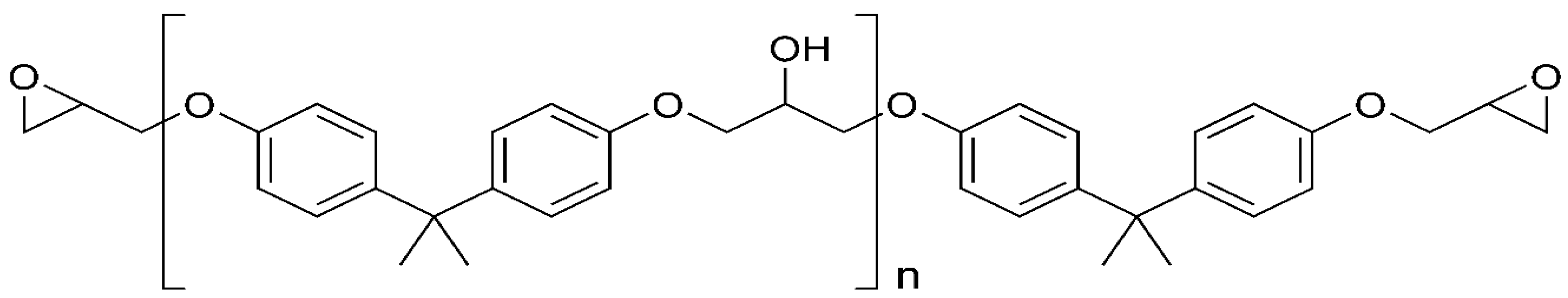
3.2. Strength Test Results—Analysis of the Effect of the Adhesive Compound Preparation Methods on the Mechanical Properties of the Adhesive
The tensile strength tests of the adhesive compounds conducted in compliance with the DIN EN ISO 527-1 standard allowed determination of the following parameters:
F—tensile force,
Et—tangent modulus, σ
γ—shear stress, σ
M—tensile stress, ε
M—relative tensile strain, and σ
B—stress at break. The results of the tangent modulus
Et of the modified adhesive compounds produced with Methods I and II (Variants I and II) are given in
Table 4.
In Method I, the highest value of
Et was obtained for the adhesive modified with 0.75% of the modifier, amounting to 464 MPa (
Table 4). The
Et value of the adhesive modified with two modifier concentrations, 0.50% and 0.75%, was higher than that of the unmodified adhesive. This difference was 2% and 10%, respectively. In the other cases, the modulus of the modified adhesives was lower than that of the unmodified adhesive. In Method II (
Table 4), the highest
Et value was obtained for the adhesive modified with the 1.00% modifier concentration, and it amounted to 1024 MPa. The
Et value obtained in the strength tests for the adhesives modified with the 0.50% and 0.75% modifier concentrations was similar to that of the unmodified adhesive. The highest difference was observed for the case with the highest modifier content (1.00%), as it amounted to 19%. In the specimens with 0.25% of the modifier, the static modulus of the modified adhesive was lower than that of the unmodified adhesive.
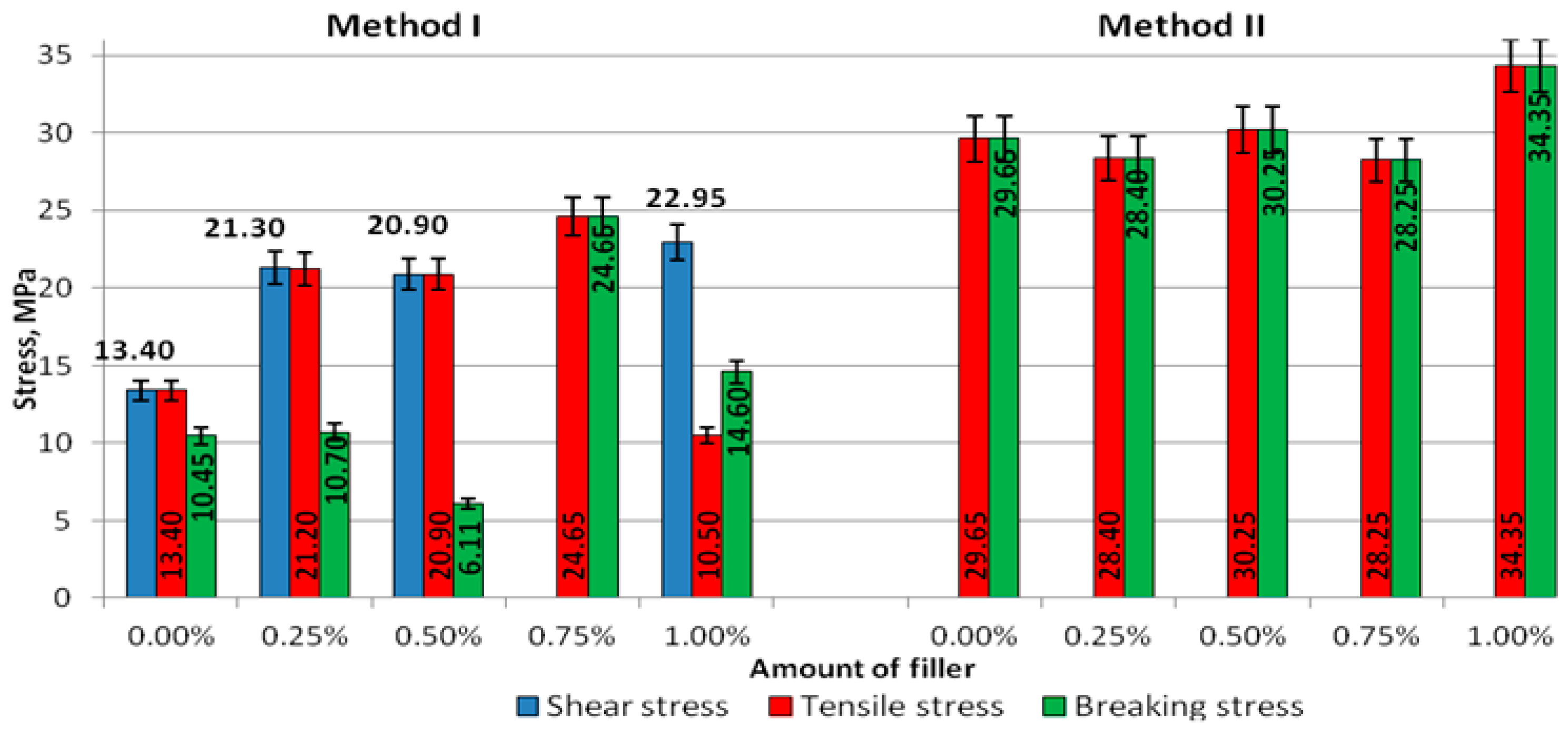
The results of stresses of the cured adhesive specimens prepared according to Methods I and II are shown in
Figure 6.
The obtained shear stresses σ
γ of the adhesive compounds prepared with Method I at concentrations of 0.25% (21.3 MPa), 0.50% (20.9 MPa), 0.75% (24.7%), and 1.00% (23.0 MPa) were higher than σ
