Shape Memory Polyurethanes Based on Zwitterionic Hard Segments
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PEG-ZSMPUs
2.3. Characterization of PEG-ZSMPUs
3. Results and Discussion
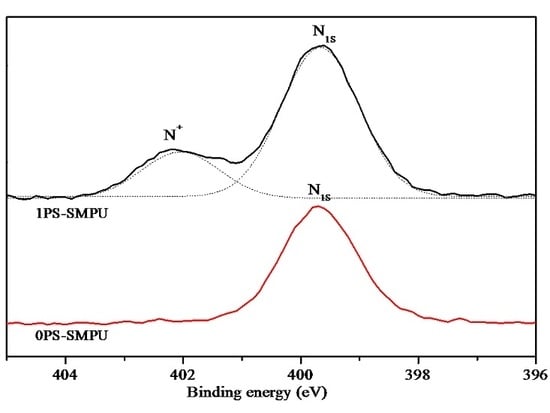
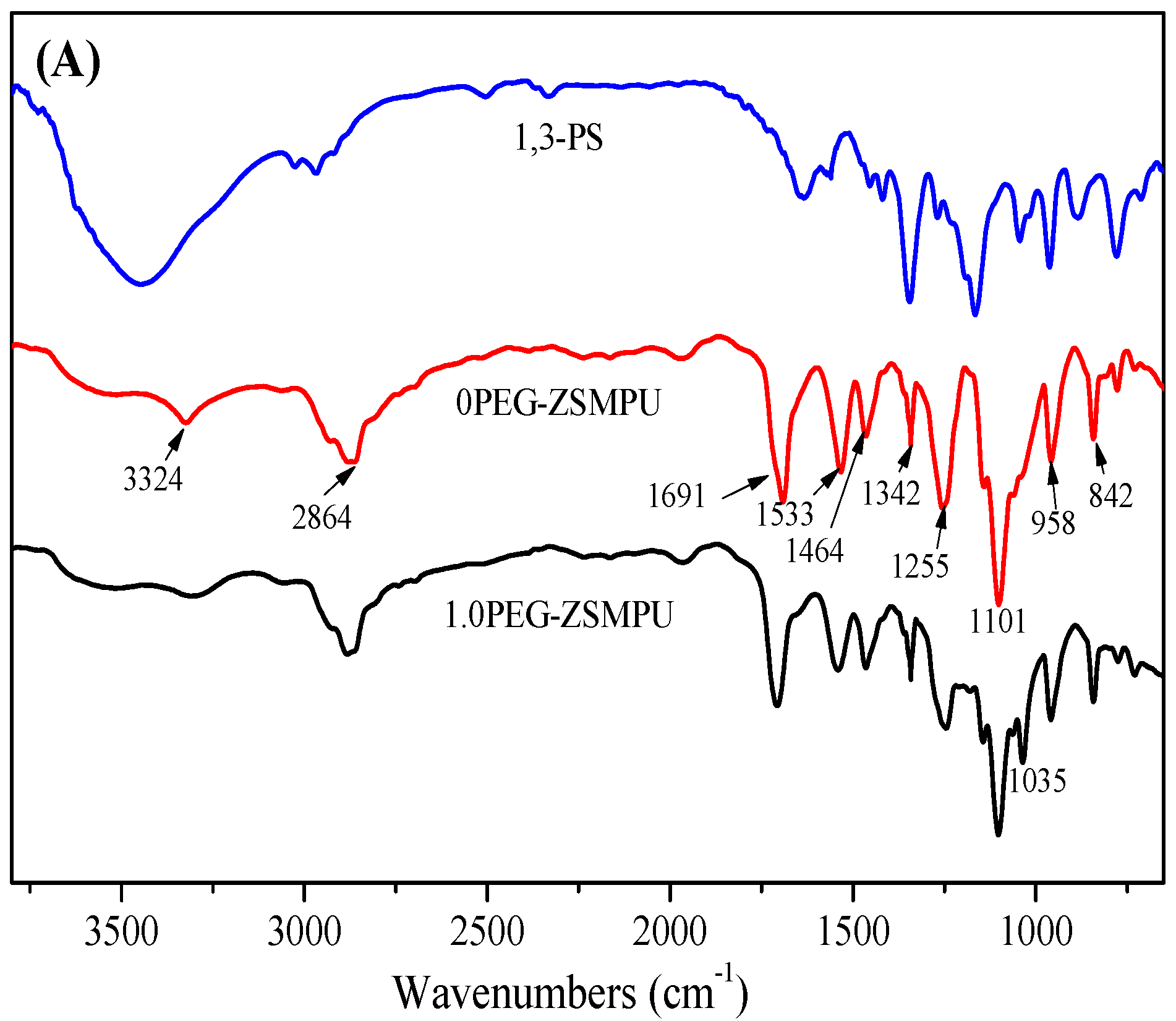
