Flexible Transparent Electrode of Hybrid Ag-Nanowire/Reduced-Graphene-Oxide Thin Film on PET Substrate Prepared Using H2/Ar Low-Damage Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
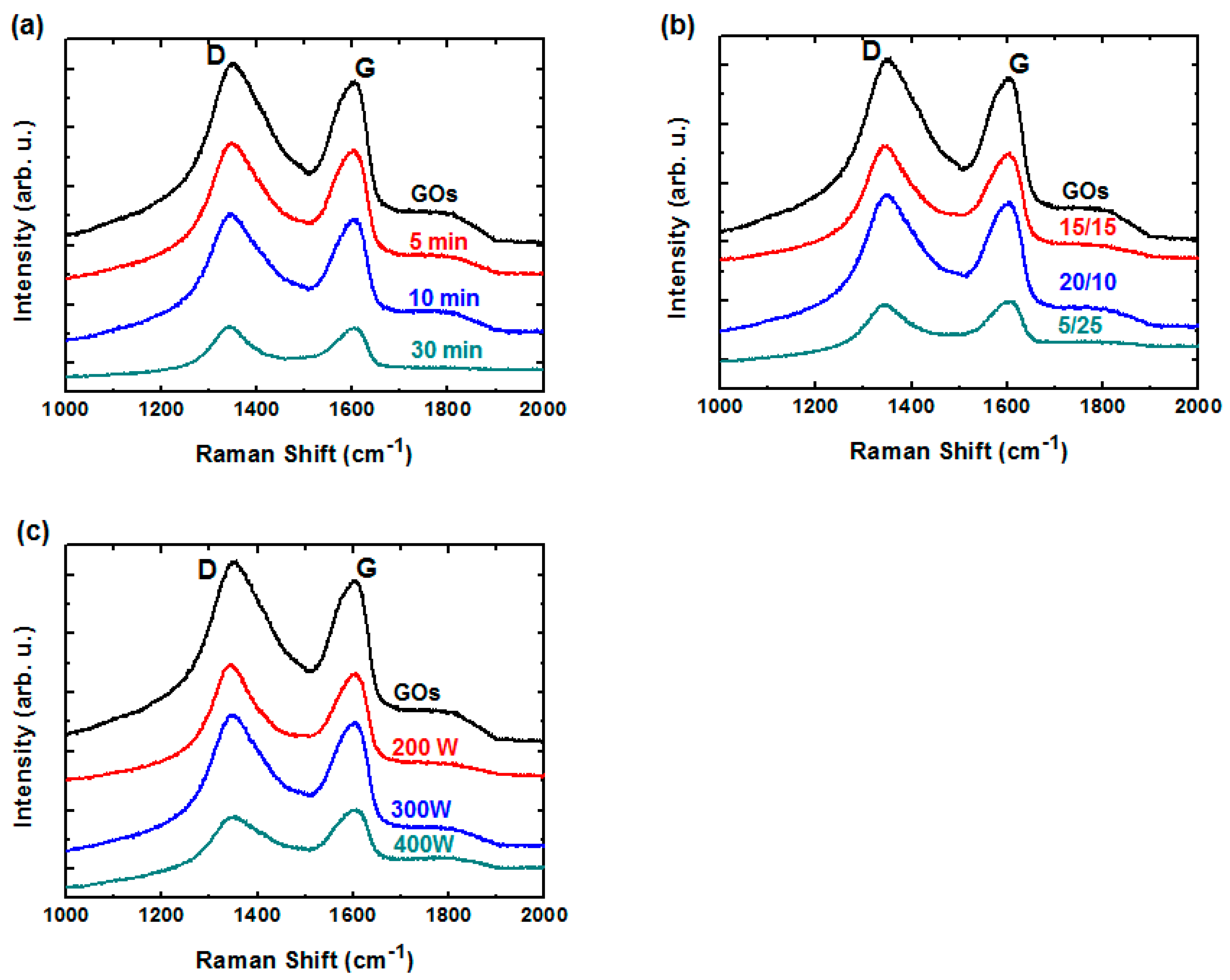
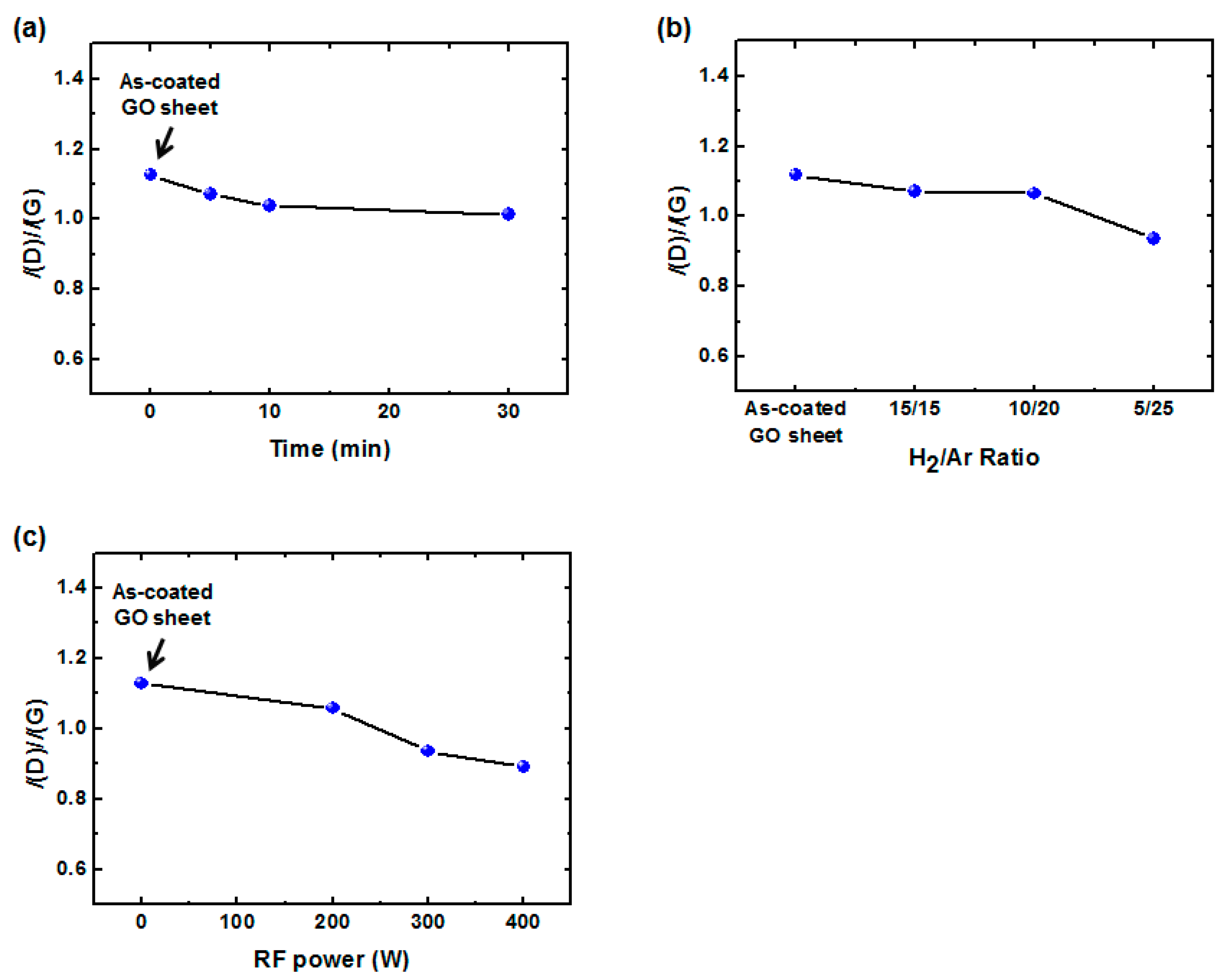
2.2. Low-Damage H2/Ar Plasma Treatment (H2/Ar-LDPT)
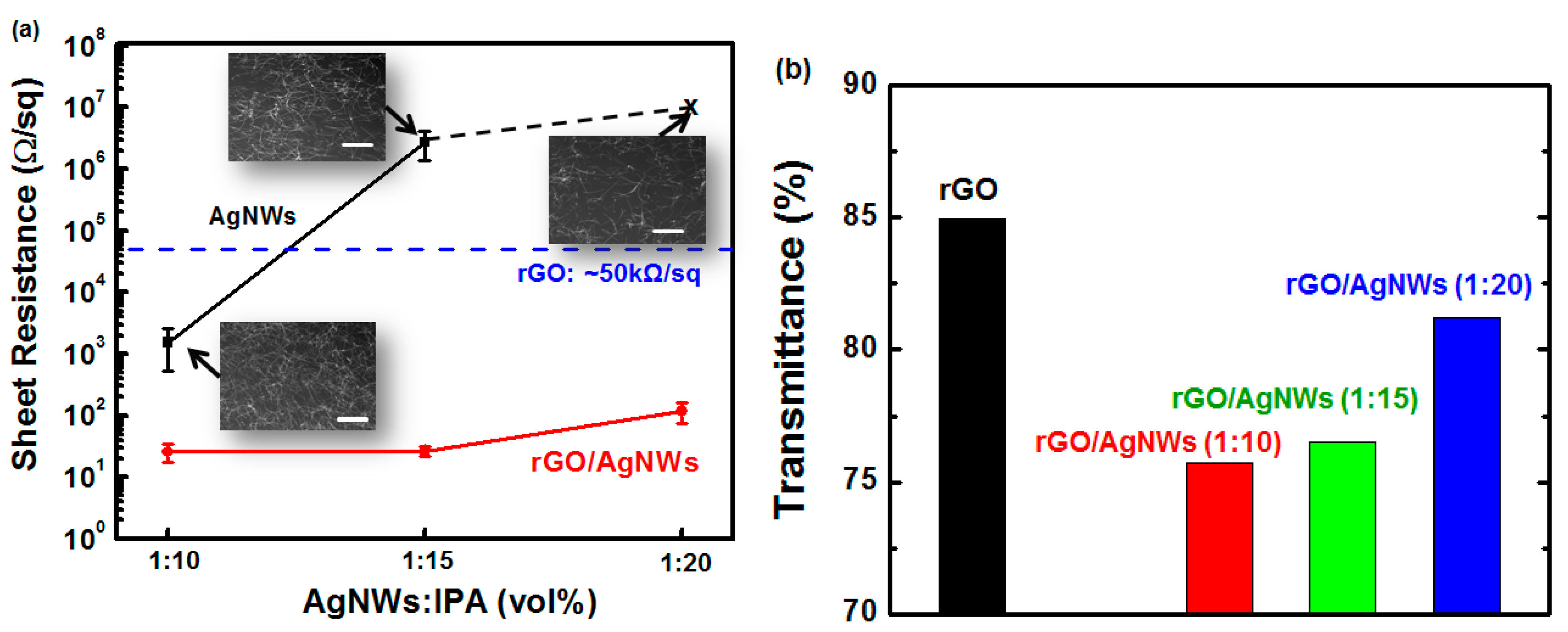
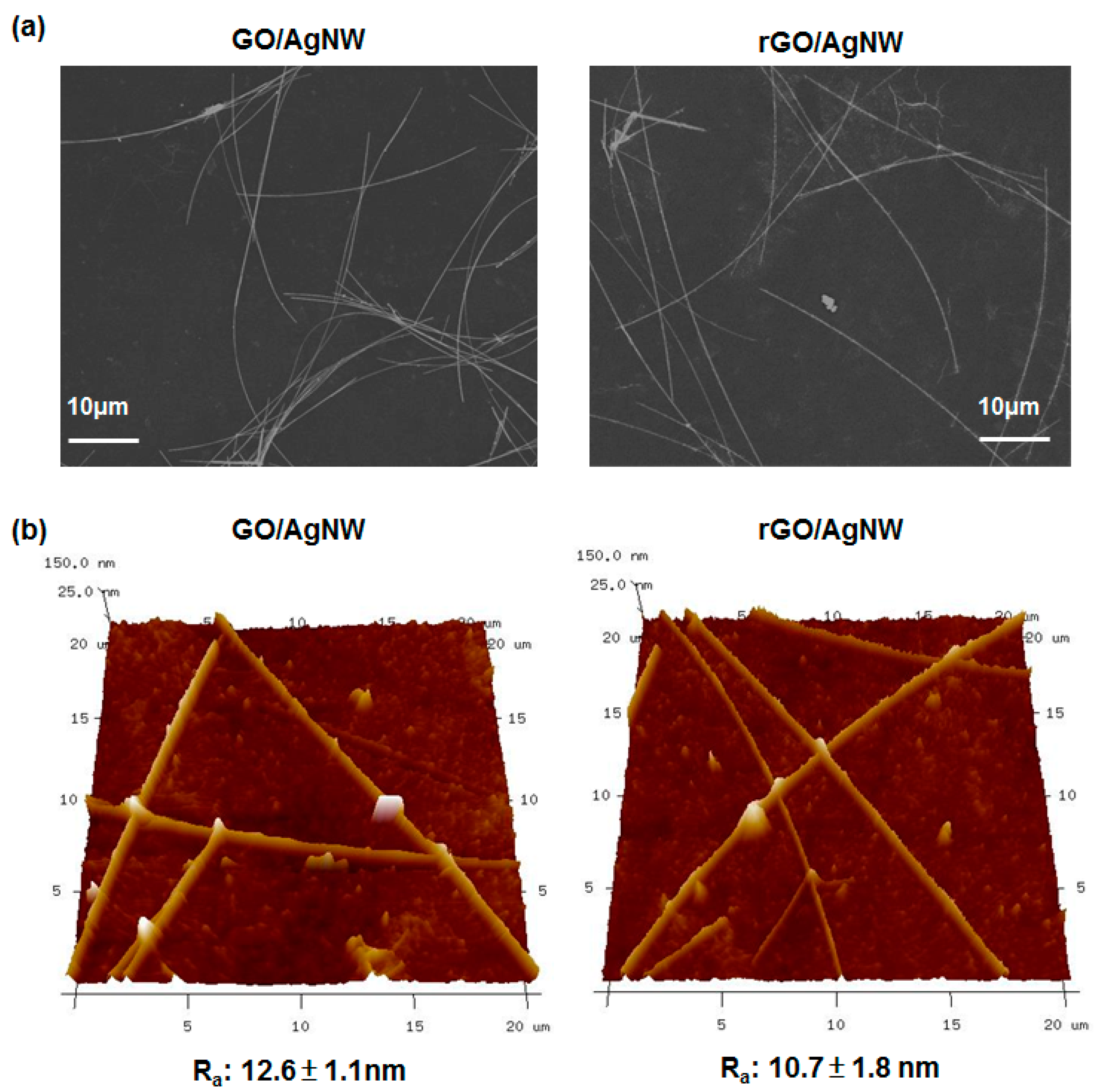
2.3. Characterization of rGO and Hybrid rGO/AgNW Thin Films
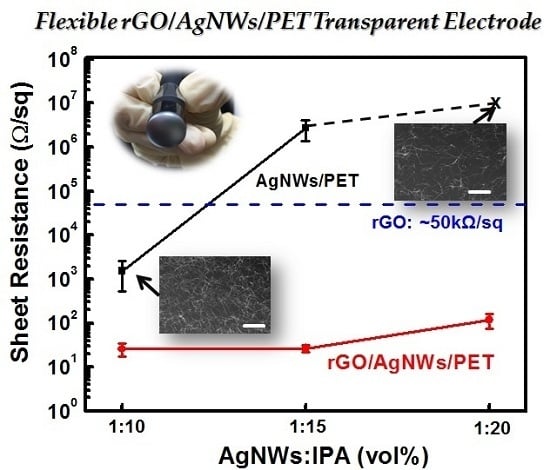
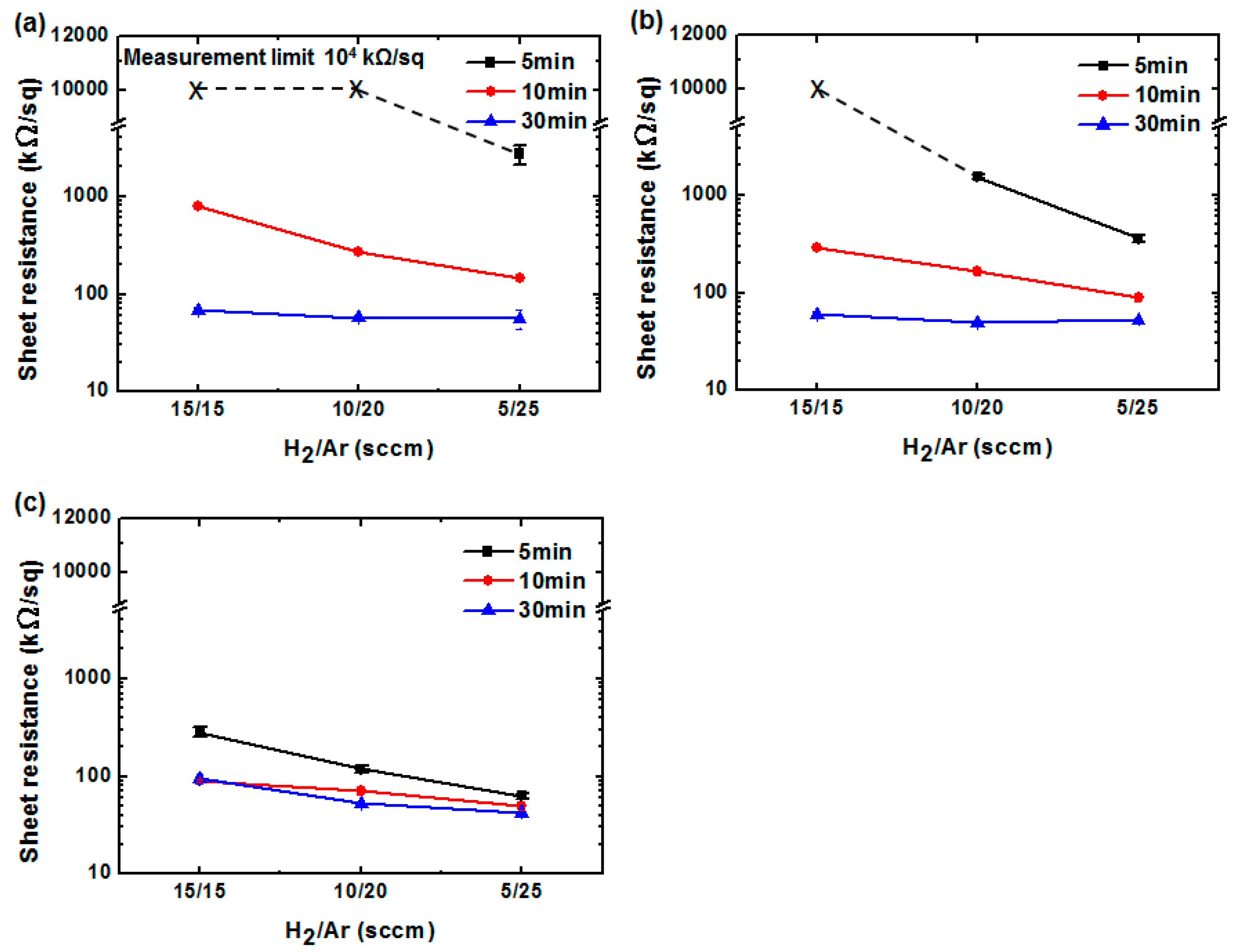
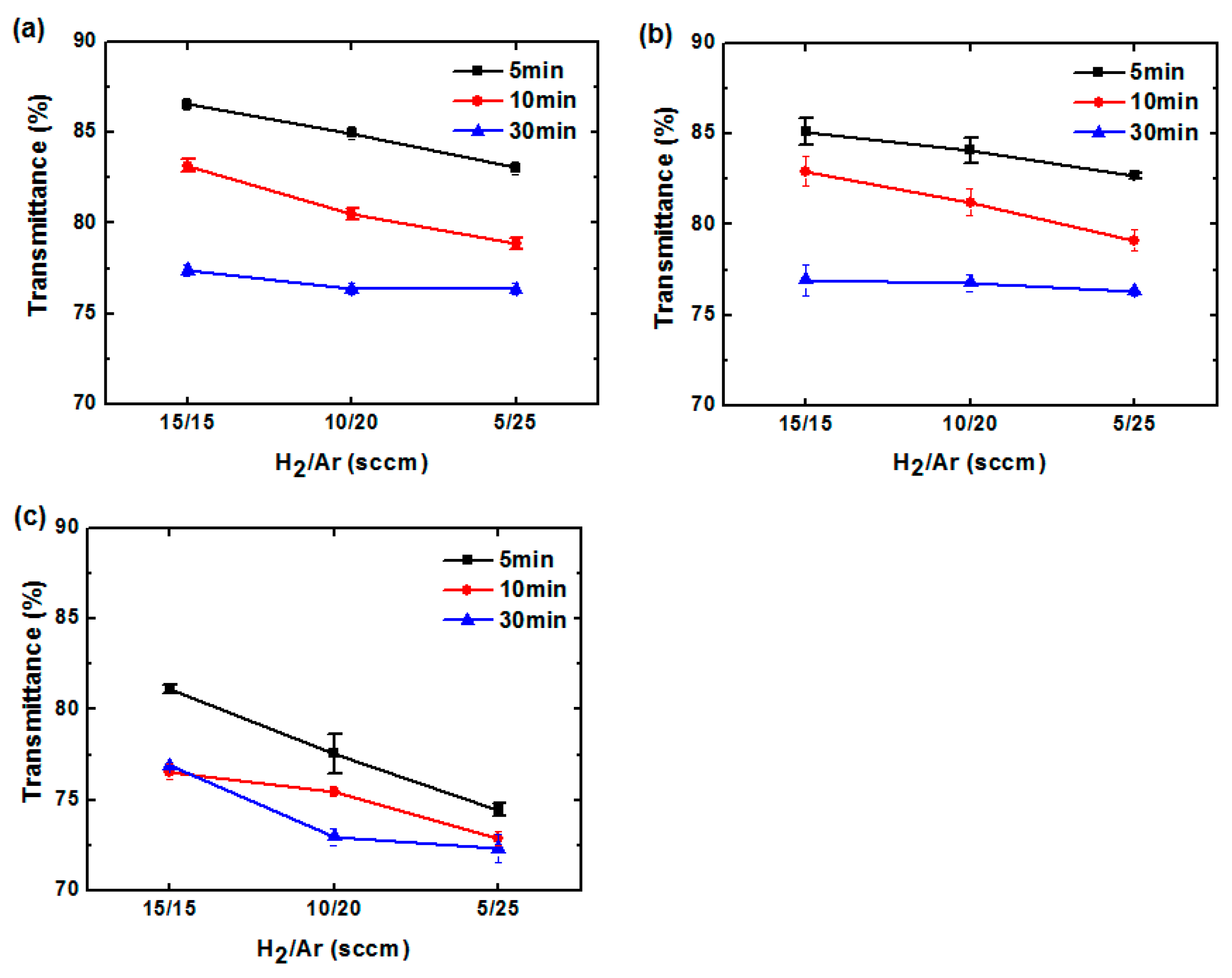
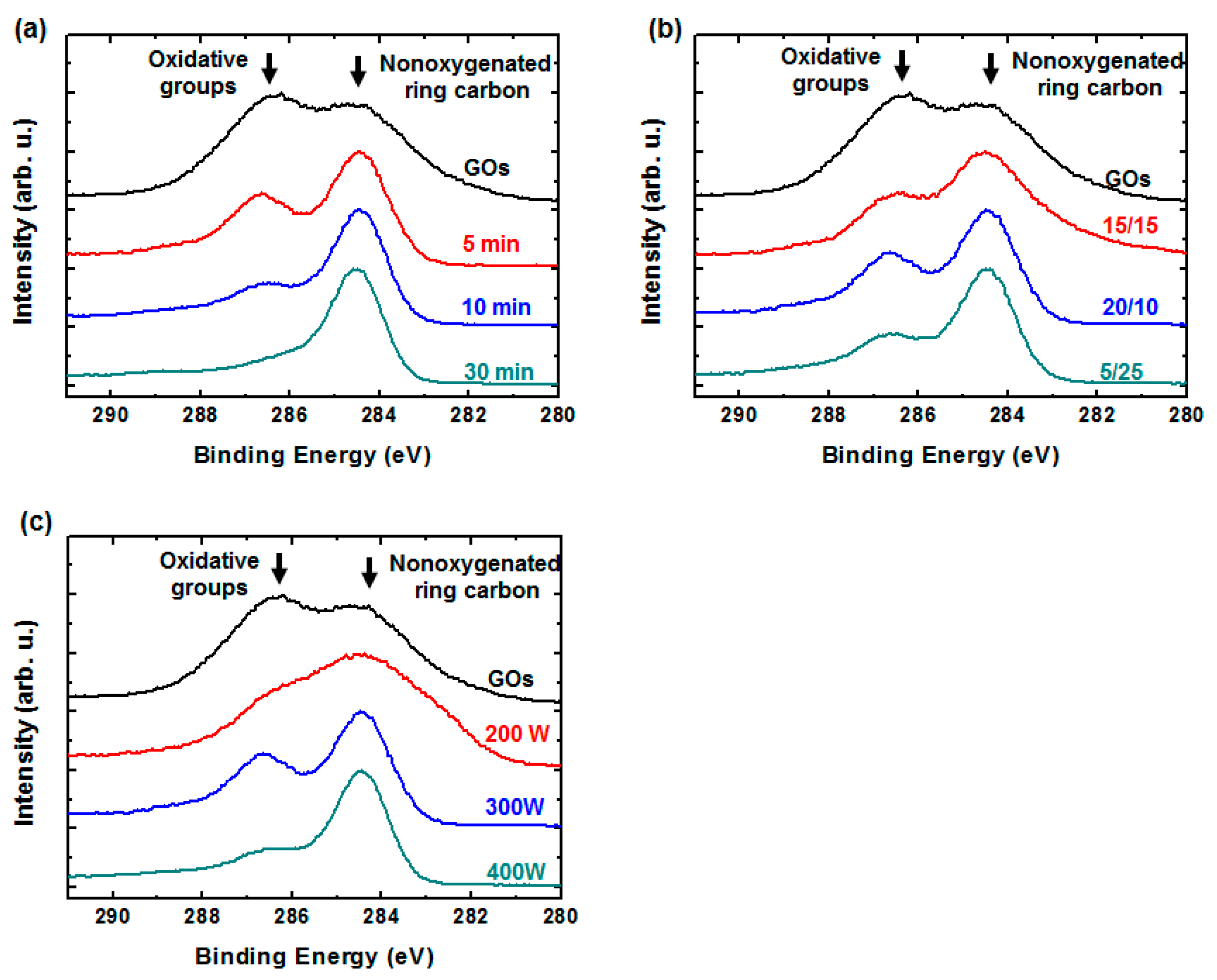
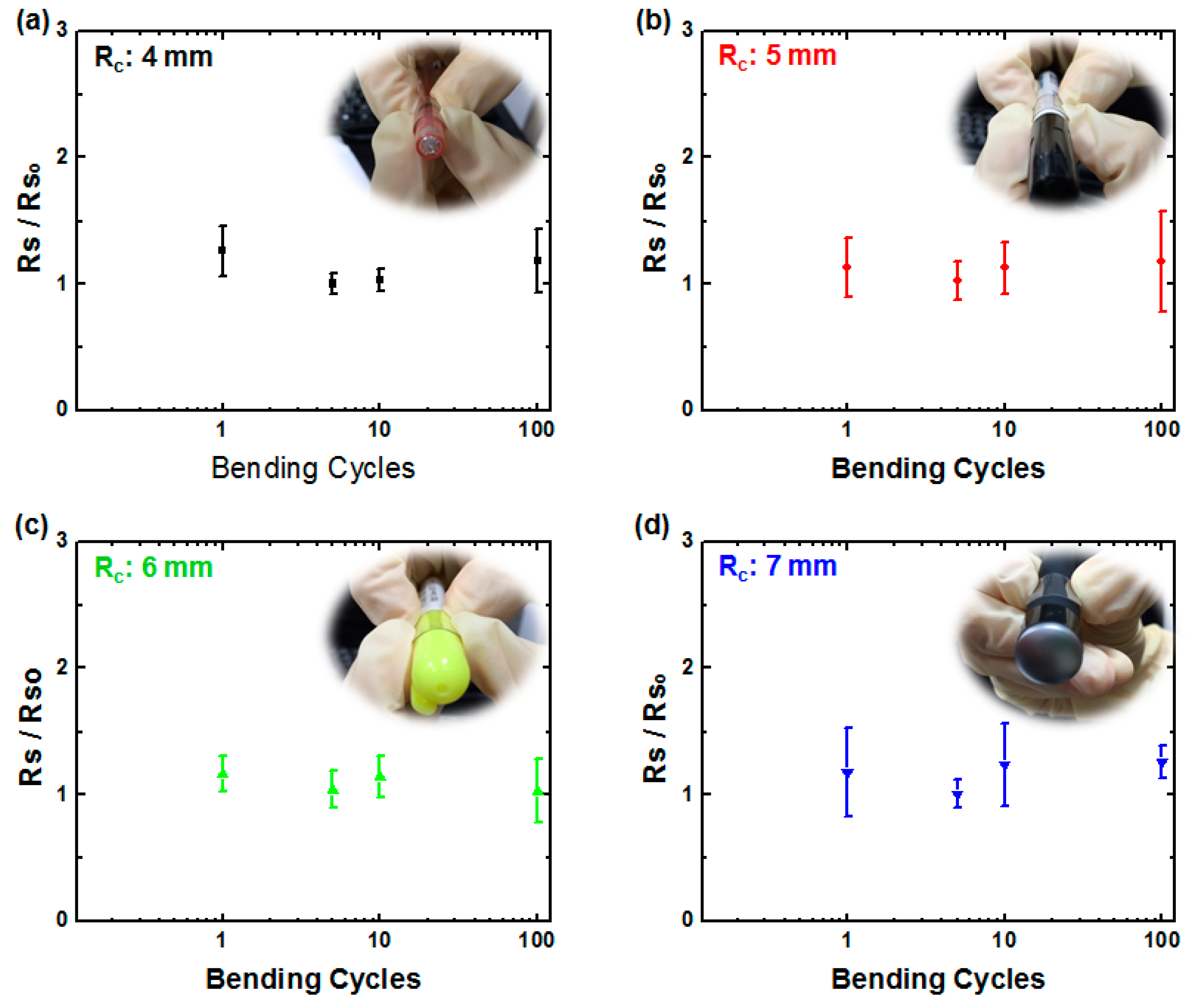
3. Results and Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldenegro-Perez, L.A.; Navarro-Rodriguez, D.; Medellin-Rodriguez, F.J.; Hsiao, B.; Avila-Ota, C.A.; Sics, I. Molecular weight and crystallization temperature effects on poly(ethylene terephthalate)(PET) homopolymers, an isothermal crystallization analysis. Polymers 2014, 6, 583–600. [Google Scholar] [CrossRef]