γ obtained in the control experiment (0%), i.e., 13.5 MPa (
Figure 6). A similar trend was observed for the tensile stress σ
M: The tensile stresses for the modified adhesive in each variant of modification were higher than those obtained for the unmodified adhesive (13.5 MPa). In Method II, the tensile stresses (σ
M) and the stresses at break (σ
B) were lower than those produced in the test with no modifier (σ
M—29.7 MPa, σ
B—29.7 MPa) only for the modifier concentration of 0.25% (σ
M—28.4 MPa, σ
B—28.4 MPa) and 0.75% (σ
M—28.3 MPa, σ
B—28.3 MPa). For the other modified adhesives, the tensile stresses (σ
M) and stresses at break (σ
B) were higher than those observed for the unmodified adhesive.
Comparing the stresses in the adhesives prepared with Methods I and II (
Figure 6), it can be seen that the tensile stresses (σ
M) and the stresses at break (σ
B) were much higher in the adhesive specimens made with Method II and the difference between them amounted almost to 18%. In Method I, the stresses measured in the zero test (σ
γ—13.4 MPa, σ
M—13.4 MPa, σ
B—10.5 MPa) were lower than those produced for the modifier concentration of 0.25% (σ
γ—21.3 MPa, σ
M—21.2 MPa, σ
B—10.7 MPa). Compared to the unmodified adhesive, higher stresses were observed in a majority of cases involving the modified adhesive, although the stress at break in the modified adhesive containing 0.50% of the modifier was much lower than that in the unmodified adhesive. In turn, the adhesive modified with 1.00% of the modifier had a lower value of tensile stress than the unmodified adhesive. Regarding the modified adhesive, the difference between the tensile stresses was 43%, while that between the stresses at break was 75%. It can be claimed that the application of Method I for preparation of the adhesive leads to significant variations in the mechanical properties of cured adhesive compounds.
