Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/β-Cyclodextrin Fibers as an Antimicrobial Wound Dressing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-CD/CA Particles
2.3. Preparation of PLA/β-CD/CA Fibers
2.4. Characterization of PLA/β-CD/CA Fibers
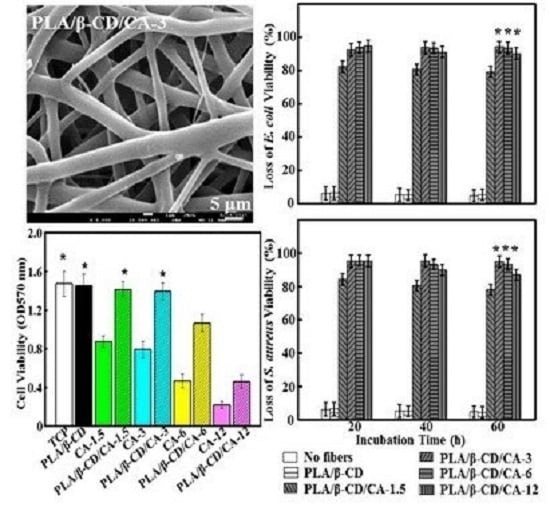
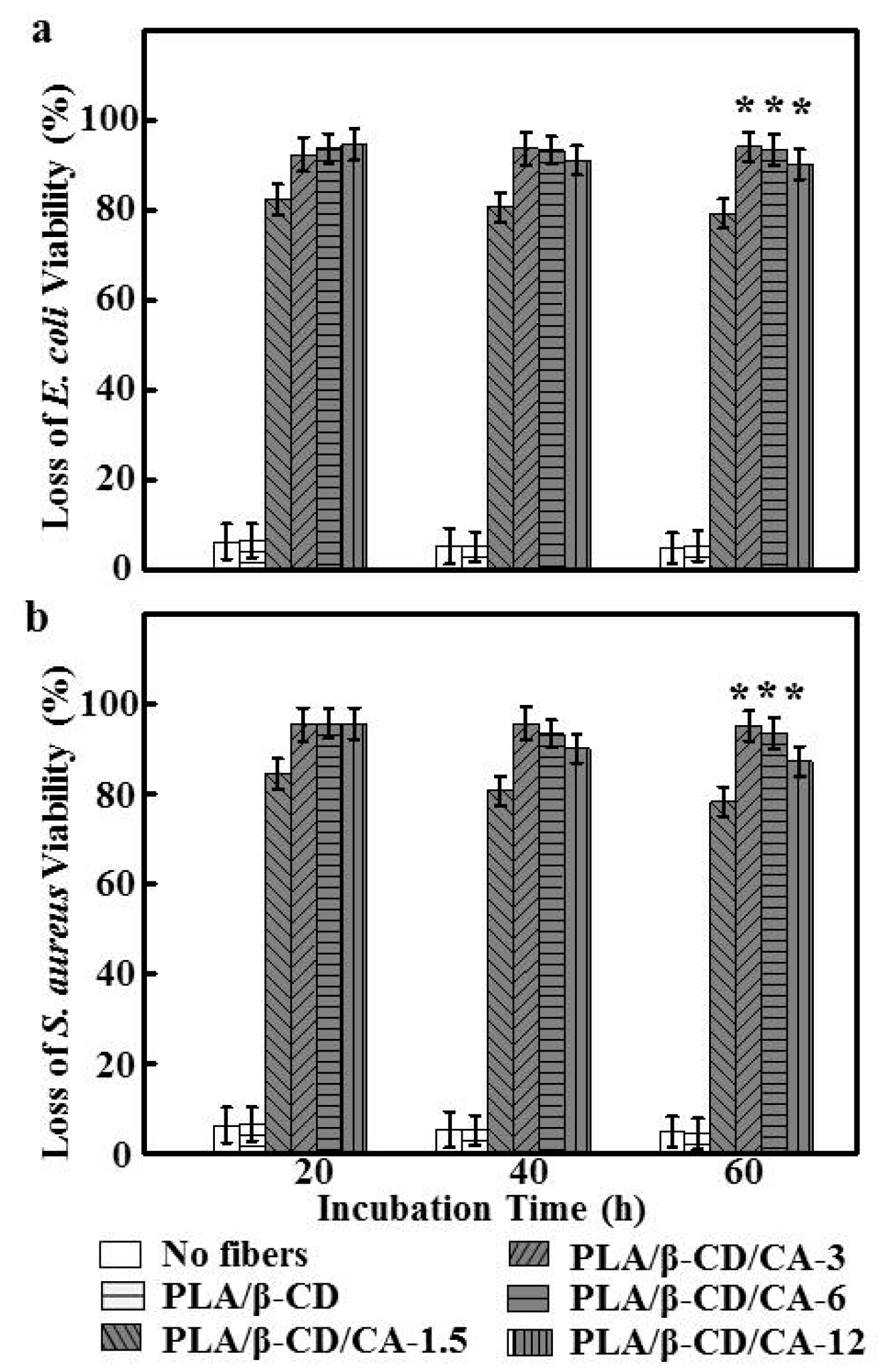
2.5. Antibacterial Test
2.6. Cell Viability Assay
2.7. Statistical Analysis
3. Results and Disccusion
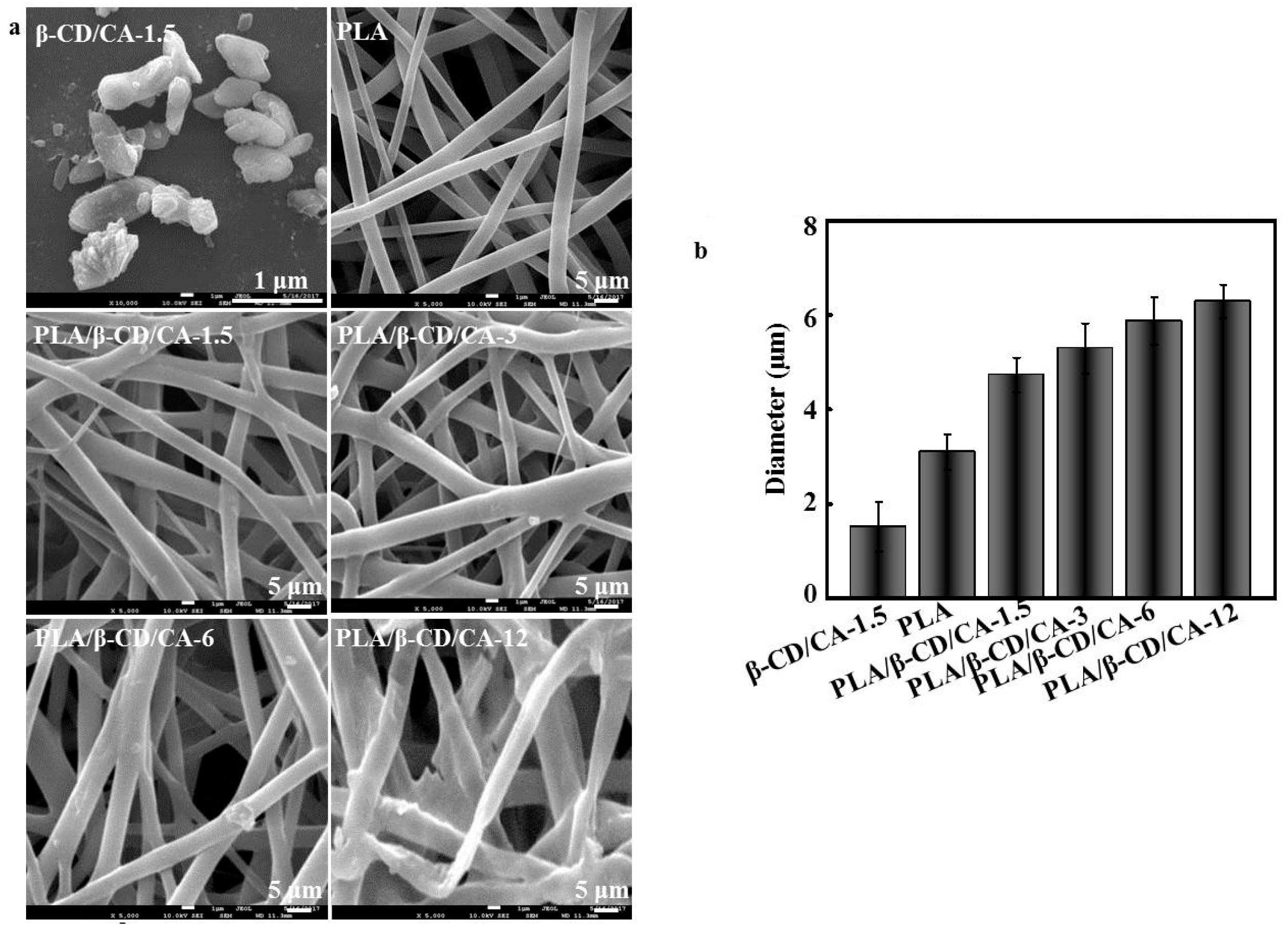
3.1. Topological Characterization of Fibrous Mats
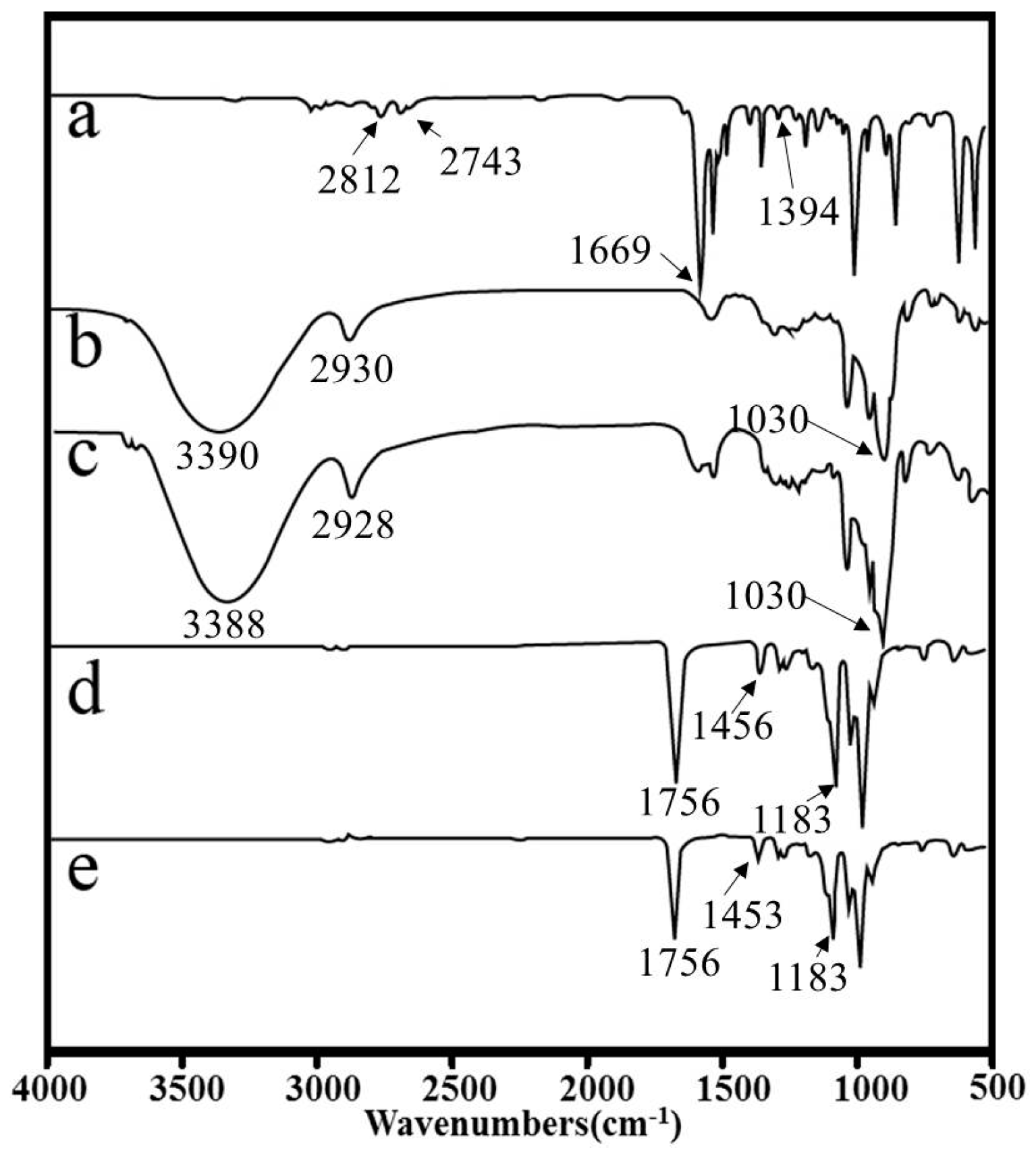
3.2. FT-IR
3.3. Physical Characteristics of Different Fibrous Mats
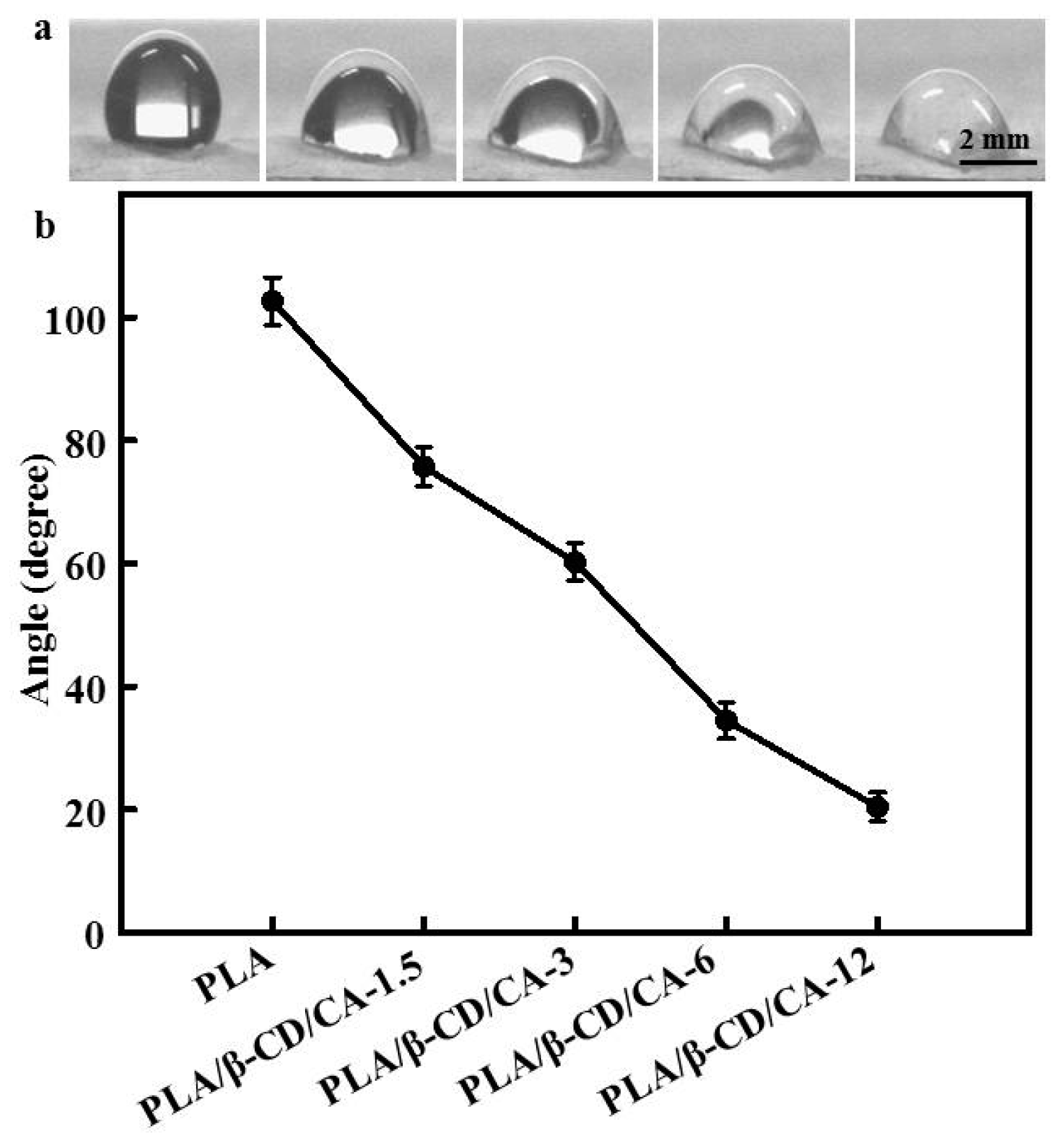
3.4. Surface Hydrophilicity of PLA/β-CD/CA Fibers
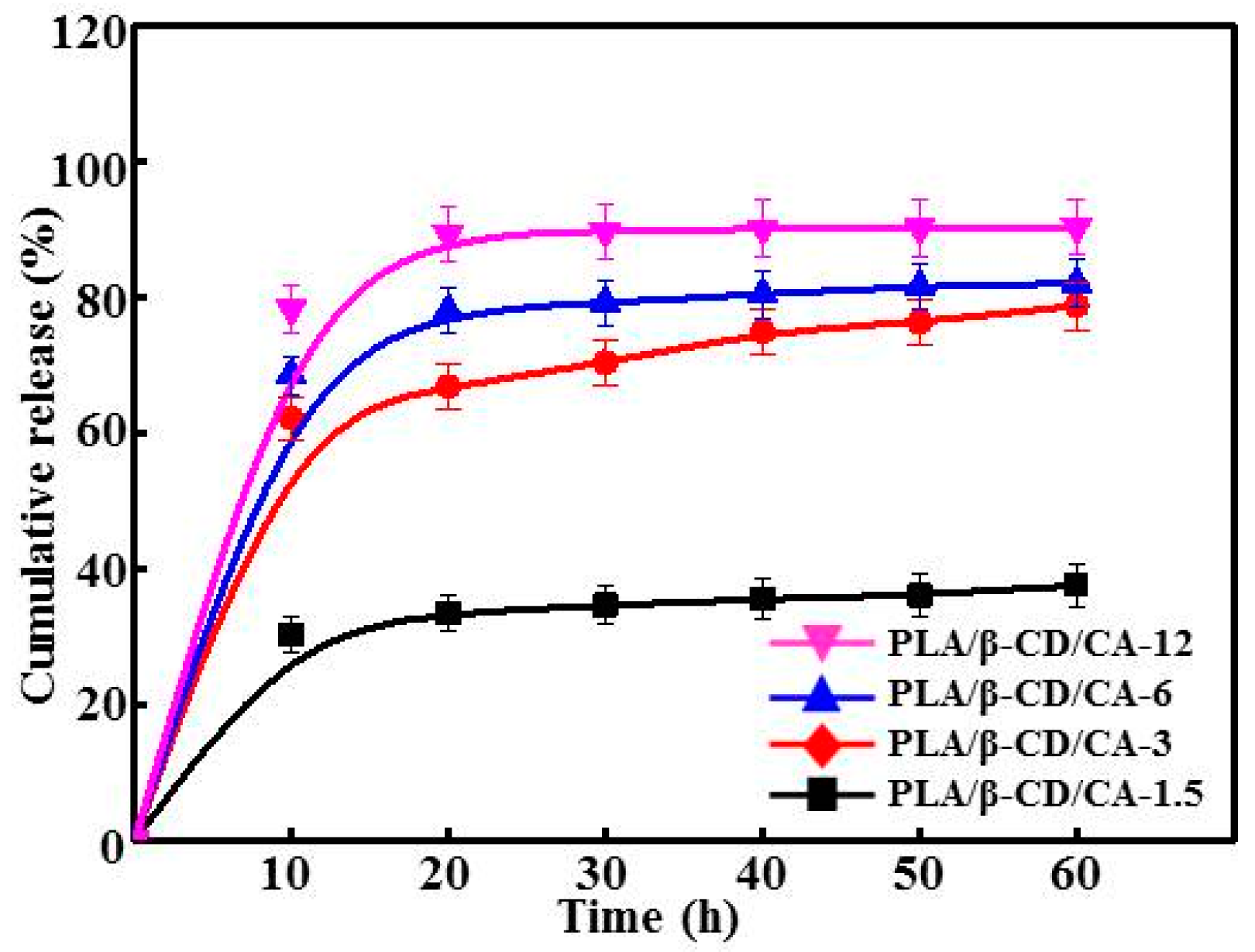
3.5. Release Characteristics of PLA/β-CD/CA Fibers
3.6. Viability of CCC-HSF-1
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hui, W.; Yuan, H.; Li, S.; Zhuo, L.; Jiang, M. Synthesis, antimicrobial activity of schiff base compounds of cinnamaldehyde and amino acids. Bioorg. Med. Chem. Lett. 2016, 26, 809–813. [Google Scholar]