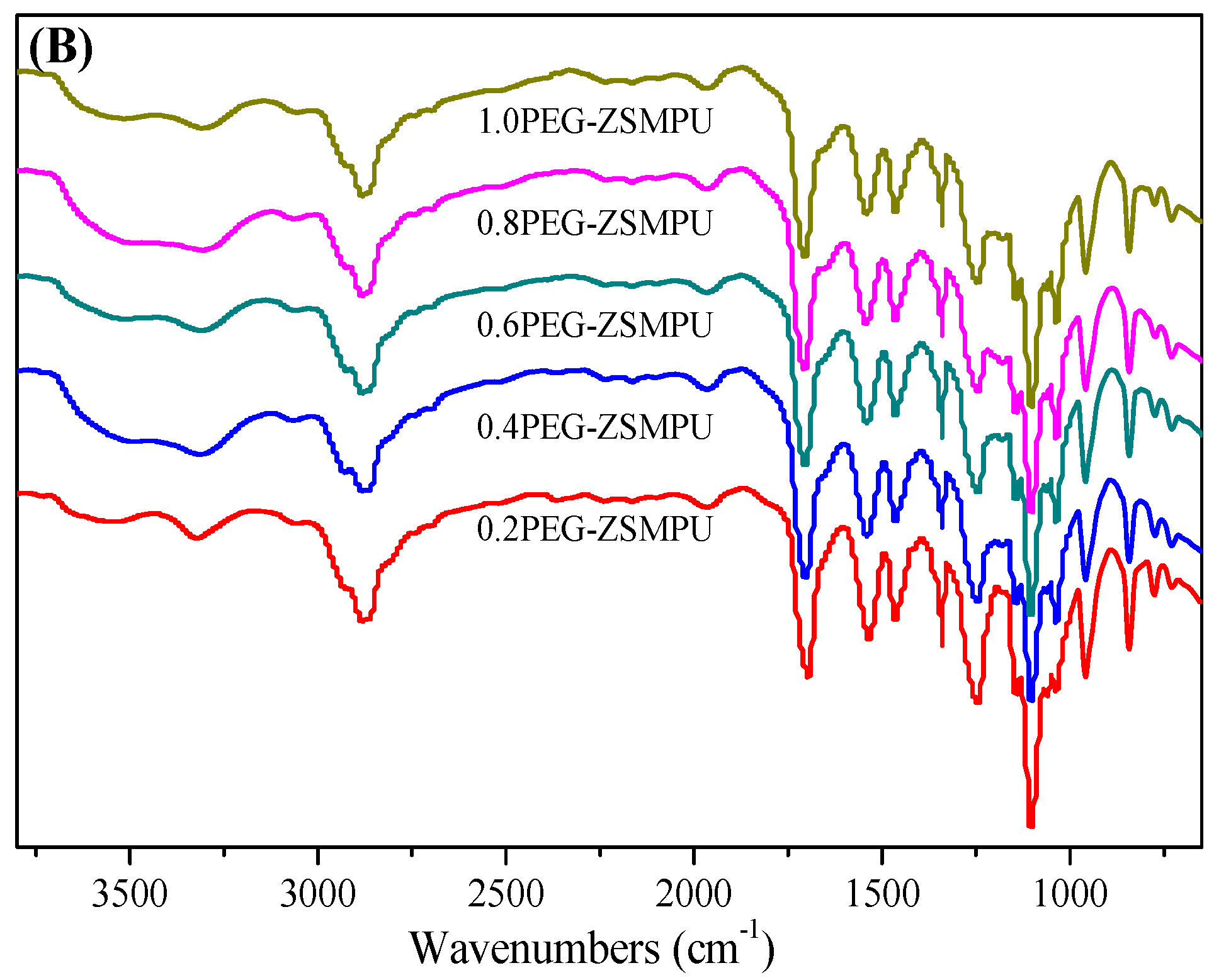
3.1. Structures of PEG-ZSMPUs
