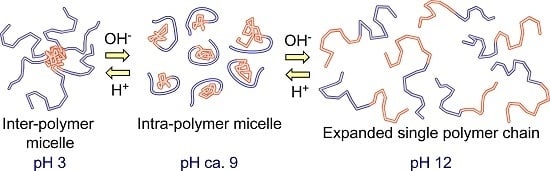
pH-Responsive Intra- and Inter-Molecularly Micelle Formation of Anionic Diblock Copolymer in Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Synthesis of Sodium 11-(Acrylamido)undecanoate (AaU)
2.3. Preparation of PAMPS Macro-Chain Transfer Agent (PAMPS Macro-CTA)
2.4. Block Copolymerization
2.5. Measurements
2.6. Preparation of Sample Solutions
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SFRP | Stable free radical polymerization |
| ATRP | Atom transfer radical polymerization |
| RAFT | Reversible addition-fragmentation chain transfer |
| PAMPS–PAaH | Poly(sodium2-(acrylamido)-2-methylpropanesulfonate)-block-poly(sodium6-(acrylamide)-hexanoate) |
| DLS | Dynamic light scattering |
| SLS | Static light scattering |
| 1H NMR | Proton neuclear magnetic resonance |
| PAMPS | Poly(sodium2-(acrylamido)-2-methylpropanesulfonate) |
| PAaU | Poly(sodium11-(acrylamide)hexanoate) |
| CPD | 4-Cyanopentanoic acid dithiobenzoate |
| AMPS | 2-(Acrylamido)-2-methylpropanesulfonic acid |
| V-501 | 4,4′-Azobis(4-cyanopentanoic acid) |
| ANS | 8-Anilino-1-naphthalenesulfonic acid ammonium salt hydrate |
| AaU | Sodium11-acrylamidohexanoate |
| PAMPS macro-CTA | Poly(sodium 2-(acrylamido)-2-methylpropanesulfonate) macro-chain transfer agent |
| GPC | Gel-permeation chromatography |
| Mn | Number-average molecular weight |
| Mw/Mn | Molecular weight distribution |
| RI | Refractiv index |
| Mw | Weight-average molecular weight |
| B | Baseline |
| β | A factor which takes into account deviations from ideal correlation |
| t | Delay time |
| τ | Relaxation time |
| D | Translational diffusion coefficient |
| q | Scattering vectr |
| n | Refractive index |
| λ | Wavelength |
| θ | Scattering angle |
| Rh | Hydrodynamic radius |
| kB | Boltzmann’s constant |
