Gamma-Irradiation Effects on the Spectral and Amplified Spontaneous Emission (ASE) Properties of Conjugated Polymers in Solution
Abstract
:1. Introduction
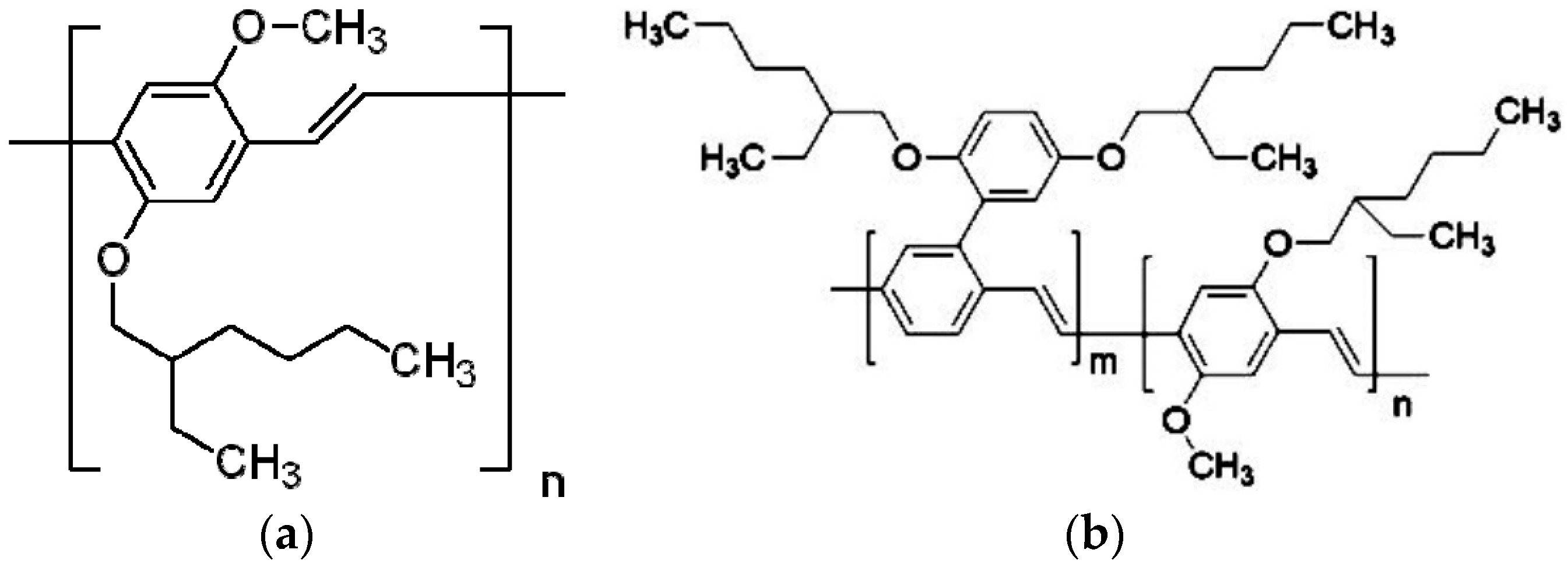
2. Materials and Methods
3. Results and Discussion
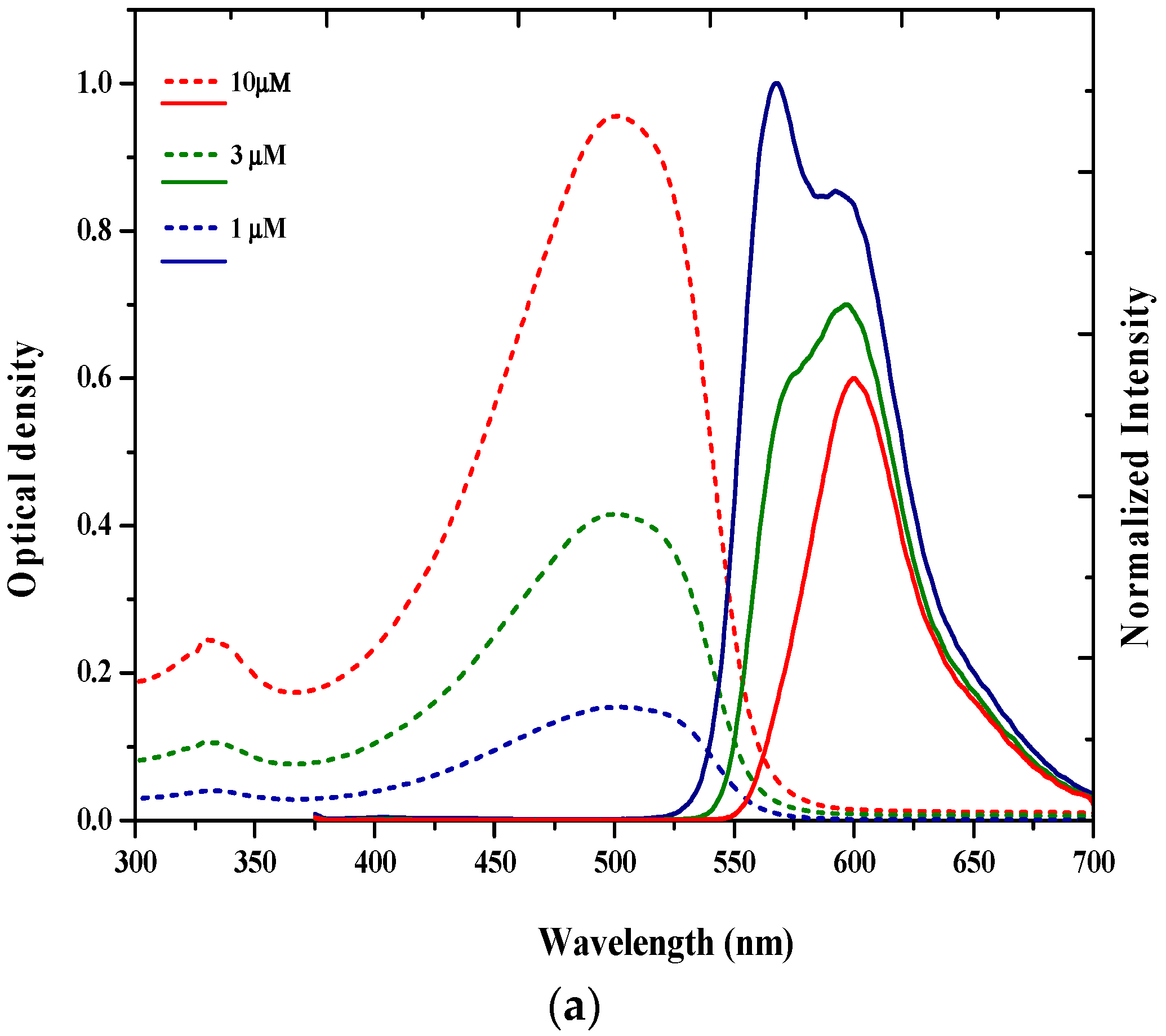
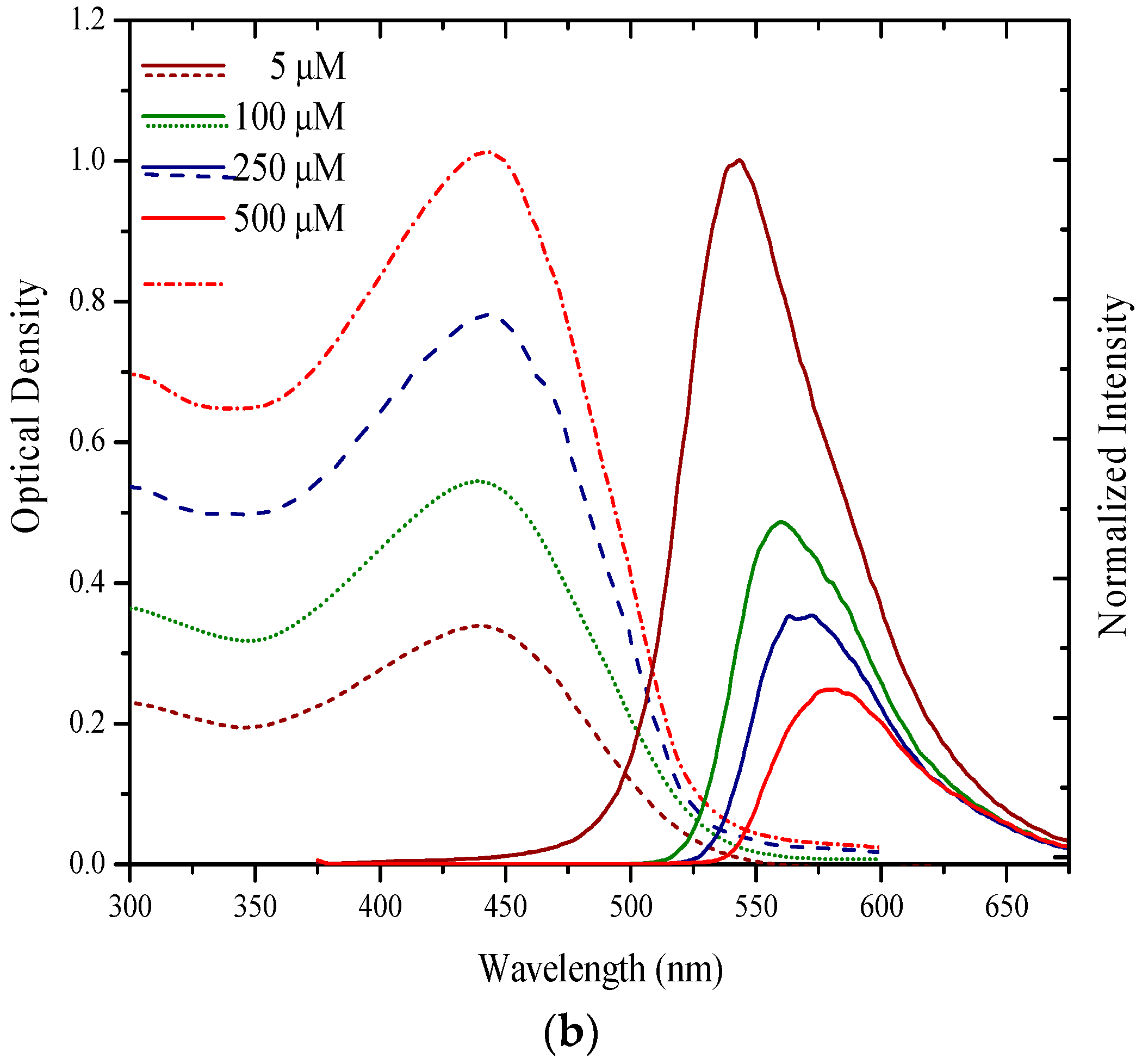
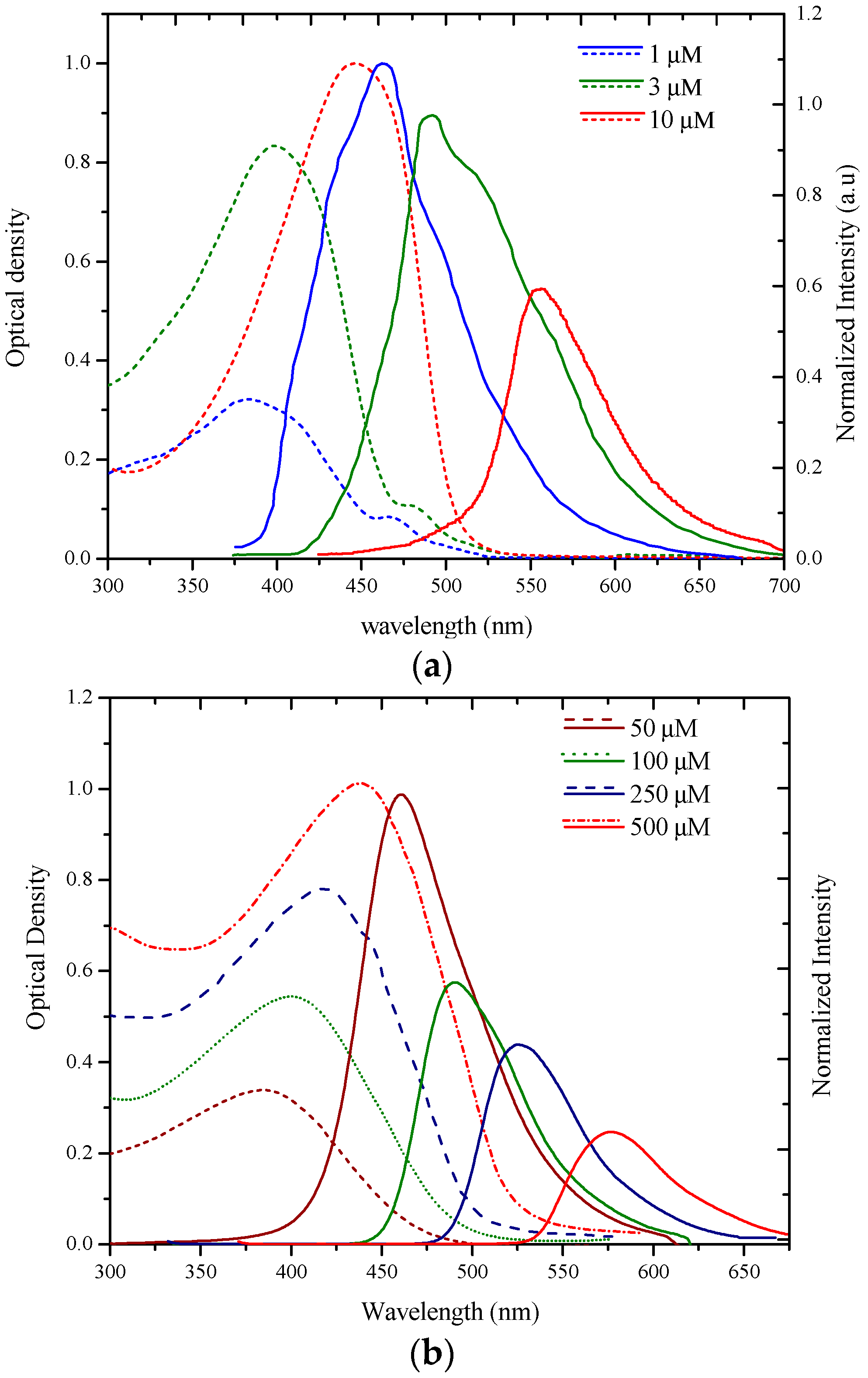
3.1. Spectral Properties
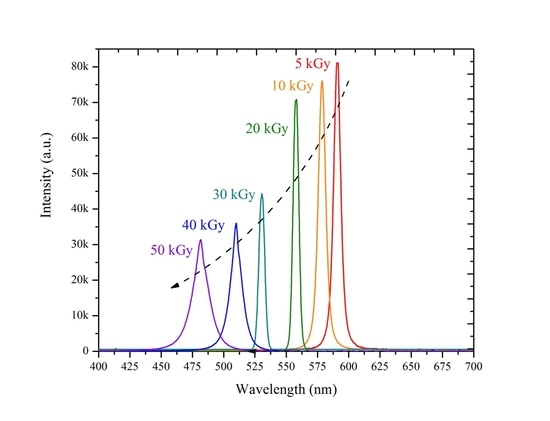
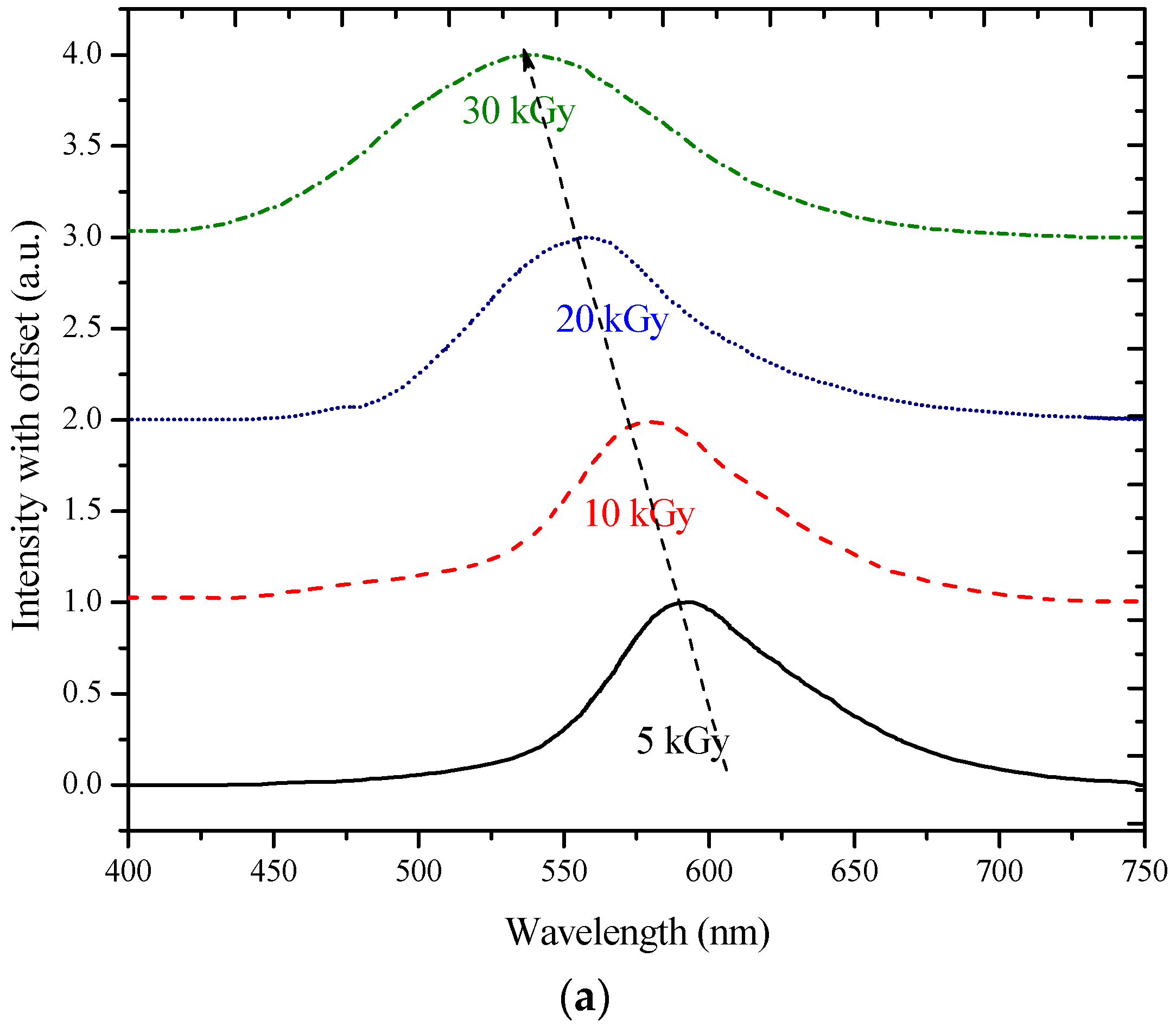
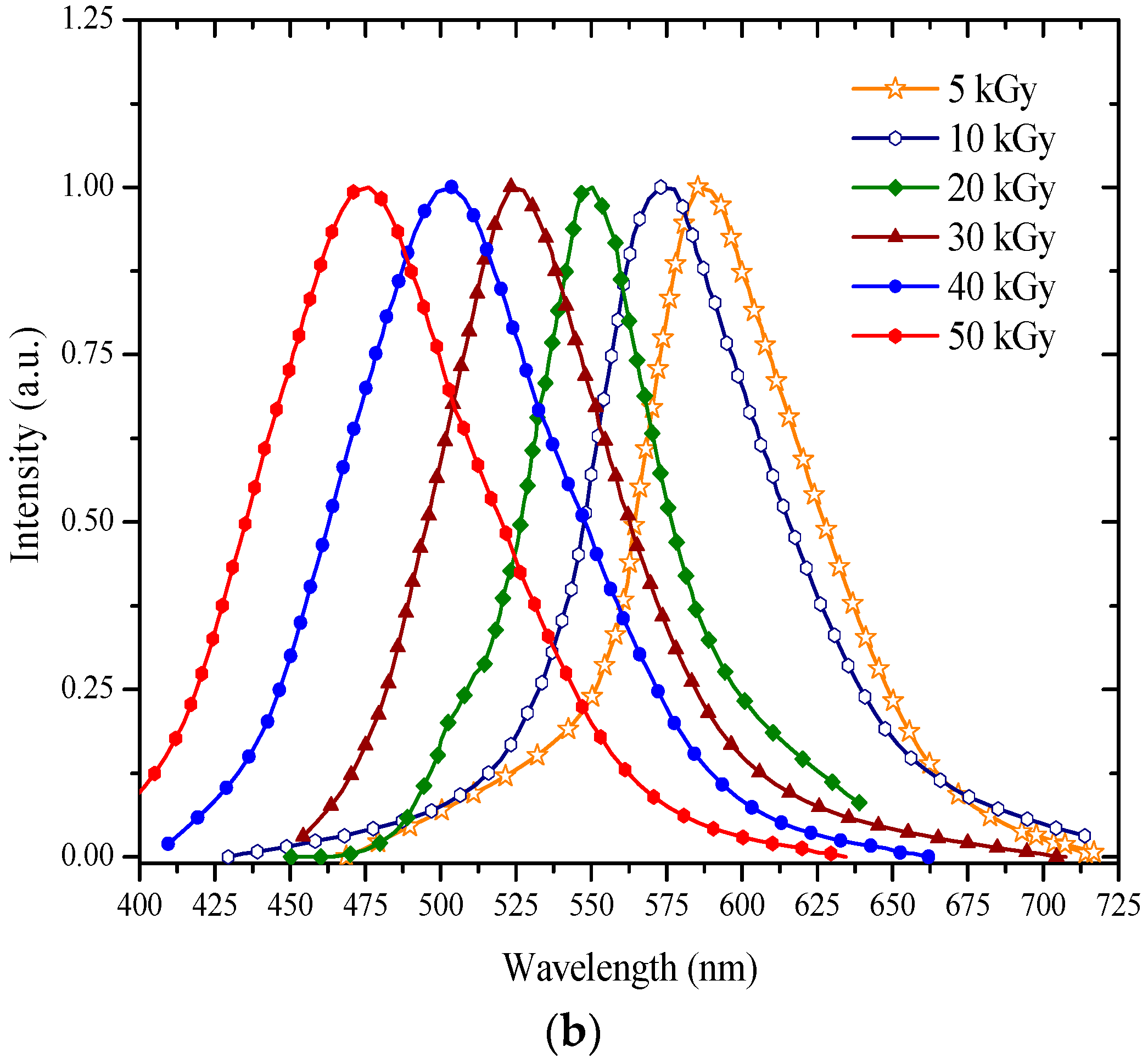
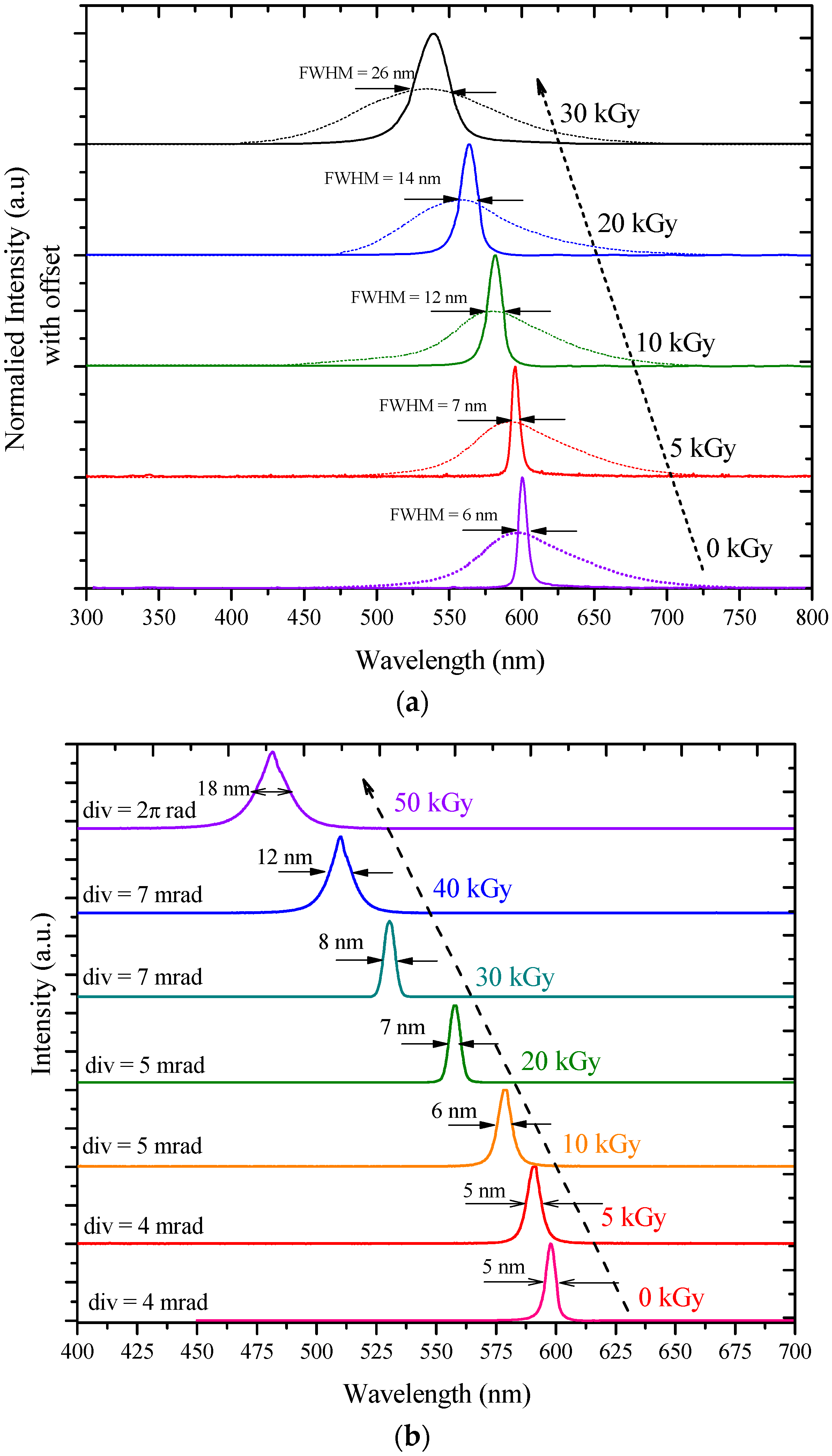
3.2. ASE Spectra
3.3. Raman Spectra
3.4. FT-IR Spectra
3.5. Swelling Experiment and Gel Permeation Chromatography (GPC)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tessler, N. Lasers based on semiconducting organic materials. Adv. Mater. 1999, 11, 363–370. [Google Scholar] [CrossRef]
- Friend, R.; Gymer, R.; Holmes, A.; Burroughes, J.; Marks, R.; Taliani, C.; Bradley, D.; Santos, D.D.; Bredas, J.; Lögdlund, M. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Ho, P.K.; Kim, J.