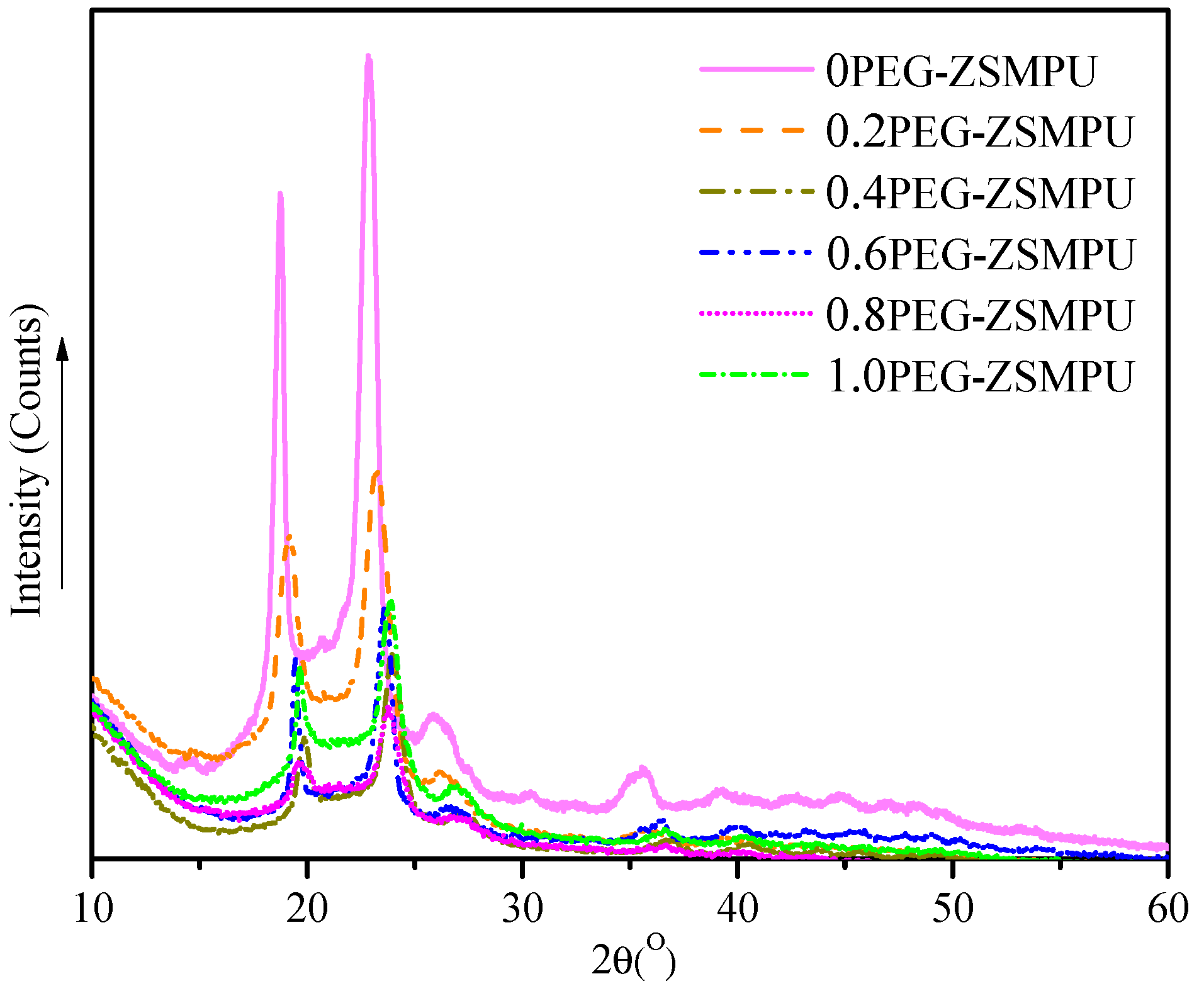
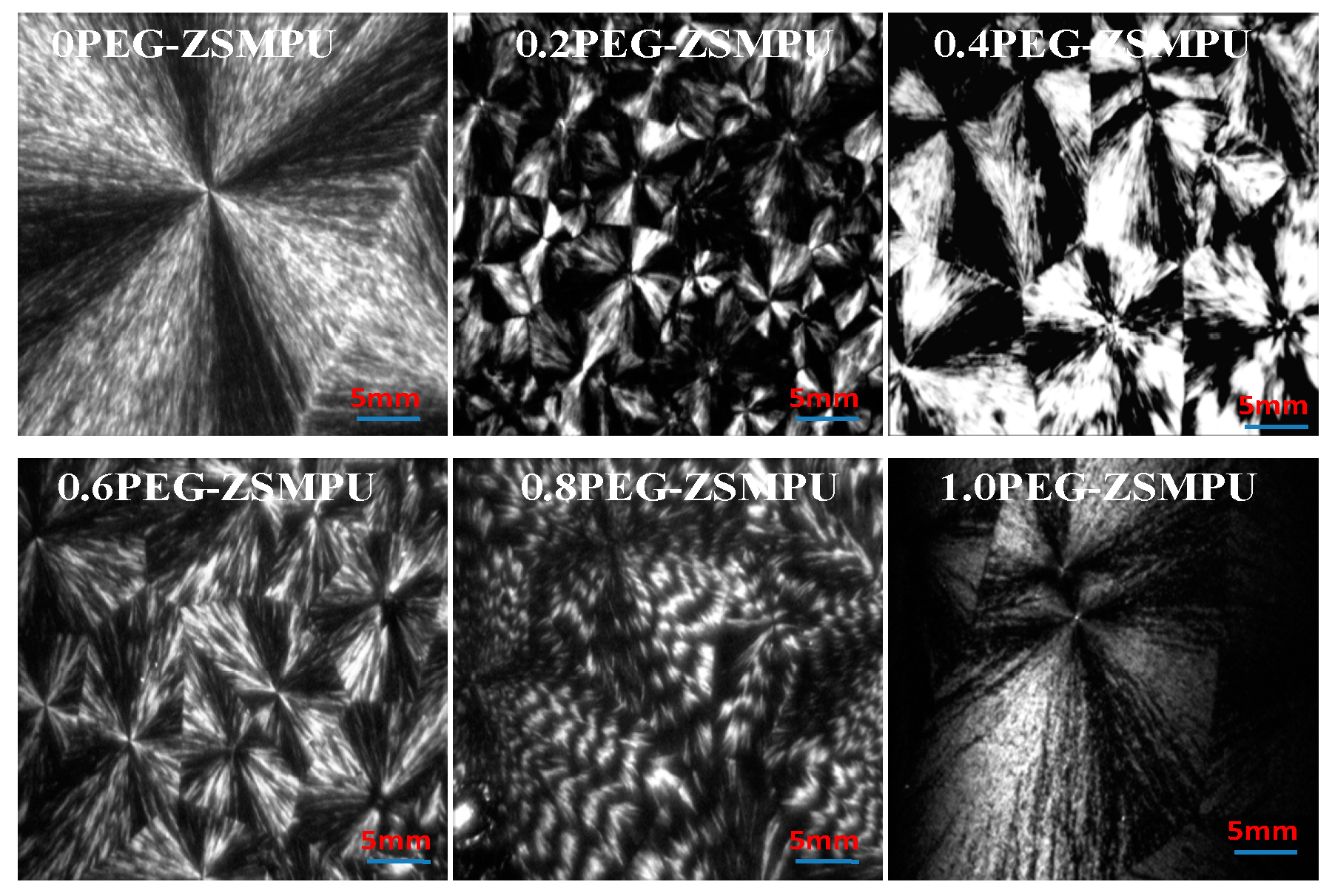