- Yue, Q.F.; Xiao, L.F.; Zhang, M.L.; Bai, X.F. The glycolysis of poly(ethylene terephthalate) waste: Lewis acidic ionic liquids as high efficient catalysts. Polymers 2013, 5, 1258–1271. [Google Scholar] [CrossRef]
- Dong, L.B.; Xu, C.J.; Huang, Z.H.; Kang, F.Y.; Yang, Q.H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Hang, T.H.; Lee, Y.; Choi, M.R.; Woo, S.H.; Bae, S.H.; Hong, B.H.; Ahn, J.H.; Lee, T.W. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 2012, 6, 105–110. [Google Scholar]
- Huang, C.H.; Yu, S.C.; Lai, Y.C.; Chi, G.C.; Yu, P. Efficiency enhancement of organic/GaAs Hybrid photovoltaic cells using transparent graphene as front electrode. IEEE J. Photovol. 2016, 6, 480–485. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zhang, H. Fabrication of graphene films with high transparent conducting characteristics. Nanoscale Res. Lett. 2013, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Antonova, I.V.; Mutilin, S.V.; Seleznev, V.A.; Soots, R.A.; Volodin, V.A.; Prinz, V.Y. Extremely high response of electrostatically exfoliated few layer graphene to ammonia adsorption. Nanotechnology 2011, 22, 285502. [Google Scholar] [CrossRef] [PubMed]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Keelogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.F.