-S.; Burroughes, J.H.; Becker, H.; Li, S.F.; Brown, T.M.; Cacialli, F.; Friend, R.H. Molecular-scale interface engineering for polymer light-emitting diodes. Nature 2000, 404, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.; Huiberts, J.; Blom, P. Simultaneous measurement of electron and hole mobilities in polymer light-emitting diodes. Appl. Phys. Lett. 2000, 77, 1852–1854. [Google Scholar] [CrossRef]
- Crone, B.; Campbell, I.; Davids, P.; Smith, D. Charge injection and transport in single-layer organic light-emitting diodes. Appl. Phys. Lett. 1998, 73, 3162–3164. [Google Scholar] [CrossRef]
- Jang, J.W.; Lee, C.E.; Lee, D.; Jin, J.-I. Transient electroluminescence study of mobility balancing in organic light-emitting diodes based on poly (p-phenylenevinylene) derivatives. Solid State Commun. 2004, 130, 265–268. [Google Scholar] [CrossRef]
- Lee, H.M.; Oh, D.K.; Lee, C.H.; Lee, C.E.; Lee, D.W.; Jin, J.I. Time-of-flight measurements of charge-carrier mobilities in a poly(p-phenylenevinylene) derivative carrying an electron-transporting moiety. Synth. Met. 2001, 119, 473–474. [Google Scholar] [CrossRef]
- Yoshino, K.; Hayashi, S.; Inuishi, Y. Effects of Electron Irradiation on Electrical Conductivity of Polyacetylene. Jpn. J. Appl. Phys. 1982, 21, L569. [Google Scholar] [CrossRef]