| T | Absolute temperature |
| η | Solvent viscosity |
| Rg | z-Average radius of gyration |
| A2 | The second virial coefficient |
| Cp | Polymer concentration |
| Rθ | Rayleigh ratio |
| K | Optical constant |
| dn/dCp | Refractive index increment against the polymer concentration |
| NA | Avogadro’s number |
| T2 | Spin-spin relaxation time |
| DP | Degree of polymerization |
| Nagg | Aggregation number |
References
- Laschewsky, A. Molecular concepts, self-organization and properties of polysoaps. Adv. Polym. Sci. 1995, 124, 1–86. [Google Scholar]
- Hurter, P.N.; Hatton, T.A. Solubilization of polycyclic aromatic hydrocarbons by poly(ethyleneoxide-propyleneoxide) block copolymer micelles: Effects of Polymer Structure. Langmuir 1992, 8, 1291–1299. [Google Scholar] [CrossRef]
- Kataoka, K.; Kwon, G.S.; Yokoyama, M.; Okano, T.; Sakurai, Y. Block copolymer micelles as vehicles for drug delivery. J. Control. Release 1993, 24, 119–132. [Google Scholar]
- Topp, M.D.C.; Dijkstra, P.J.; Talsma, H.; Feijen, J. Thermosensitive micelle-forming block copolymers of poly(ethyleneglycol) and poly(N-isopropylacrylamide). Macromolecules 1997, 30, 8518–8520. [Google Scholar] [CrossRef]
- Bütün, V.; Armes, S.P.; Billingham, N.C.; Tuzar, Z.; Rankin, A.; Eastoe, J.; Heenan, R.K. The remarkable “flip-flop” self-assembly of diblock copolymer in aqueous solution. Macromolecules 2001, 34, 1503–1511. [Google Scholar] [CrossRef]
- Paz Báñez, M.V.; Robinson, K.L.; Bütün, V.; Armes, S.P. Use of oxyanion-initiated polymerization for the synthesis of amine methacrylate-based homopolymers and block copolymers. Polymer 2001, 42, 29–37. [Google Scholar] [CrossRef]