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2012, 45, 1558–1565. [Google Scholar] [CrossRef]
- Rajagopalan, B.; Chung, J.S. Reduced chemically modified graphene oxide for supercapacitor electrode. Nanoscale Res. Lett. 2014, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.F.; Zhao, J.P.; Du, J.H.; Ren, W.C.; Cheng, H.M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, T.; Liu, Y.H.; Qin, G.Q.; Wang, X.P.; Chen, T.S. Glucose-reduced graphene oxide with excellent biocompatibility and photothermal efficiency as well as drug loading. Nanoscale Res. Lett. 2016, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, F.; Liu, Y.L.; Ma, N.; Wang, Z.Q.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Fan, X.B.; Peng, W.C.; Li, Y.; Li, X.Y.; Wang, S.L.; Zhang, G.L.; Zhang, F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Lopez, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical vapor deposition repair of graphene oxide: A route to highly conductive graphene monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar] [CrossRef]
- Dai, H.J.; Li, X.L.; Zhang, G.Y.; Bai, X.D.; Sun, X.M.; Wang, W.R.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir-Blodgett films. Nat. Nanotechnol. 2008, 3, 538–542. [Google Scholar]
- Khai, T.V.; Kwon, Y.J.; Kim, S.S.; Shim, K.B.; Kim, H.W. High-quality graphene thin films synthesized by H2 ambient-annealing of reduced graphene oxide sheets. J. Ceram. Proc. Res. 2013, 14, 355–362. [Google Scholar]
- Grimm, S.; Schweiger, M.; Eigler, S.; Zaumseil, J. High-quality reduced graphene oxide by CVD-assisted annealing. J. Phys. Chem. C 2016, 120, 3036–3041. [Google Scholar] [CrossRef]
- Chen, Y.N.; Fu, K.; Zhu, S.Z.; Luo, W.; Wang, Y.B.; Li, Y.J.; Hitz, E.; Yao, Y.; Dai, J.; Wan, J.; et al. Reduced gphene oxide films with ultrahigh conductivity as Li-ion battery current collectors. Nano Lett. 2016, 16, 3616–3623. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Huang, G.S.; Cheng, Y.C.; Cao, R.G.; Ding, F.; Schwingenschlogl, U.; Mei, Y. Thinning and functionalization of few-layer graphene sheets by CF4 plasma treatment. Nanoscale Res. Lett. 