- Kudoh, H.; Sasuga, T.; Seguchi, T.; Katsumura, Y. High-energy-ion-irradiation effects on polymer materials: 3. The sensitivity of cellulose triacetate and poly(methyl methacrylate). Polymer 1996, 37, 2903–2908. [Google Scholar] [CrossRef]
- Graham, S.; Friend, R.; Fung, S.; Moratti, S. The effect of X-ray irradiation on poly(p-phenylene vinylene) and derivatives. Synth. Met. 1997, 84, 903–904. [Google Scholar] [CrossRef]
- Silva, E.; Borin, J.; Nicolucci, P.; Graeff, C.; Netto, T.G.; Bianchi, R. Low dose ionizing radiation detection using conjugated polymers. Appl. Phys. Lett. 2005, 86, 131902. [Google Scholar] [CrossRef]
- Scherf, U.; Riechel, S.; Lemmer, U.; Mahrt, R. Conjugated polymers: Lasing and stimulated emission. Curr. Opin. Solid State Mater. Sci. 2001, 5, 143–154. [Google Scholar] [CrossRef]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- Chapiro, A. Chemical modifications in irradiated polymers. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1988, 32, 111–114. [Google Scholar] [CrossRef]
- Lee, E.H. Ion-beam modification of polymeric materials–fundamental principles and applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 151, 29–41. [Google Scholar] [CrossRef]