- Qin, Y.; Liu, D.; Wu, Y.; Yuan, M.; Li, L.; Yang, J. Effect of PLA/PCL/cinnamaldehyde antimicrobial packaging on physicochemical and microbial quality of button mushroom (agaricus bisporus). Postharvest Biol. Technol. 2015, 99, 73–79. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Lopez-Carballo, G.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread foodstuffs. Int. J. Food Microbiol. 2013, 166, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; De Benedictis, V.M.; Madaghiele, M.; Corcione, C.E.; Maffezzoli, A. Nanostructured active chitosan-based films for food packaging applications: Effect of graphene stacks on mechanical properties. Measurement 2016, 90, 418–423. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fabra, M.J.; Castro-Mayorga, J.L.; Bourbon, A.I.; Pastrana, L.M.; Vicente, A.A.; Lagaron, J.M. Use of electrospinning to develop antimicrobial biodegradable multilayer systems: Encapsulation of cinnamaldehyde and their physicochemical characterization. Food Bioprocess Technol. 2016, 9, 1874–1884. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. Biol. Interact. 2009, 179, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Wei, B.; Lv, G.; Ji, H.; Li, S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem. 2012, 130, 229–235. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar] [PubMed]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L.). Food Chem. Toxicol. 2013, 53, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Sofokleous, P.; Chang, M.W.; Ge, B.Y.; Stride, E.; Edirisinghe, M. Controlled preparation of drug-exchange phase loaded polymeric fibres by co-axial electrospinning. Bioinspir. Biomim. Nanbiomater. 2011, 1, 48–56. [Google Scholar]
- Sofokleous, P.; Stride, E.; Bonfield, W.; Edirisinghe, M. Design, construction and performance of a portable handheld electrohydrodynamic multi-needle spray gun for biomedical applications. Mater. Sci. Eng. C 2013, 33, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Holban, A.M.; Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Modified wound dressing with phyto-nanostructured coating to prevent staphylococcal and pseudomonal biofilm development. Nanoscale Res. Lett. 2012, 7, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Chifiriuc, M.C.; Saviuc, C.; Grumezescu, V.; Hristu, R.; Mihaiescu, D.E.; Andronescu, E. Hybrid nanomaterial for stabilizing the antibiofilm activity of Eugenia carryophyllata essential oil. IEEE Trans. Nanobiosci. 2012, 11, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Liu, G.; Gu, Z.; Hong, Y.; Cheng, L.; Li, C. Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. J. Control. Release 2017, 252, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Electrospinning chitosan/poly(ethylene oxide) solutions with essential oils: Correlating solution rheology to nanofiber formation. Carbohydr. Polym. 