118, 834–840. [Google Scholar] [CrossRef]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z. Intumescent flame retardant-montmorillonite synergism in ABS nanocomposites. Appl. Clay Sci. 2008, 42, 238–245. [Google Scholar] [CrossRef]
- Tata, J.; Alongi, J.; Carosio, F.; Frache, A. Optimization of the procedure to burn textile fabrics by cone calorimeter: Part I. Combustion behavior of polyester. Fire Mater. 2011, 35, 397–409. [Google Scholar] [CrossRef]
- Norrish, K. The swelling of montmorillonite. Discuss. Faraday Soc. 1954, 18, 120–134. [Google Scholar] [CrossRef]
- Zheng, Y.; Zaoui, A. How water and counterions diffuse into the hydrated montmorillonite. Solid State Ion. 2011, 203, 80–85. [Google Scholar] [CrossRef]
- Carosio, F.; Kochumalayil, J.; Cuttica, F.; Camino, G.; Berglund, L. Oriented clay nanopaper from biobased components--mechanisms for superior fire protection properties. ACS Appl. Mater. Interfaces 2015, 7, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Camino, G.; Malucelli, G. Heating rate effect on char yield from cotton, poly(ethylene terephthalate) and blend fabrics. Carbohydr. Polym. 2013, 92, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.J.; Horrocks, A.R.; Alderson, A. The sensitisation of thermal decomposition of ammonium polyphosphate by selected metal ions and their potential for improved cotton fabric flame retardancy. Polym. Degrad. Stab. 2005, 88, 114–122. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers.3. Effect of urea on the ammonium polyphosphate pentaerythritol system. Polym. Degrad. Stab. 1984, 7, 221–229. [Google Scholar] [CrossRef]