- Adel, M.; Amir, O.; Kalish, R.; Feldman, L.C. Ion-beam-induced hydrogen release from a-C:H: A bulk molecular recombination model. J. Appl. Phys. 1989, 66, 3248–3251. [Google Scholar] [CrossRef]
- De Jong, M.; Maas, A.; van Ijzendoorn, L.; Klein, S.; de Voigt, M. A model for ion-irradiation induced hydrogen loss from organic materials. J. Appl. Phys. 1997, 82, 1058–1064. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z.-I. How the π conjugation length affects the fluorescence emission efficiency. J. Am. Chem. Soc. 2008, 130, 13867–13869. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhao, Y.; Li, Y.; Pei, Q. Photoluminescence quenching of conjugated polymer nanocomposites for gamma ray detection. Nanotechnology 2008, 19, 505503. [Google Scholar] [CrossRef] [PubMed]
- Sonkawade, R.; Kumar, V.; Kumar, L.; Annapoorni, S.; Vaijapurkar, S.; Dhaliwal, A. Effects of gamma ray and neutron radiation on polyanilne conducting polymer. Indian J. Pure Appl. Phys. 2010, 48, 453–456. [Google Scholar]
- Yang, G.; Chen, X.; Wang, Y.; Feng, S. Lasing characteristic of organic octagonal quasicrystal slabs with single-defect microcavity at low-index contrast. Opt. Express 2013, 21, 11457–11464. [Google Scholar] [CrossRef] [PubMed]