2016, 139, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhou, S.; Li, X.; Zhao, J.; Yuan, M. In vitro degradation and release profiles for poly–dl–lactide-poly(ethylene glycol) microspheres containing human serum albumin. J. Control. Release 2001, 71, 165–173. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of poly(lactic acid)/chitosan fibers loaded with essential oil for antimicrobial applications. Nanomaterials 2017, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, X.; Wang, S.; Hu, K. Electrospun poly(lactic-co-glycolic acid)/multiwalled carbon nanotube nanofibers for cardiac tissue engineering. J. Biomater. Tissue Eng. 2016, 6, 719–728. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, R. Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol. 2017, 103, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, M.; Stride, E.; Edirisinghe, M.; Harker, A. Electrosprayed nanoparticle delivery system for controlled release. Mater. Sci. Eng. C 2016, 66, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ji, H.; Tan, D.; Teng, Y.; Dong, G.; Zhou, J.; Zhang, M. Silver nanoparticles decorated, flexible SiO2 nanofibers with excellent antibacterial effect as reusable wound cover. Colloids Surf. A 2011, 387, 57–64. [Google Scholar] [CrossRef]
- Vega-Lugo, A.C.; Lim, L.T. Controlled release of allyl isothiocyanate using soy protein and poly(lactic acid) electrospun fibers. Food Res. Int. 2009, 42, 933–940. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Fernandez-Saiz, P.; Ocio, M.J. Using ATR-FTIR spectroscopy to design active antimicrobial food packaging structures based on high molecular weight chitosan polysaccharide. J. Agric. Food Chem. 2007, 55, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial electrospun poly(lactic acid) (PLA) nanofibrous webs incorporating triclosan/cyclodextrin inclusion complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Rodriguez, K.; Han, J.H.; Kim, Y.T. Improved thermal stability of polylactic acid (PLA) composite film via PLA–β-cyclodextrin-inclusion complex systems. Int. J. Biol. Macromol. 2015, 81, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Ilangakoon, U.E.; Mahalingam, S.; Wang, K.; Cheong, Y.K.; Canales, E.; Ren, G.G.; Ciric, L. Gyrospun antimicrobial nanoparticle loaded fibrous polymeric filters. Mater. Sci. Eng. C 2017, 74, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mahalingam, S.; Rohn, J.L.; Ren, G.; Edirisinghe, M. Physio-chemical and antibacterial characteristics of pressure spun nylon