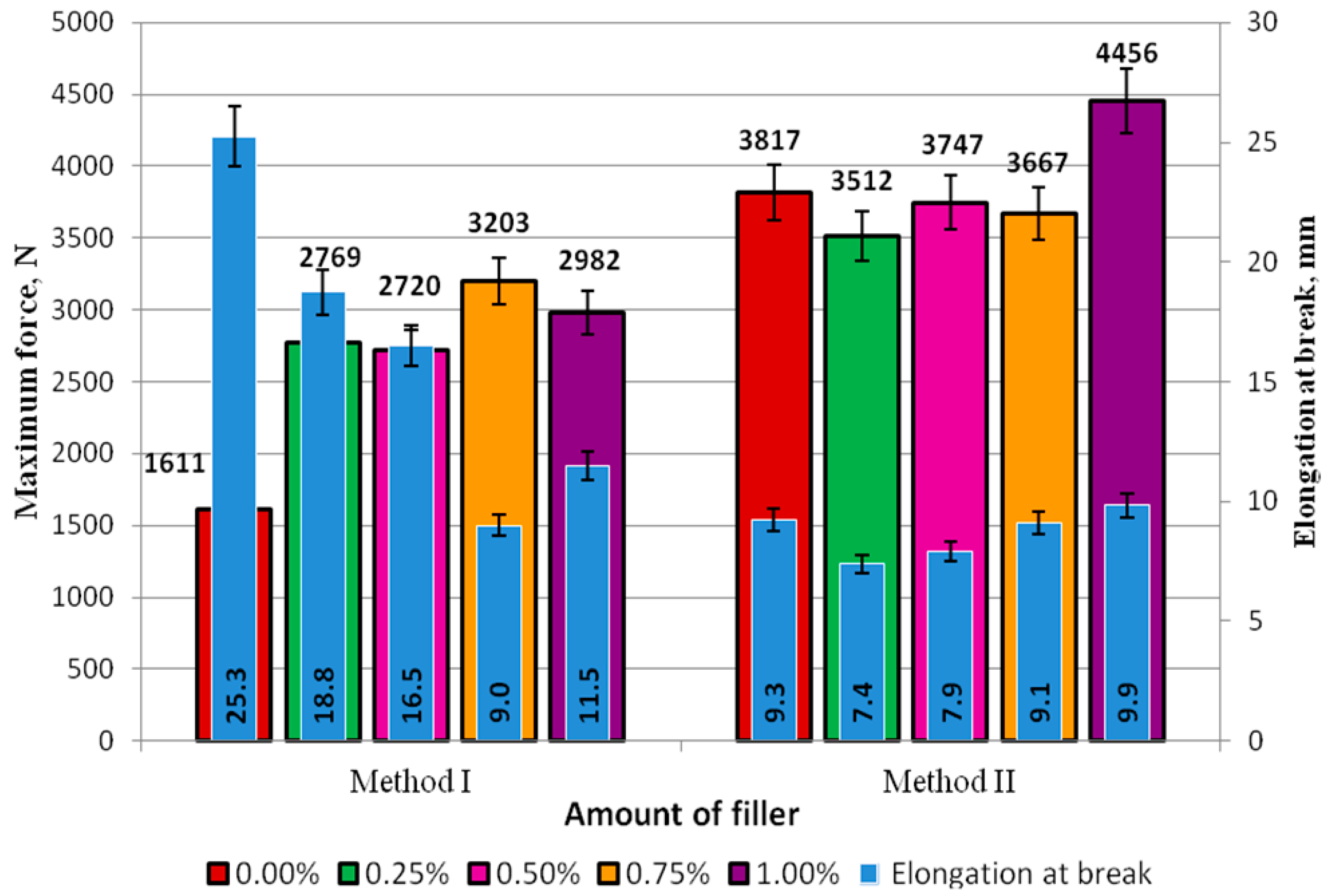
The standard force and elongation of the adhesive specimens made with Methods I and II are compared in
Figure 7.