- Georges, M.K.; Veregin, R.P.M.; Kazmaier, P.M.; Hamer, G.K. Narrow molecular weight resins by a free-radical polymerization process. Macromolecules 1993, 26, 2987–2988. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotriz-(triphenylphosphin)ruthenium (II)/methylaluminium bis(2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. “Living”/controlled radical polymerization. Transition-metal-catalyzed atom transfer radical polymerization in the presence of conventional radical initiator. Macromolecules 1995, 28, 7572–7573. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Yusa, S.; Shimada, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. pH-Responsive micellization of amphiphilic diblock copolymers synthesized via reversible addition-fragmentation chain transfer polymerization. Macromolecules 2003, 36, 4208–4215. [Google Scholar] [CrossRef]
- Mitsukami, Y.; Donovan, M.S.; Lowe, A.B.; McCormick, C.L. Water-soluble polymers 81. Direct synthesis of hydrophilic styrenic-based homopolymers and copolymers in aqueous solution via RAFT. Macromolecules 2001, 34, 2248–2256. [Google Scholar] [CrossRef]
- Jakeš, J. Testing of the constrained regularization method of inverting Laplace transform on simulated very wide quasielastic light scattering autocorrelation functions. Czech. J. Phys. B 1988, 38, 1305–1316. [Google Scholar] [CrossRef]
- Brown, W.; Nicolai, T.; Hvidt, S.; Stepanek, P. Relaxation time distributions of entangled polymer solutions from dynamic light scattering and dynamic mechanical measurements. Macromolecules 1990, 23, 357–359. [Google Scholar] [CrossRef]
- Zimm, B.H. Apparatus and methods for measurement and interpretation of the angular variation of light scattering; preliminary results on polystyrene solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Nielsen, S.O. Acidity measurements with the glass electrode in H2O–D2O mixtures. J. Phys. Chem. 1960, 64, 632–637. [Google Scholar] [CrossRef]