nanofibres embedded with functional silver nanoparticles. Mater. Sci. Eng. C 2015, 56, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Chung, O.H.; Park, J.S. Coaxial electrospun poly(lactic acid)/chitosan (core/shell) composite nanofibers and their antibacterial activity. Carbohydr. Polym. 2011, 86, 1799–1806. [Google Scholar] [CrossRef]
- Saquing, C.D.; Manasco, J.L.; Khan, S.A. Electrospun nanoparticle-nanofiber composites via a one-step synthesis. Small 2009, 5, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Lai, C.; Reneker, D.H.; Qiu, H.; Ye, Y.; Hou, H. Electrospun polymer nanofibres with small diameters. Nanotechnology 2006, 17, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Moore, C.A.; Elahi, E.N.; Smart, A.T.; Hotchkiss, S.A. Human skin absorption and metabolism of the contact allergens, cinnamic aldehyde, and cinnamic alcohol. Toxicol. Appl. Pharm. 2000, 168, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Lin, I.F.; Chen, C.J.; Chen, S.T.; Chang, S.T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Ka, H.; Park, H.J.; Jung, H.J.; Choi, J.W.; Cho, K.S.; Ha, J.; Lee, K.T. Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 2003, 196, 143–152. [Google Scholar] [CrossRef]

| Sample | Viscosity (mPa·s) | Conductivity (µS/cm) | Strain (%) | Stress (MPa) | Young’s modulus |