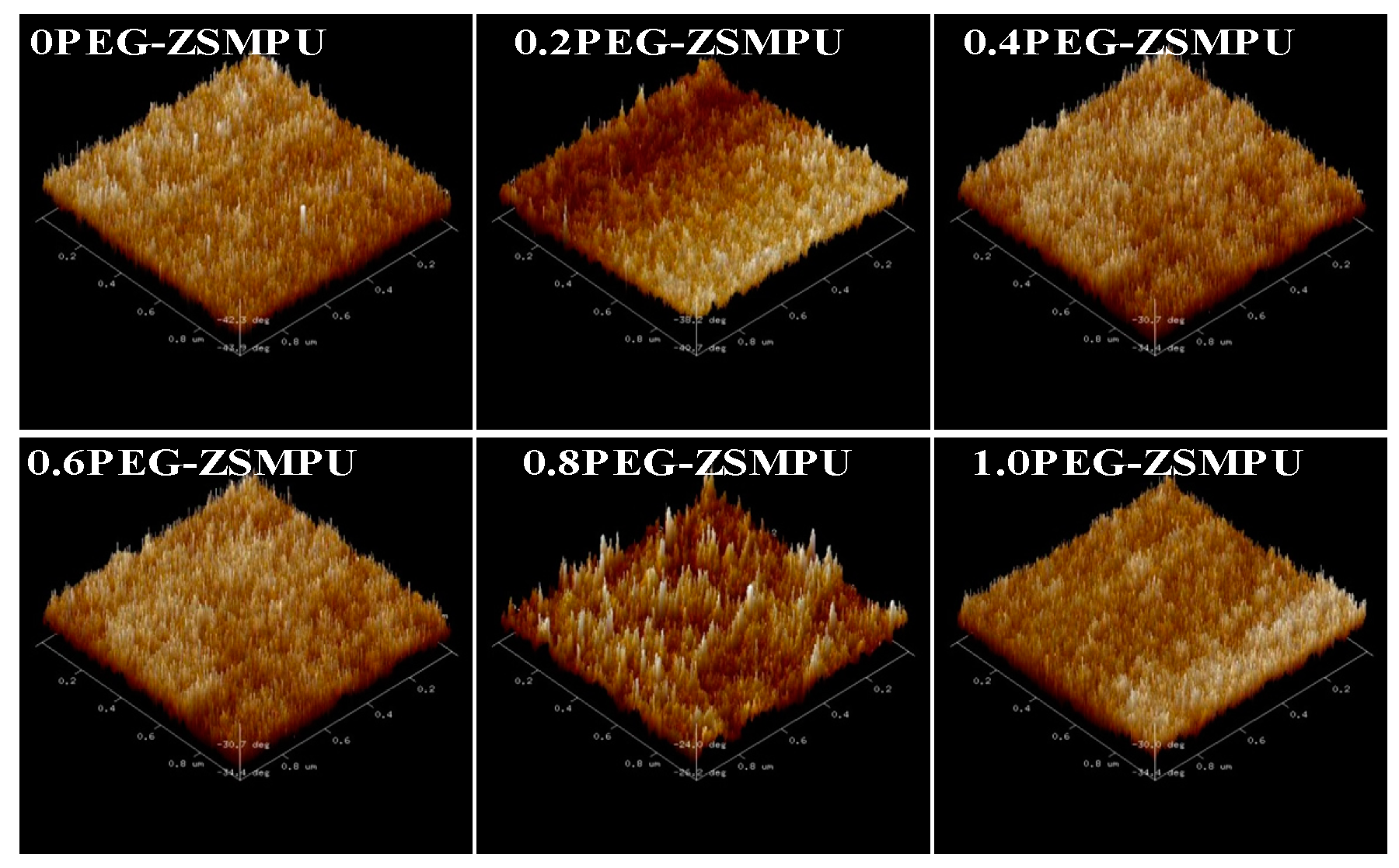
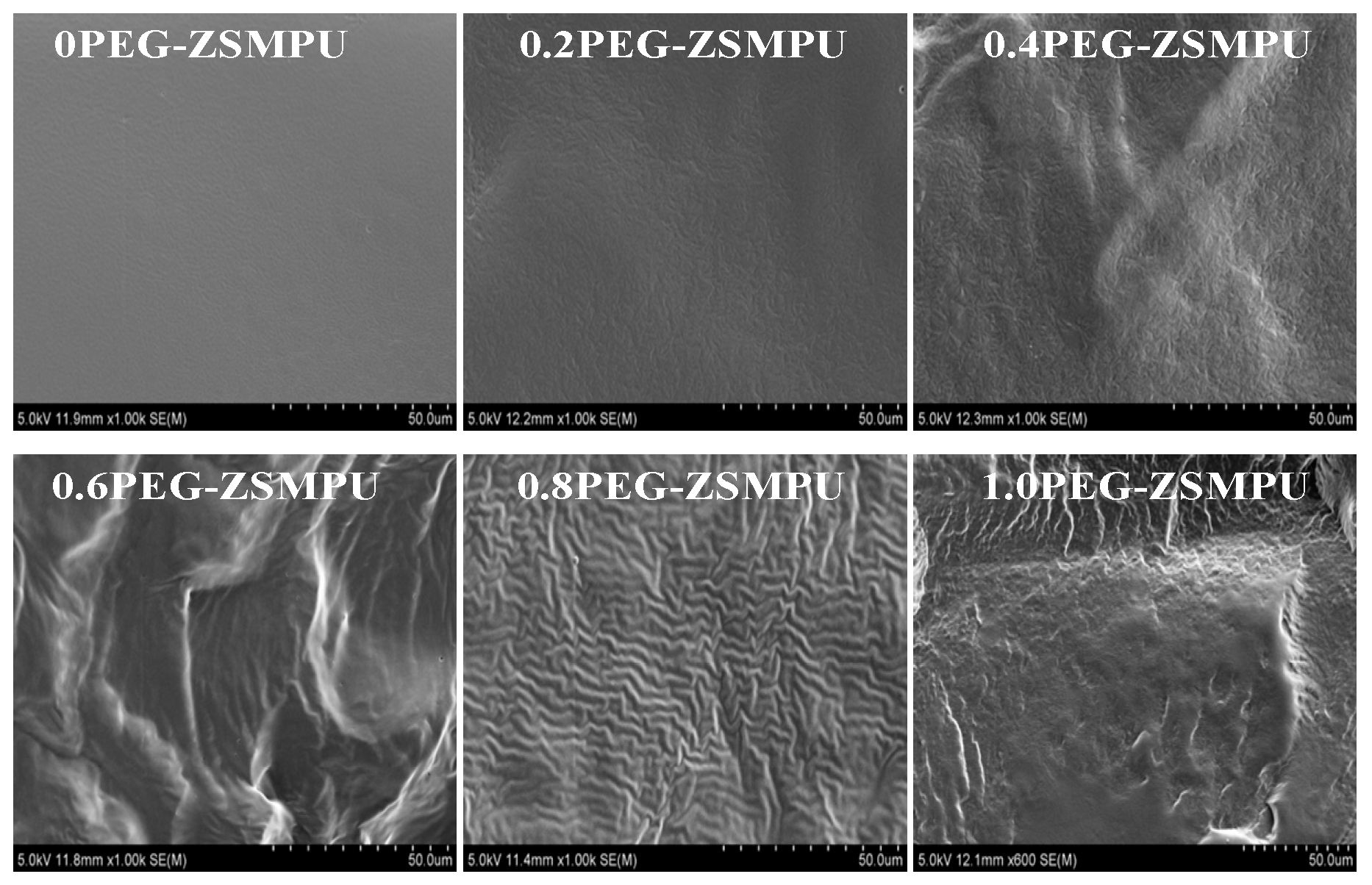
3.2. Morphology