In Method I for preparation of the adhesive (
Figure 7), the highest elongation at break, was produced for the unmodified adhesive and it amounted to 25.3 mm. It was observed that with the increasing modifier concentration in the adhesive compound, the elongation at break decreased. The correlation coefficient of these quantities is 0.85. It can be claimed that an increase in the modifier content leads to a decrease in the elongation and thus reduced elasticity of the adhesive. The difference between the highest (18.8 mm at 0.25%) and the smallest elongation at break (9.0 mm at 0.75%) was 9.8 mm, which almost amounted to 50%.
In Method II for preparation of the adhesive compound (
Figure 7), no significant differences were observed with respect to elongation at break. The elongation at break ranged from 7.4 mm (at 0.25% modifier content) to 9.9 mm (at 1.00% modifier content), and the difference between these quantities was 25%. An increase in the elongation at break was observed with the increasing modifier content. The correlation coefficient is 0.95. It can therefore be predicted that an increase in the modifier content (lyophilized fungal preparation) leads to a higher elasticity level of the adhesive. The elongation at break of the unmodified adhesive was smaller only for the adhesive compound with the highest modifier content tested, i.e., 1.00%. This difference amounted to 6%.
In Method I (
Figure 7), the maximum force in the control experiment was 1611 N, which was lower than the force obtained for the specimens with concentrations of 0.25% (2769 N), 0.50% (2720 N), 0.75% (3203 N), and 1.00% (2982 N). Regarding the modified adhesive, the highest force, i.e., 3203 N, was obtained for the specimens with 0.75% of the modifier; this value of force was almost half as high as the force obtained for the unmodified adhesive specimens (1611 N). In Method II, only the results of the tests involving the 0.25% and 0.75% modifier concentrations were lower than the results of the maximum force of the referential specimens of the unmodified adhesive (3817 N).
The values of elongation at break were higher for the specimens obtained with Method I, compared to those obtained with Method II; still, among all the modifier concentrations, they were smaller than the results obtained in the 0.00% test (25.3 mm). In Method II, the elongation at break of Epidian 53/PAC with the 1.00% modifier concentration (9.9 mm) was higher by 0.50 mm than that yielded in the control experiment.
The results given in
Figure 7 reveal that the failure forces were much higher in the adhesive specimens prepared with Method II, and the difference between these values for the respective modifier concentrations was as follows: 0.25%–21%, 0.50%–28%, 0.75%–13%, and 1.00%–32%. It can be claimed that the application of Method II yielded similar results, although with a smaller scatter of values than in Method I. Based on the results obtained, it seems that the addition of modifiers affects both growth and reduction of various mechanical properties, which is dependent on the method of preparation of the adhesives. In the case of Method II, it can be noted that the increasing level of modifier addition causes an increase in both the breaking force and elongation. The analysis of tensile strength shows that the increasing addition of the modifier results in a significant increase in the strength when Method I is applied and absence of a significant impact in a majority of cases in Method II. The knowledge of these values and relationships will allow a design of adhesive joints in accordance with their intended use. In cases where greater strength is required, an adhesive prepared with Method II can be used, whereas Method I will ensure greater flexibility. Addition of modifiers designed to improve aging resistance is also important.
3.3. Strength Test Results—The Effect of Seasoning of the Adhesive Compounds on the Mechanical Properties of the Adhesive
The results of the mechanical properties of the adhesive compounds produced with Method I after the two-month seasoning (Variant III—
Table 3) are given in
Table 5.
The lowest values of failure force, static modulus, shear stress (σy), tensile stress (σM), and stress at break (σB) were measured for the unmodified adhesive. In the control test, the tensile stresses and the stresses at break had the same value of 8.8 MPa, only exceeding the results obtained in the test with 1% of the modifier. In all tested cases of the modified adhesives (except for the stress at break of the adhesive modified with 1.00% modifier), the mechanical properties of the adhesive specimens subjected to seasoning for 2 months at a temperature of 50 °C and 50% humidity were higher than those noted for the unmodified adhesive. For all the modifier concentrations, the tensile stresses were higher, ranging from 2.1 MPa to 4.4 MPa, while the stresses at break ranged from 1.38 MPa to 2.6 MPa, compared to the control test results (σM—8.8 MPa, σB—8.8 MPa). It can therefore be stated that the addition of the lyophilized fungal preparation has a positive effect on the mechanical properties of the adhesive during the seasoning period.
The highest tensile stress was observed for the adhesive containing 0.75% of the modifier (13.2 MPa); yet, in the other cases, the results do not differ significantly. This value was higher by 33% than that of the referential specimens. As for the modified adhesives, the lowest stresses were obtained for the adhesive with the highest modifier concentration, i.e., 1.00%.