2012, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Maultzsch, J.; Han, M.Y.; Kim, P.; Brus, L.E. Raman spectroscopy of lithographically patterned graphene nanoribbons. ACS Nano 2011, 5, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Samukawa, S. Selective in-plane nitrogen doping of graphene by an energy-controlled neutral beam. Nanotechnology 2015, 26, 485602. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Su, C.Y.; Okada, T.; Li, L.J.; Ho, K.I.; Li, P.W.; Chen, I.H.; Chou, C.; Lai, C.S.; Samukawa, S. Ultra-low-edge-defect graphene nanoribbons patterned by neutral beam. Carbon 2013, 61, 229–235. [Google Scholar] [CrossRef]
- McEvoy, N.; Nolan, H.; Kumar, N.A.; Hallam, T.; Duesberg, G.S. Functionalisation of graphene surfaces with downstream plasma treatments. Carbon 2013, 54, 283–290. [Google Scholar] [CrossRef]
- Jinnai, B.; Uesugi, T.; Koyama, K.; Kato, K.; Yasuda, A.; Maeda, S.; Momose, H.; Samukawa, S. Decisive factors affecting plasma resistance and roughness formation in ArF photoresist. J. Phys. D 2010, 43, 395204. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, I.S.; Ho, K.I.; Lin, Y.T.; Chou, C.; Chan, C.F.; Lai, C.S. High polarization and low-repulsion HfO2 thin film for alkali metal ion detections by plasma system with a complementary filter. IEEE Sens. J. 2013, 13, 2459–2465. [Google Scholar] [CrossRef]
- Huang, C.H.; Lin, C.T.; Wang, J.C.; Chou, C.; Ye, Y.R.; Cheng, B.M.; Lai, C.S. Tunable bandgap energy of fluorinated nanocrystals for flash memory applications produced by low-damage plasma treatment. Nanotechnology 2012, 23, 475201. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Su, C.Y.; Lai, C.S.; Samukawa, S. Ultra-low-damage radical treatment for the highly controllable oxidation of large-scale graphene sheets. Carbon 2014, 73, 244–251. [Google Scholar] [CrossRef]
- Cheng, H.E.; Wang, Y.Y.; Wu, P.C.; Huang, C.H. Preparation of large-area graphene oxide sheets with a high density of carboxyl groups using O2/H2 low-damage plasma. Surf. Coat. Technol. 2016, 303, 170–175. [Google Scholar] [CrossRef]
- Lee, S.W.; Mattevi, C.; Chhowalla, M.; Sankaran, R.M. Plasma-assisted reduction of graphene oxide at low temperature and atmospheric pressure for flexible conductor applications. Phys. Chem. Lett. 2012, 3, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Henson, A.B.; Gersten, S.; Shagam, Y.; Narevicius, J.; Narevicius, E. Observation of resonances in penning ionization reactions at sub-Kelvin temperatures inmerged beams. Science 2012, 338, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.-P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001. [Google Scholar] [CrossRef] [PubMed]
- Triambulo, R.E.; Cheong, H.-G.; Park, J.-W. All-solution-processed foldable transparent electrodes of Ag nanowire mesh and metal matrix films for flexible electronics. Org. Electron. 2014, 15, 2685. [Google Scholar] [CrossRef]
- Seong, B.; Chae, I.; Lee, H.; Nguyen, V.D.; Byun, D. Spontaneous self-welding of silver nanowire networks. Phys. Chem. Chem. Phys. 2015, 17, 7629. [Google Scholar] [CrossRef] [PubMed]
- Coskun, S.; Ates, E.S.; Unalan, H.E. Optimization of silver nanowire networks for polymer light emitting diode electrodes. Nanotechnology 2013, 24, 125202. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.T.; Vora, P.M.; Mele, E.J.; Johnson, A.T.C.; Kikkawa, J.M. Photoluminescence and band gap modulation in graphene oxide. Appl. Phys. Lett. 2008, 94, 111909. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Subrahmanyam, K.S.; Matte, H.S.S.R.; Abdulhakeem, B.; Govindaraj, A.; Das, B.; Kumar, P.; Ghosh, A.; Late, D.J. A study of the synthetic methods and properties of graphenes. Sci. Technol. Adv. Mater. 2010, 11, 054502. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Wang, Y.-Y.; Lu, T.-H.; Li, Y.-C. Flexible Transparent Electrode of Hybrid Ag-Nanowire/Reduced-Graphene-Oxide Thin Film on PET Substrate Prepared Using H2/Ar Low-Damage Plasma. Polymers 2017, 9, 28. https://doi.org/10.3390/polym9010028
Huang C-H, Wang Y-Y, Lu T-H, Li Y-C. Flexible Transparent Electrode of Hybrid Ag-Nanowire/Reduced-Graphene-Oxide Thin Film on PET Substrate Prepared Using H2/Ar Low-Damage Plasma. Polymers. 2017; 9(1):28. https://doi.org/10.3390/polym9010028
Chicago/Turabian StyleHuang, Chi-Hsien, Yin-Yin Wang, Tsung-Han Lu, and Yen-Cheng Li. 2017. "Flexible Transparent Electrode of Hybrid Ag-Nanowire/Reduced-Graphene-Oxide Thin Film on PET Substrate Prepared Using H2/Ar Low-Damage Plasma" Polymers 9, no. 1: 28. https://doi.org/10.3390/polym9010028