- Price, D.; Horrocks, A.R.; Akalin, M.; Faroq, A.A. Influence of flame retardants on the mechanism of pyrolysis of cotton (cellulose) fabrics in air. J. Anal. Appl. Pyrolysis 1997, 40–41, 511–524. [Google Scholar] [CrossRef]
- Mamleev, V.; Bourbigot, S.; Le Bras, M.; Yvon, J. The facts and hypotheses relating to the phenomenological model of cellulose pyrolysis interdependence of the steps. J. Anal. Appl. Pyrolysis 2009, 84, 1–17. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R.; Price, D.; Coleman, G.V. Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J. Macromol. Sci. RMC 1996, C36, 721–794. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Soares, S.; Camino, G.; Levchik, S. Comparative-study of the thermal-decomposition of pure cellulose and pulp paper. Polym. Degrad. Stab. 1995, 49, 275–283. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Influence of layer by layer coatings containing octapropylammonium polyhedral oligomeric silsesquioxane and ammonium polyphosphate on the thermal stability and flammability of acrylic fabrics. J. Anal. Appl. Pyrolysis 2016, 119, 114–123. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]

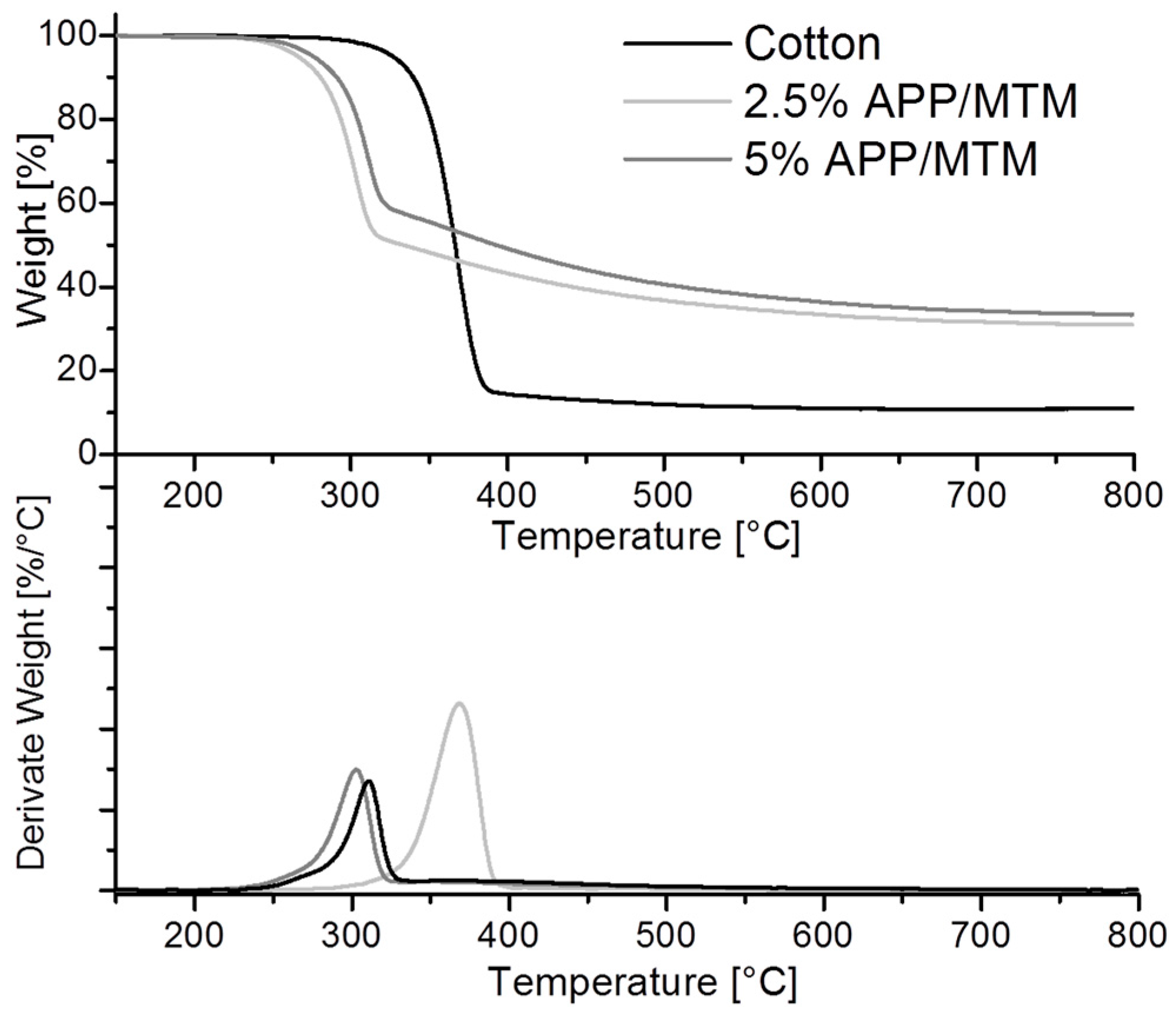
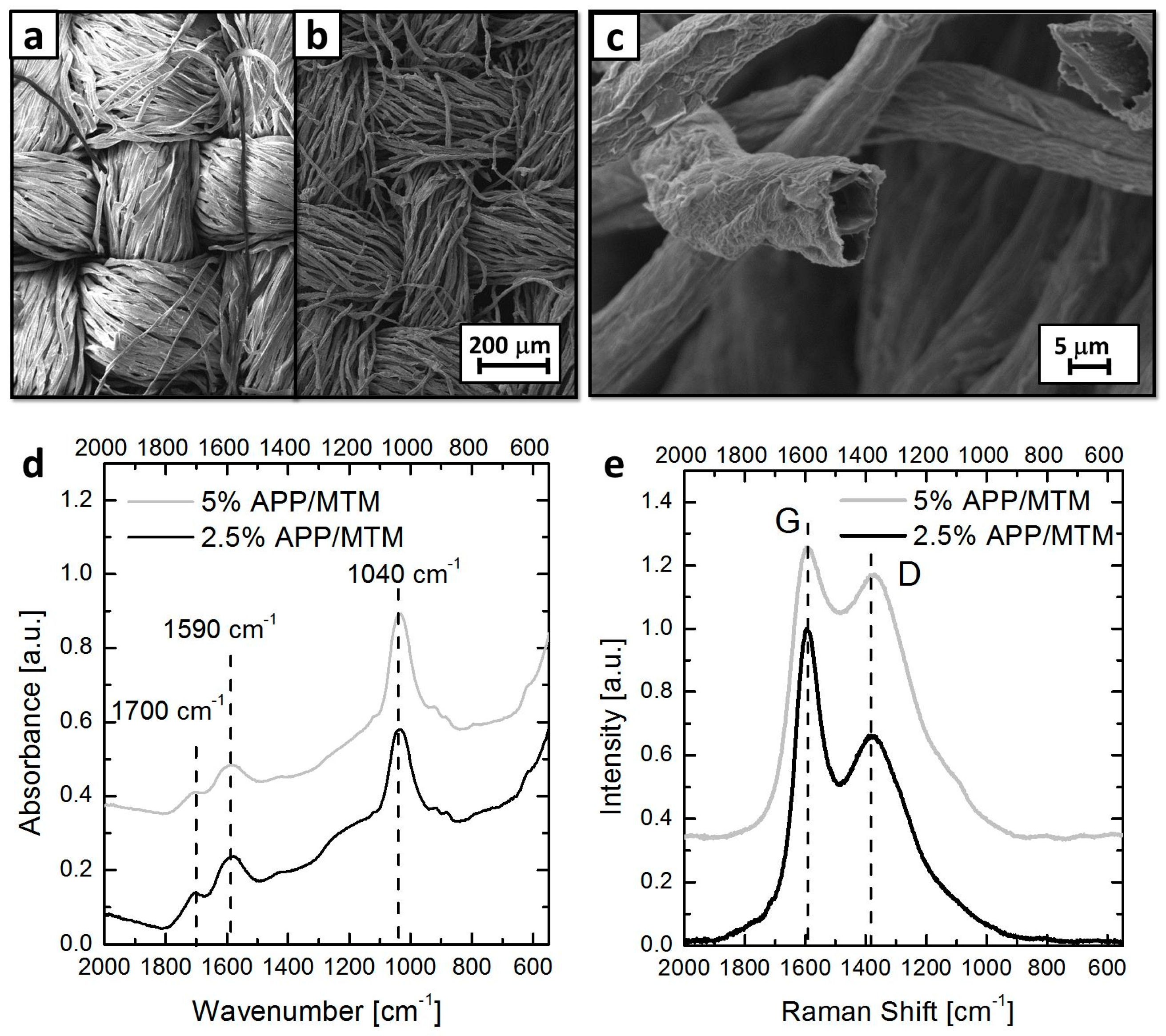

| Sample | T *onset5% (°C) | T *max (°C) | Residue at 800 °C (%) |
|---|---|---|---|
| Cotton | 327 | 369 | 11 |
| 2.5% APP/MTM | 266 | 301 | 31 |
| 5% APP/MTM | 276 | 310 | 34 |