Comparison of the results of tensile strength for the adhesive specimens subjected to climatic chamber seasoning (Variant III,
Table 5) with those for the adhesive specimens made with the same method but seasoned at ambient temperature for 7 days (Variant I;
Figure 6) revealed that the stresses significantly decrease after the climatic chamber seasoning. The smallest differences were observed with respect to stresses at break. The stress at break in the modified adhesive containing 0.50% of the modifier after the seasoning was higher by over 40% (10.7 MPa) than the stress of break of the adhesive after 7 days of curing (6.1 MPa). The stress at break was also higher for the adhesive modified with 0.25% of the modifier; however, this difference was not significant and only amounted to 6%. For this reason, it can be claimed that the seasoning of the modified adhesives does not lead to reduction of some of their mechanical properties; it was observed in the case of the unmodified adhesive that each of the tested quantities decreased after the seasoning.
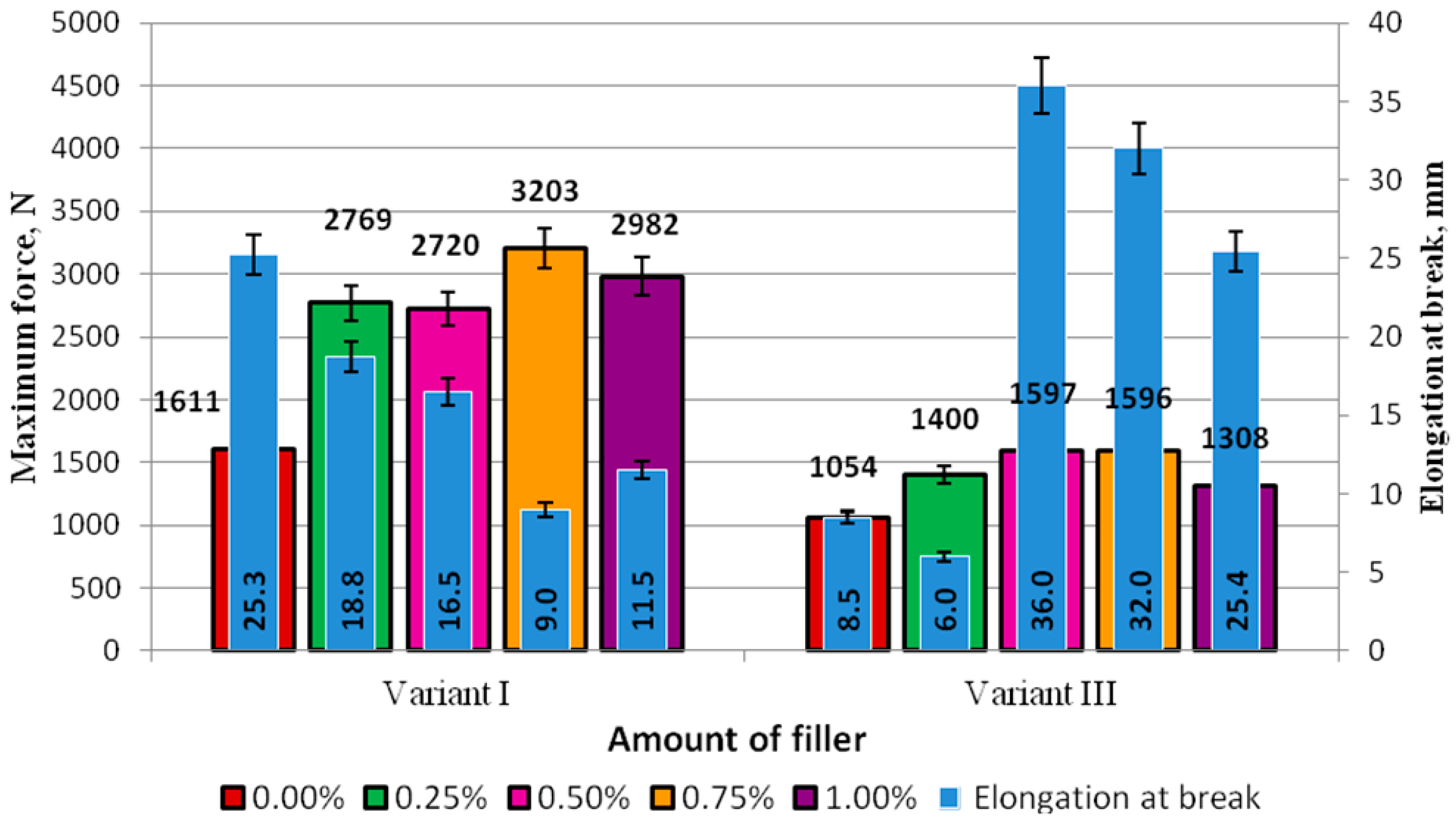
The standard force and elongation at break of the adhesive specimens prepared according to Method I and subjected to seasoning for 7 days (Variant I) and two months (Variant III) are compared in
Figure 8. The diagram in
Figure 8 shows that the values of elongation at break of the specimens prepared with the modifier concentrations of 0.50% (36.0 mm), 0.75% (32.5 mm), and 1% (25.4 mm) were not only much higher than the elongation results obtained in the control experiment (8.5 mm) but also the stresses yielded in the experiments. In the control experiments, the stresses were equal to 8.5 MPa and only exceeded the stresses of the specimens with the modifier concentration of 1.00% (7.3 MPa).