|---|---|---|---|---|---|
| PLA | 129.15 ± 12.61 a | 0.037 ± 0.063 a | 96.74 ± 12.54 a | 4.42 ± 0.92 a | 84.62 ± 9.74 a |
| PLA/β-CD/CA-1.5 | 118.46 ± 10.19 a | 0.039 ± 0.065 a | 91.25 ± 11.81 a | 4.31 ± 0.89 a | 76.47 ± 8.27 a |
| PLA/β-CD/CA-3 | 97.82 ± 7.18 b | 0.043 ± 0.069 a | 88.63 ± 10.17 a | 4.27 ± 0.87 a | 73.88 ± 8.01 a |
| PLA/β-CD/CA-6 | 70.48 ± 5.53 b | 0.049 ± 0.071 a | 76.36 ± 8.34 a | 4.02 ± 0.82 a | 64.21 ± 7.48 b |
| PLA/β-CD/CA-12 | 45.27 ± 3.54 c | 0.058 ± 0.079 a | 70.49 ± 7.87 a | 3.63 ± 0.74 a | 52.74 ± 5.94 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liang, X.; Zhang, R.; Lan, W.; Qin, W. Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/β-Cyclodextrin Fibers as an Antimicrobial Wound Dressing. Polymers 2017, 9, 464. https://doi.org/10.3390/polym9100464
Liu Y, Liang X, Zhang R, Lan W, Qin W. Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/β-Cyclodextrin Fibers as an Antimicrobial Wound Dressing. Polymers. 2017; 9(10):464. https://doi.org/10.3390/polym9100464
Chicago/Turabian StyleLiu, Yaowen, Xue Liang, Rong Zhang, Wenting Lan, and Wen Qin. 2017. "Fabrication of Electrospun Polylactic Acid/Cinnamaldehyde/β-Cyclodextrin Fibers as an Antimicrobial Wound Dressing" Polymers 9, no. 10: 464. https://doi.org/10.3390/polym9100464