- Ibnaouf, K.; Prasad, S.; Masilamani, V.; AlSalhi, M.; Mustapha, N.; Alyamani, A. Triple amplified spontaneous emissions from a conjugated copolymer BEHP-co-MEH–PPV in solution. Phys. E Low Dimens. Syst. Nanostruct. 2013, 53, 66–71. [Google Scholar] [CrossRef]
- Mustapha, N.; Ibnaouf, K.; Fekkai, Z.; Hennache, A.; Prasad, S.; Alyamani, A. Improved efficiency of solar cells based on BEHP-co-MEH–PPV doped with ZnO nanoparticles. Opt. Int. J. Light Electron Opt. 2013, 124, 5524–5527. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Al Salhi, M.; Hamdan, A.; Zaman, M.; El Mir, L. Influence of the solvent environments on the spectral features of CdSe quantum dots with and without ZnS shell. J. Lumin. 2014, 149, 369–373. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.; AlSalhi, M.; Masilamani, V. Laser from the dimer state of a conjugated polymer (PFO) in solution. Polymer 2014, 55, 727–732. [Google Scholar] [CrossRef]
- Ugelstad, J.; Mork, P.; Kaggerud, K.H.; Ellingsen, T.; Berge, A. Swelling of oligomer-polymer particles. New methods of preparation. Adv. Colloid Interface Sci. 1980, 13, 101–140. [Google Scholar] [CrossRef]