The elongation at break of the adhesive specimens modified with 0.50% of the modifier was higher by 0.56% than that of the same adhesive that was not subjected to seasoning. An even more significant difference, i.e., 72%, was observed regarding the elongation of the adhesive with 0.75% of the modifier. The elongation of the adhesive with 1.00% of the modifier after seasoning was higher by 55% than that of the modified adhesive with the same modifier content and cured for 7 days.
An analysis of the results of the failure force demonstrated that this force was smaller for all adhesives subjected to seasoning, and the differences between the values of this parameter were as follows: Unmodified adhesive (0%)—34%, adhesive with 0.25% modifier—41%, adhesive with 0.50% modifier—41%, adhesive with 0.75% modifier—50%, and adhesive with 1.00% modifier—54%. The results demonstrate that the maximum force decreased nearly by half for all modified adhesives compared to the modified adhesives that were not subjected to seasoning. It can therefore be claimed that although seasoning has a negative effect on the failure force of the adhesive, it leads to a higher elasticity level of the adhesive. In the majority of cases, the elongation at break increased by over 50% (and even by over 70%), compared to that yielded for the modified adhesives that were not subjected to seasoning. It was observed that the elongation of the modified adhesives after seasoning even increased by nearly 30% (0.50% modifier) and 27% (0.75% modifier) compared to the elongation results obtained for the unmodified adhesive cured for 7 days at ambient temperature (25.3 mm). These results are similar to those yielded for the adhesive with 1.00% of the modifier. It was also observed that, in the two cases of the modified adhesives subjected to seasoning, the value of the maximum force was similar to that obtained for the modified adhesive that was not subjected to seasoning.
On the basis of the results, it can be suspected that an increase in the modifier content will lead to higher elasticity of the adhesive. This applies primarily to Method II for preparation of modified adhesives. This may ensure greater beneficial behavior during the use of elements produced with these adhesive compositions and adhesive joints, i.e., better adaptation to changes in the shape of the elements and adhesive joints under the influence of external factors. Moreover, the increase in the quantity of the modifier decreases internal stresses in the cured adhesive composition, which contributes to better fuctioning of such a material in terms of the impact of external loads. The use of modifiers prevents material brittleness, which is a cause of destruction of such materials or adhesive joints caused by the impact of external stresses.
3.4. Microscopic Results
The effects of the adhesive compound preparation method applied (described in
Table 2) and subjecting the adhesive compounds to seasoning (listed in
Table 3) on the adhesive structure are given in
Table 6.
As for Method I, it was observed that the biologically modified adhesive mixture of Epidian 53/PAC/1:1 applied to the micro slides exhibited changes at its edge at the 0.50% and 0.75% modifier contents compared to the results yielded in the control experiments. In Method II and the 0.25% modifier content, significant changes were observed in the specimen edge compared to those obtained in the 0% test. In the solution with the 0.50% and 1% modifier contents, the changes were smaller than those in the control experiment. In Method I, the changes were insignificant for the contents of 0.25% and 1.00%, and in Method II, the same was observed for the modifier content of 0.75%.
In Variant III (Method I and seasoning for 2 months), wherein the micro slides with the modifier were subjected to seasoning, the most significant changes, compared to the control experiment (no modifier applied), were observed for the adhesive specimens modified with the modifier contents of 0.50% and 0.75%.