of PEG-ZSMPUs
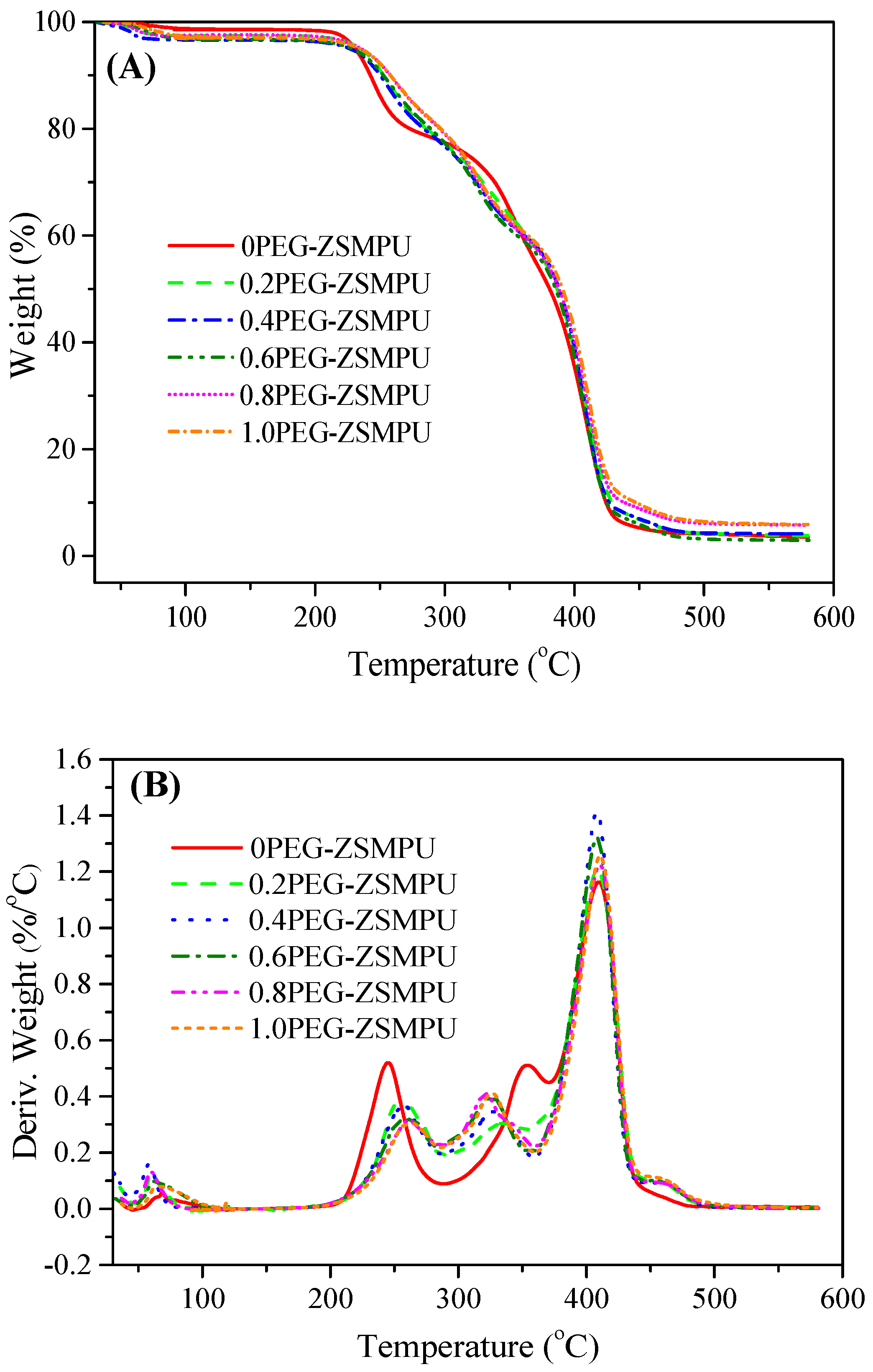
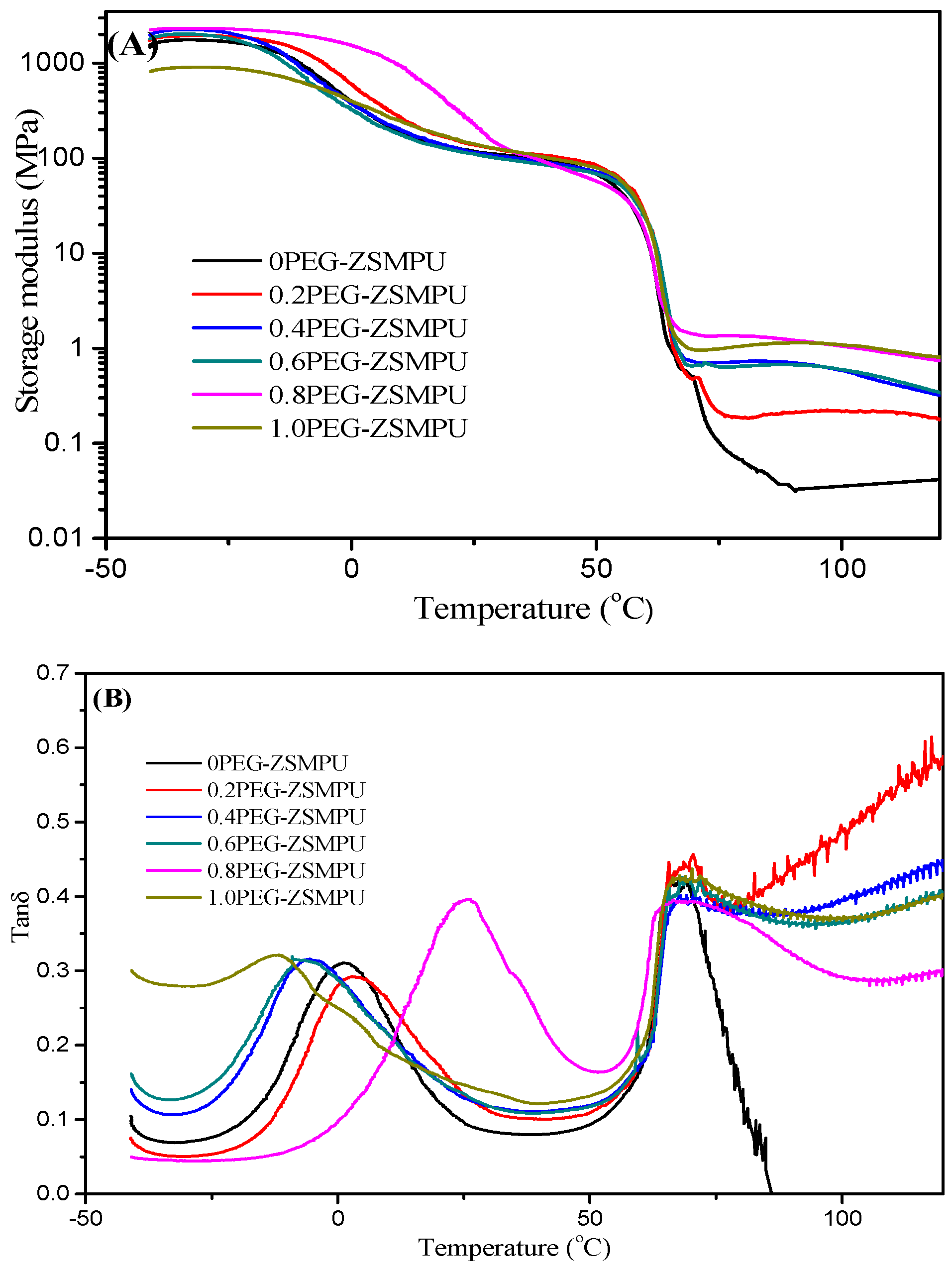