- Alsalhi, M.; Ibnaouf, K.; Masilamani, V.; Yassin, O. Excimer state of a conjugate polymer (MEH–PPV) in liquid solutions. Laser Phys. 2007, 17, 1361–1366. [Google Scholar] [CrossRef]
- Masilamani, V.; Chandrasekar, V.; Sivaram, B.; Sivasankar, B.; Natarajan, S. Simultaneous dual band laser emission from two conformations of DAMC. Opt. Commun. 1986, 59, 203–207. [Google Scholar] [CrossRef]
- Ibnaouf, K.; Prasad, S.; Masilamani, V.; AlSalhi, M. Evidence for amplified spontaneous emission from double excimer of conjugated polymer (PDHF) in a liquid solution. Polymer 2013, 54, 2401–2405. [Google Scholar] [CrossRef]
- Wegmann, G.; Giessen, H.; Greiner, A.; Mahrt, R. Laser emission from a solid conjugated polymer: Gain, tunability, and coherence. Phys. Rev. B 1998, 57, R4218. [Google Scholar] [CrossRef]
- Bazani, D.L.M.; Lima, J.P.H.; de Andrade, A.M. MEH–PPV thin films for radiation sensor applications. IEEE Sens. J. 2009, 9, 748–751. [Google Scholar] [CrossRef]