- Konishi, T.; Yoshizaki, T.; Yamakawa, H. On the “universal constants” ρ and φ of flexible polymers. Macromolecules 1991, 24, 5614–5622. [Google Scholar] [CrossRef]
- Slavik, J. Anilinonaphthalene sulfonate as a probe of membrane composition and function. Biochim. Biophys. Acta. 1982, 694, 1–25. [Google Scholar] [CrossRef]
- Steinschulte, A.A.; Schulte, B.; Rütten, S.; Eckert, T.; Okuda, J.; Möller, M.; Schneider, S.; Borisov, O.V.; Plamper, F.A. Effects of architecture on the stability of thermosensitive unimolecular micelles. Phys. Chem. Chem. Phys. 2014, 16, 4917–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
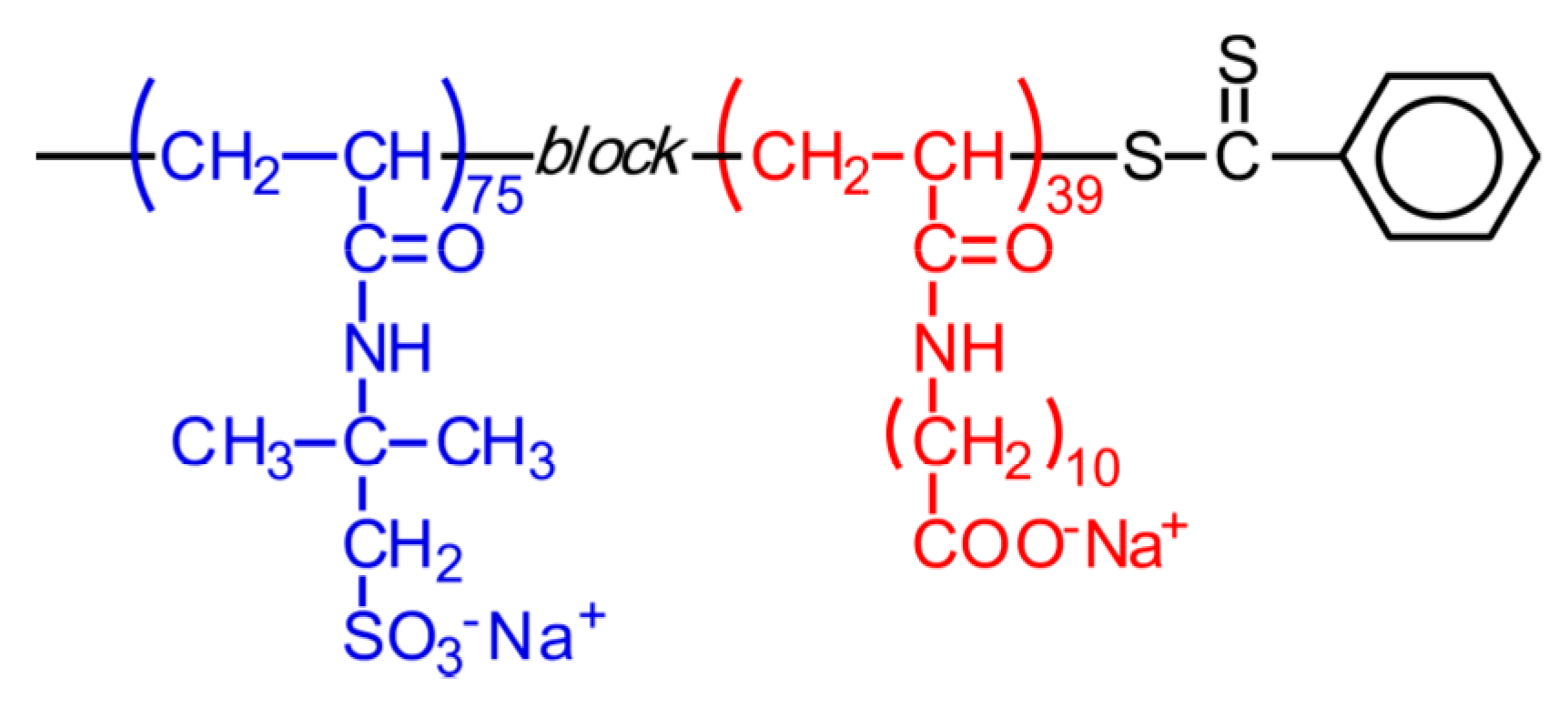
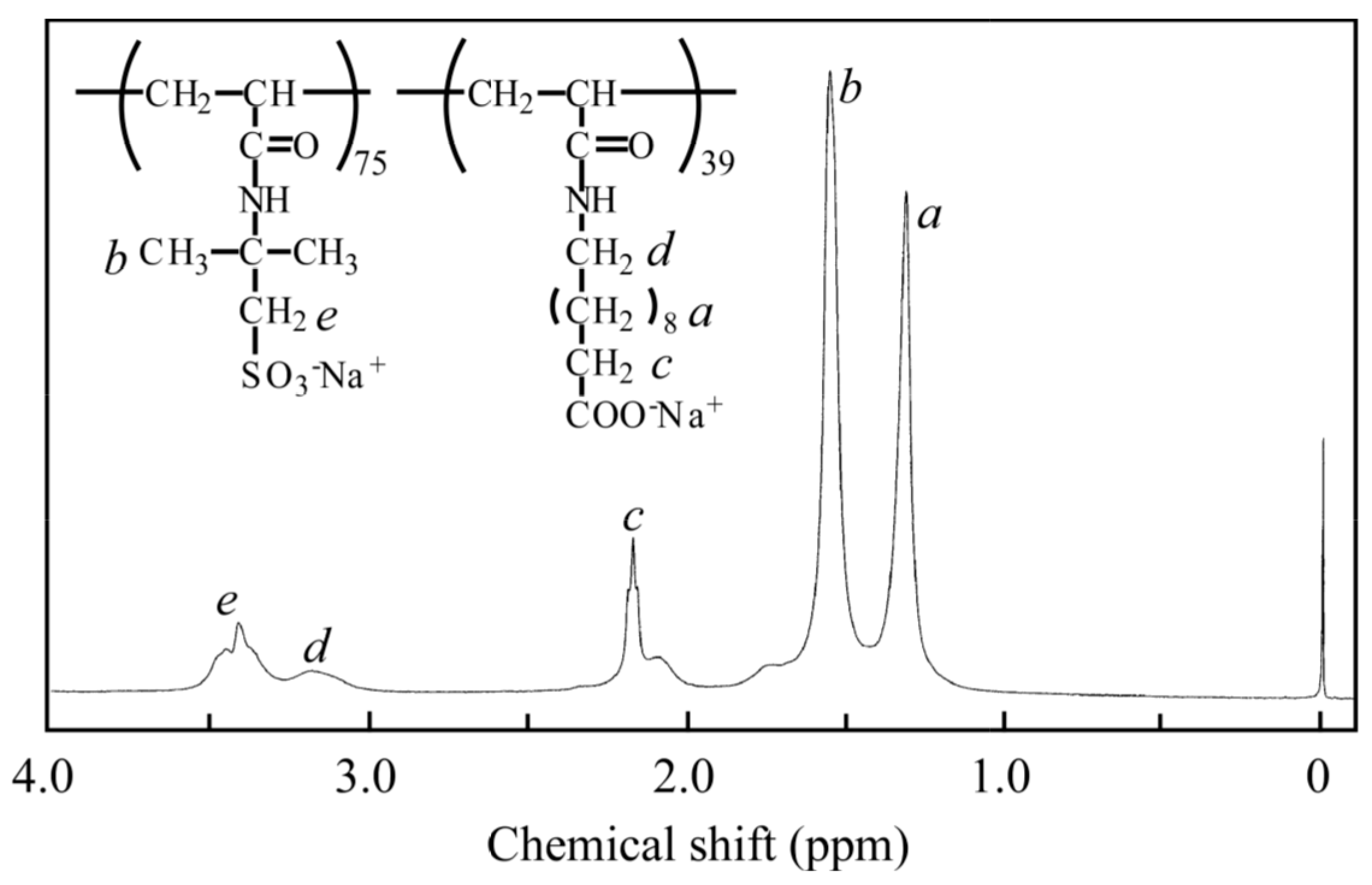






| Sample | DP of PAMPS a | DP of PAaU b | Mn a × 10−4 | Mw a × 10−4 | Mw/Mn a |
|---|---|---|---|---|---|
| PAMPS–PAaU | 75 | 39 | 7.63 | 9.38 | 1.23 |
| pH | Mw a × 10−4 | A2 a × 104m (mol·mL·g−2) | Rg a (nm) | Rh b (nm) | Rg/Rh | Nagg c |
|---|---|---|---|---|---|---|
| 3 | 78.2 | 1.65 | 15.0 | 12.5 | 1.20 | 9.2 |
| 9 | 6.1 | 15.6 | 12.0 | 5.1 | 2.35 | 1 |
| 12 | 8.5 | 8.86 | 17.8 | 11.2 | 1.59 | 1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizusaki, M.; Shimada, Y.; Morishima, Y.; Yusa, S.-i. pH-Responsive Intra- and Inter-Molecularly Micelle Formation of Anionic Diblock Copolymer in Water. Polymers 2016, 8, 56. https://doi.org/10.3390/polym8020056
Mizusaki M, Shimada Y, Morishima Y, Yusa S-i. pH-Responsive Intra- and Inter-Molecularly Micelle Formation of Anionic Diblock Copolymer in Water. Polymers. 2016; 8(2):56. https://doi.org/10.3390/polym8020056
Chicago/Turabian StyleMizusaki, Masanobu, Yoshihiko Shimada, Yotaro Morishima, and Shin-ichi Yusa. 2016. "pH-Responsive Intra- and Inter-Molecularly Micelle Formation of Anionic Diblock Copolymer in Water" Polymers 8, no. 2: 56. https://doi.org/10.3390/polym8020056