| Sample | T *onset5% (°C) | T *max1 (°C) | T *max2 (°C) | Residue at 400 °C (%) | Residue at 800 °C (%) |
|---|---|---|---|---|---|
| Cotton | 324 | 350 | 506 | 18 | 2 |
| 2.5% APP/MTM | 263 | 296 | 508 | 39 | 4 |
| 5% APP/MTM | 280 | 313 | 607 | 48 | 5 |
| Sample | Combustion Rate ± σ (mm/s) | Afterglow | Residue ± σ (%) |
|---|---|---|---|
| Cotton | 1.7 ± 0.04 | Yes | 0 |
| 2.5% APP/MTM | 1.6 ± 0.30 | No | 78 ± 5 |
| 5% APP/MTM | 1.3 ± 0.08 | No | 85 ± 2 |
| Sample | TTI ± σ (s) | pkHRR ± σ (kW/m2) | THR ± σ (MJ/m2) | TSR ± σ (m2/m2) | Residue (%) |
|---|---|---|---|---|---|
| Cotton | 36 ± 2 | 61 ± 4 | 1.0 ± 0.1 | 25 ± 8 | 0 |
| 2.5% APP/MTM | 22 ± 5 | 38 ± 7 | 0.38 ± 0.05 | 13 ± 4 | 13 |
| 5% APP/MTM | N.A. * | N.A. * | N.A. * | 40 ± 6 | 19 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alongi, J.; Carosio, F. All-Inorganic Intumescent Nanocoating Containing Montmorillonite Nanoplatelets in Ammonium Polyphosphate Matrix Capable of Preventing Cotton Ignition. Polymers 2016, 8, 430. https://doi.org/10.3390/polym8120430
Alongi J, Carosio F. All-Inorganic Intumescent Nanocoating Containing Montmorillonite Nanoplatelets in Ammonium Polyphosphate Matrix Capable of Preventing Cotton Ignition. Polymers. 2016; 8(12):430. https://doi.org/10.3390/polym8120430
Chicago/Turabian StyleAlongi, Jenny, and Federico Carosio. 2016. "All-Inorganic Intumescent Nanocoating Containing Montmorillonite Nanoplatelets in Ammonium Polyphosphate Matrix Capable of Preventing Cotton Ignition" Polymers 8, no. 12: 430. https://doi.org/10.3390/polym8120430