- Choi, D.H.; Cho, M.J.; Han, K.I.; Chang, I.-H.; Song, J.S.; Kim, J.H.; Paek, S.-H.; Choi, S.-H. Luminescence properties of MEH–PPV and its crosslinked polymer: Effect of crosslink on photoluminescence and electroluminescence. Synth. Met. 2006, 156, 685–689. [Google Scholar] [CrossRef]
- Wu, C.; McNeill, J. Swelling-controlled polymer phase and fluorescence properties of polyfluorene nanoparticles. Langmuir 2008, 24, 5855–5861. [Google Scholar] [CrossRef] [PubMed]
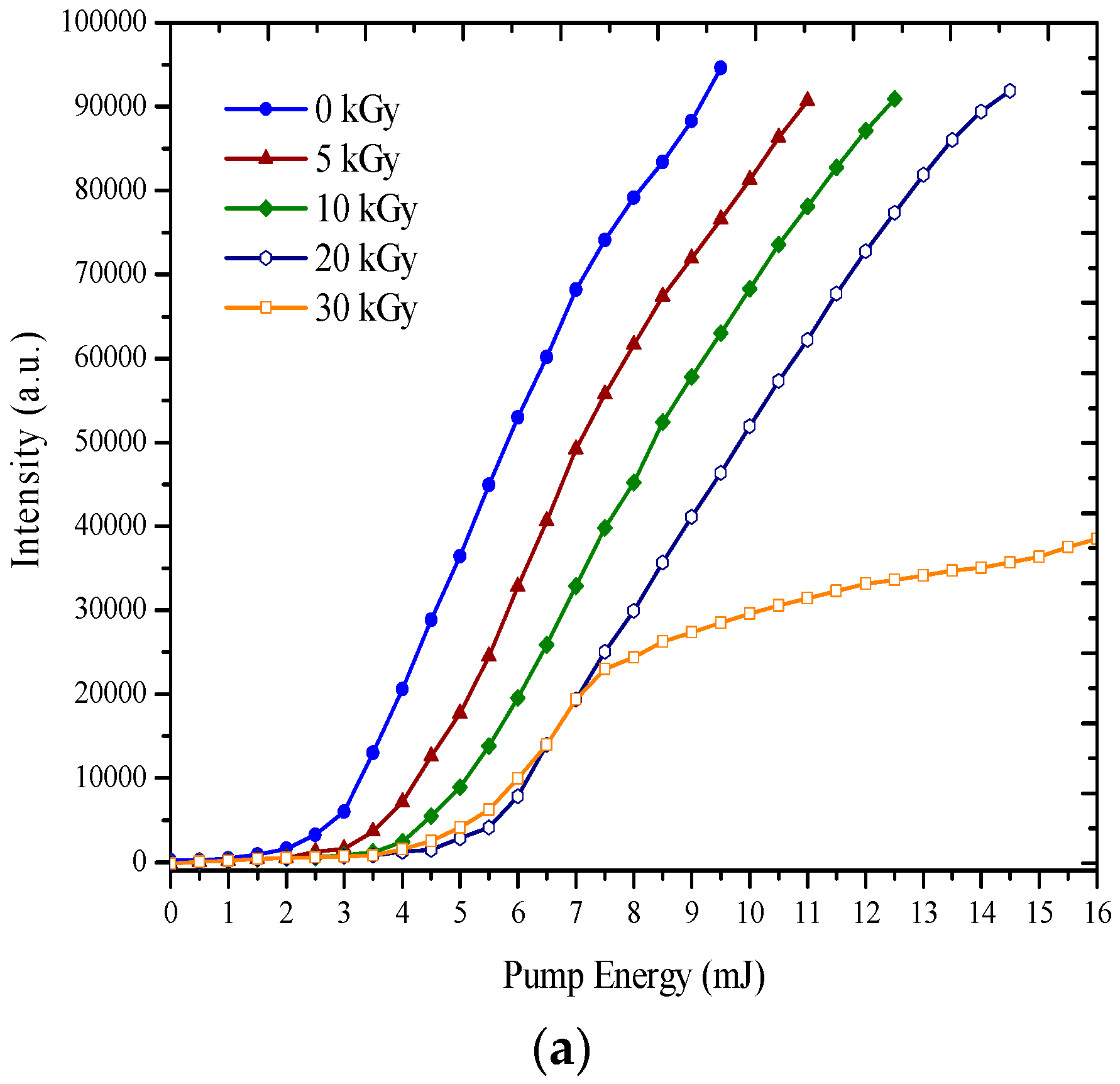
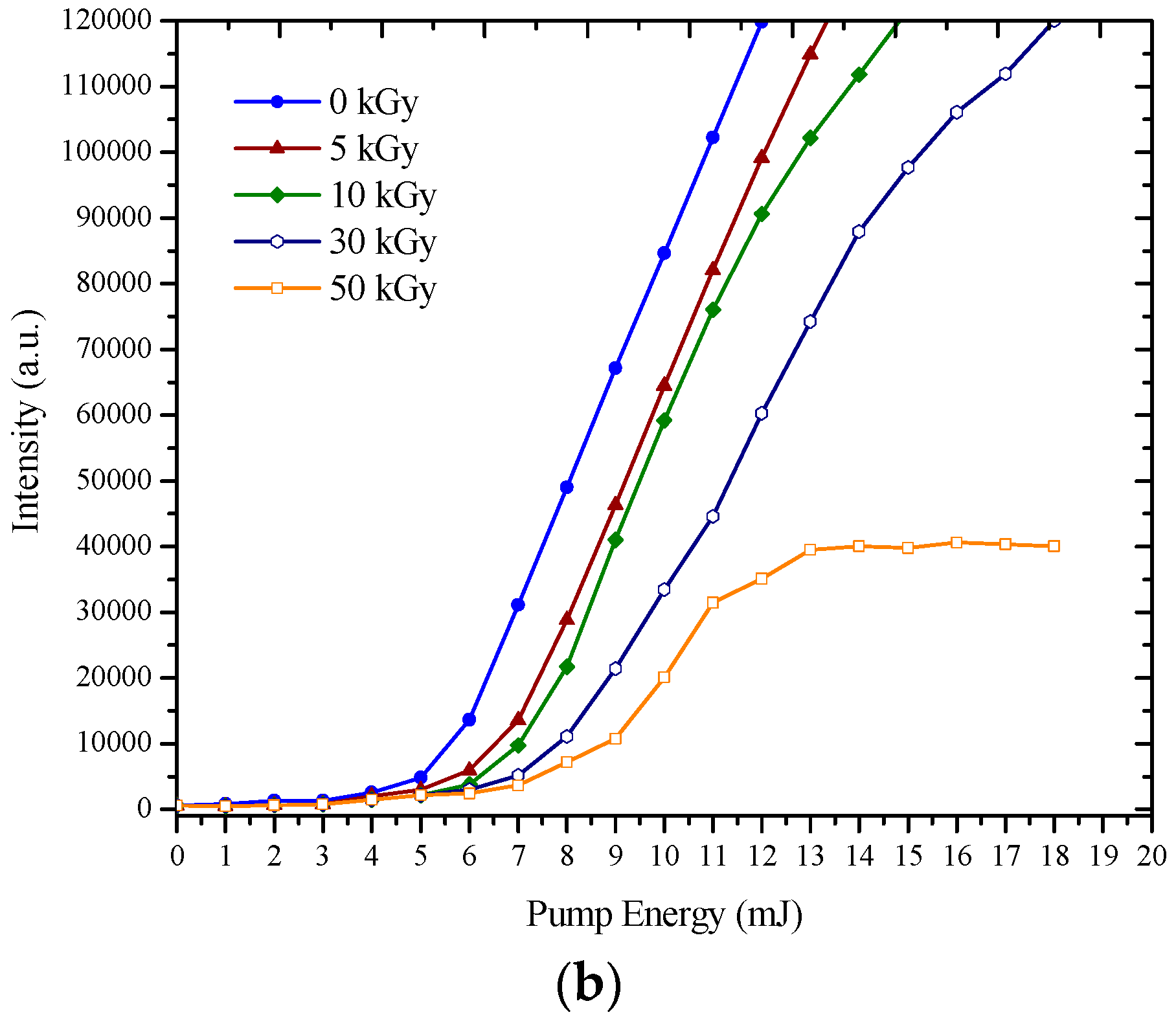
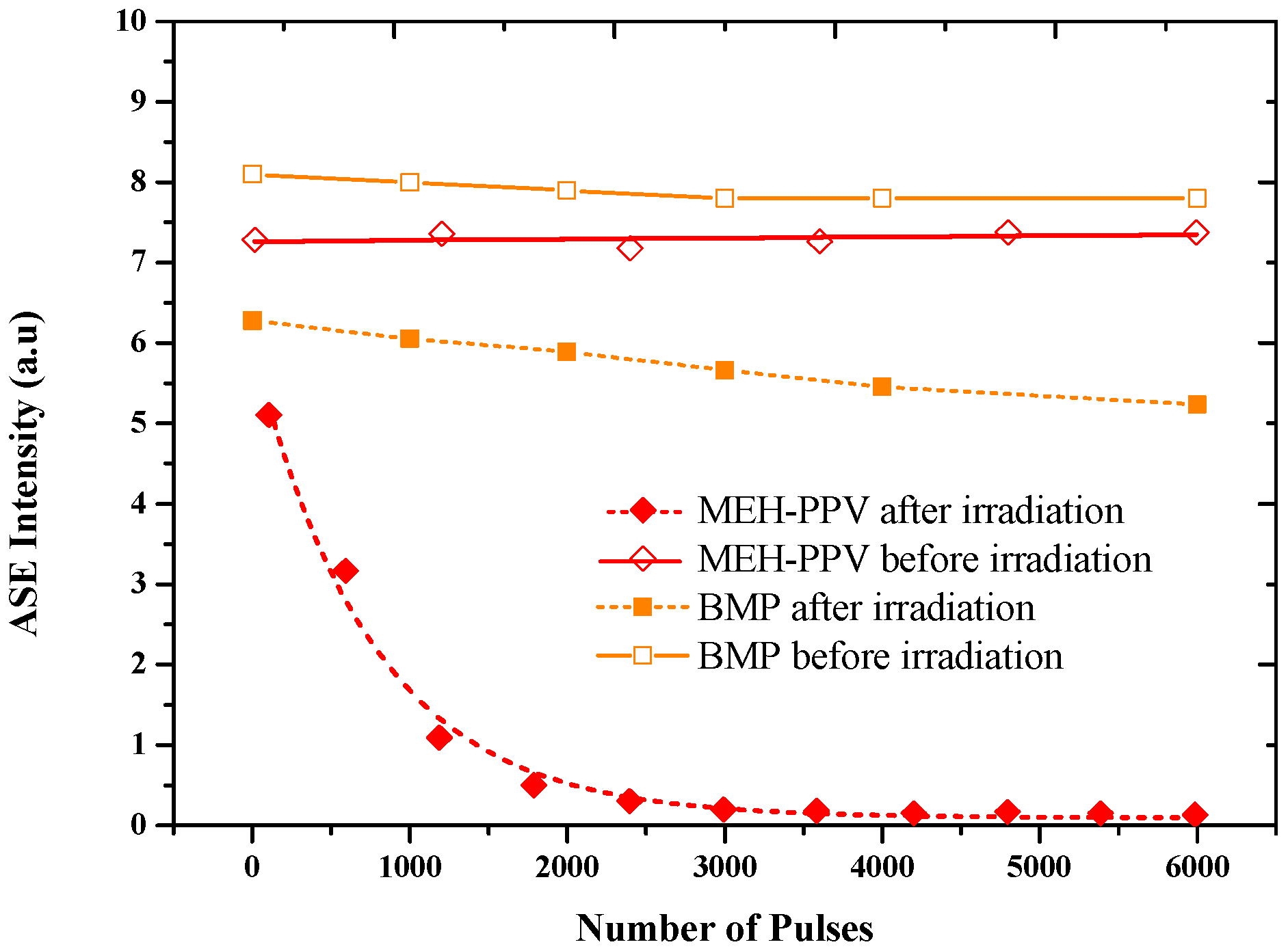

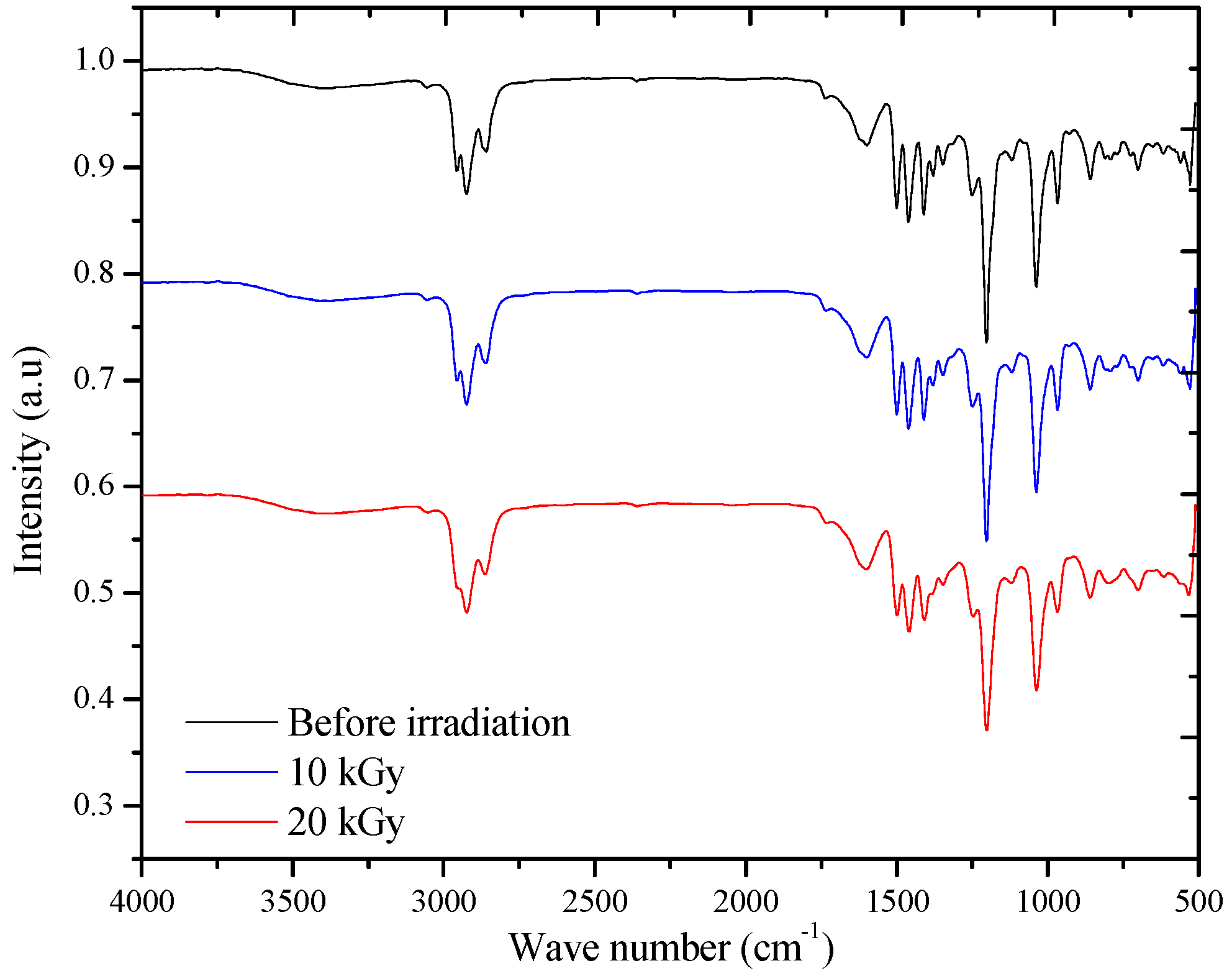
| BEHP-co-MEH–PPV | ||||||
|---|---|---|---|---|---|---|
| Dose kGy | Fluorescence peak | ASE peak wavelength (nm) | Peak intensity (a.u.) | Blue shift | FWHM | |
| a | 0 | 595 | 598 | 102,045 | 0 | 5 |
| 5 | 585 | 590 | 81,081 | 5 | 5 | |
| 10 | 573 | 579 | 76,091 | 19 | 7 | |
| 20 | 550 | 557 | 70,832 | 41 | 7 | |
| 30 | 525 | 530 | 44,390 | 68 | 8 | |
| 40 | 504 | 510 | 36,067 | 88 | 12 | |
| 50 | 476 | 481 | 31,434 | 117 | 17 | |
| MEH–PPV | ||||||
| b | 0 | 595 | 600 | 78,985 | 0 | 6 |
| 5 | 590 | 595 | 61,224 | 5 | 7 | |
| 10 | 578 | 582 | 45,012 | 18 | 12 | |
| 20 | 556 | 563 | 29,587 | 37 | 14 | |
| 30 | 536 | 540 | 23,971 | 60 | 28 | |
| MEH–PPV | ||||
|---|---|---|---|---|
| Dose kGy | Quantum yield | Molecular weight (Mw) | Polydispersity index PDI = | |
| a | 0 kGy | 0.43 | 120,830 | 1.6 |
| 10 kGy | 0.32 | 144,508 | 2.5 | |
| 20 kGy | 0.27 | 159,204 | 3.6 | |
| BMP | ||||
| b | 0 kGy | 0.46 | 132,030 | 2.4 |
| 10 kGy | 0.35 | 142,310 | 3.2 | |
| 20 kGy | 0.28 | 156,025 | 4.1 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. AlSalhi, M.; Prasad, S.; Devaraj, D.; S. Abo Mustafa, Z. Gamma-Irradiation Effects on the Spectral and Amplified Spontaneous Emission (ASE) Properties of Conjugated Polymers in Solution. Polymers 2017, 9, 7. https://doi.org/10.3390/polym9010007
S. AlSalhi M, Prasad S, Devaraj D, S. Abo Mustafa Z. Gamma-Irradiation Effects on the Spectral and Amplified Spontaneous Emission (ASE) Properties of Conjugated Polymers in Solution. Polymers. 2017; 9(1):7. https://doi.org/10.3390/polym9010007
Chicago/Turabian StyleS. AlSalhi, Mohamad, Saradh Prasad, D. Devaraj, and Ziad S. Abo Mustafa. 2017. "Gamma-Irradiation Effects on the Spectral and Amplified Spontaneous Emission (ASE) Properties of Conjugated Polymers in Solution" Polymers 9, no. 1: 7. https://doi.org/10.3390/polym9010007