3.3. Thermal Properties of PEG-ZSMPUs
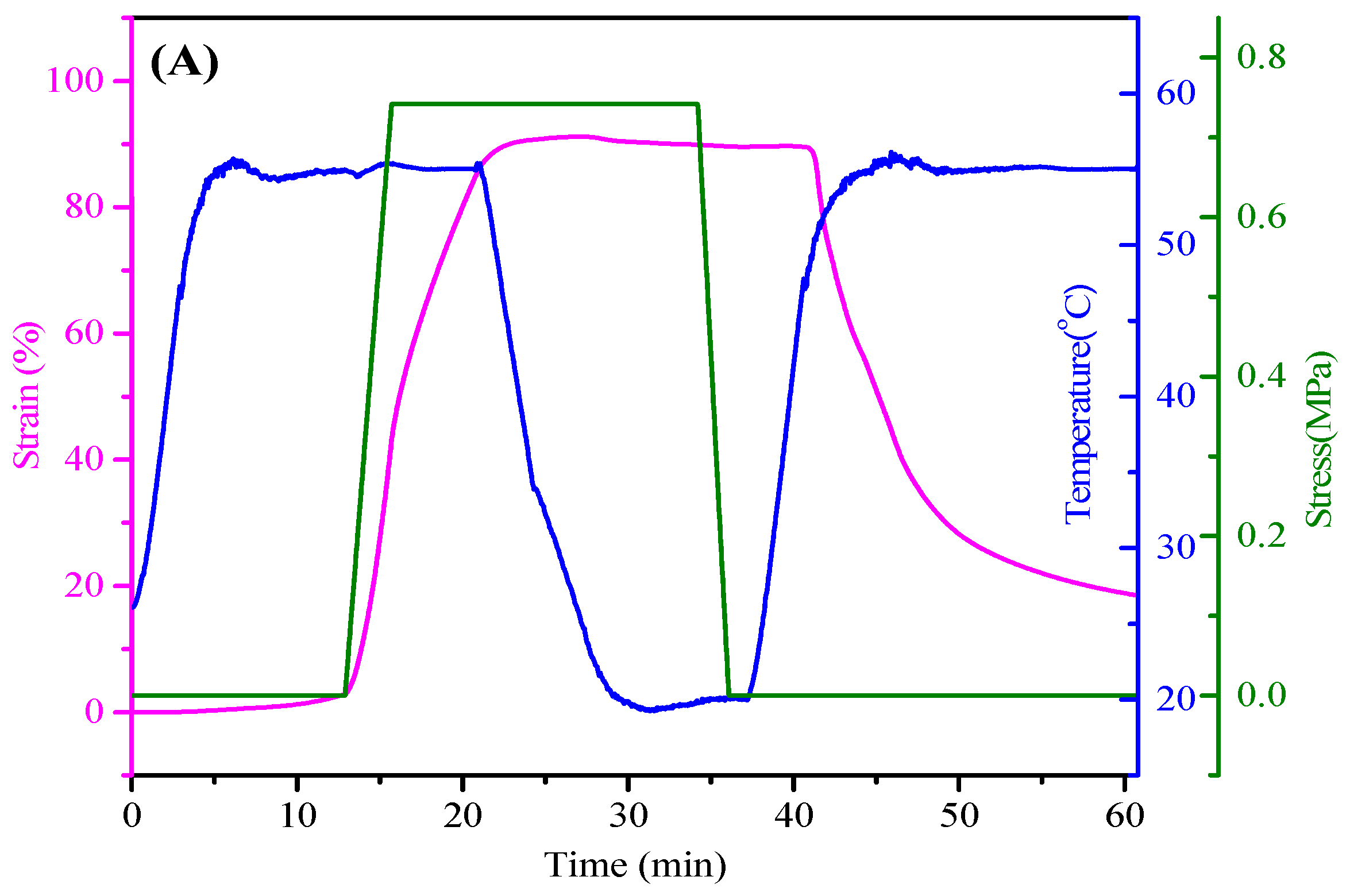
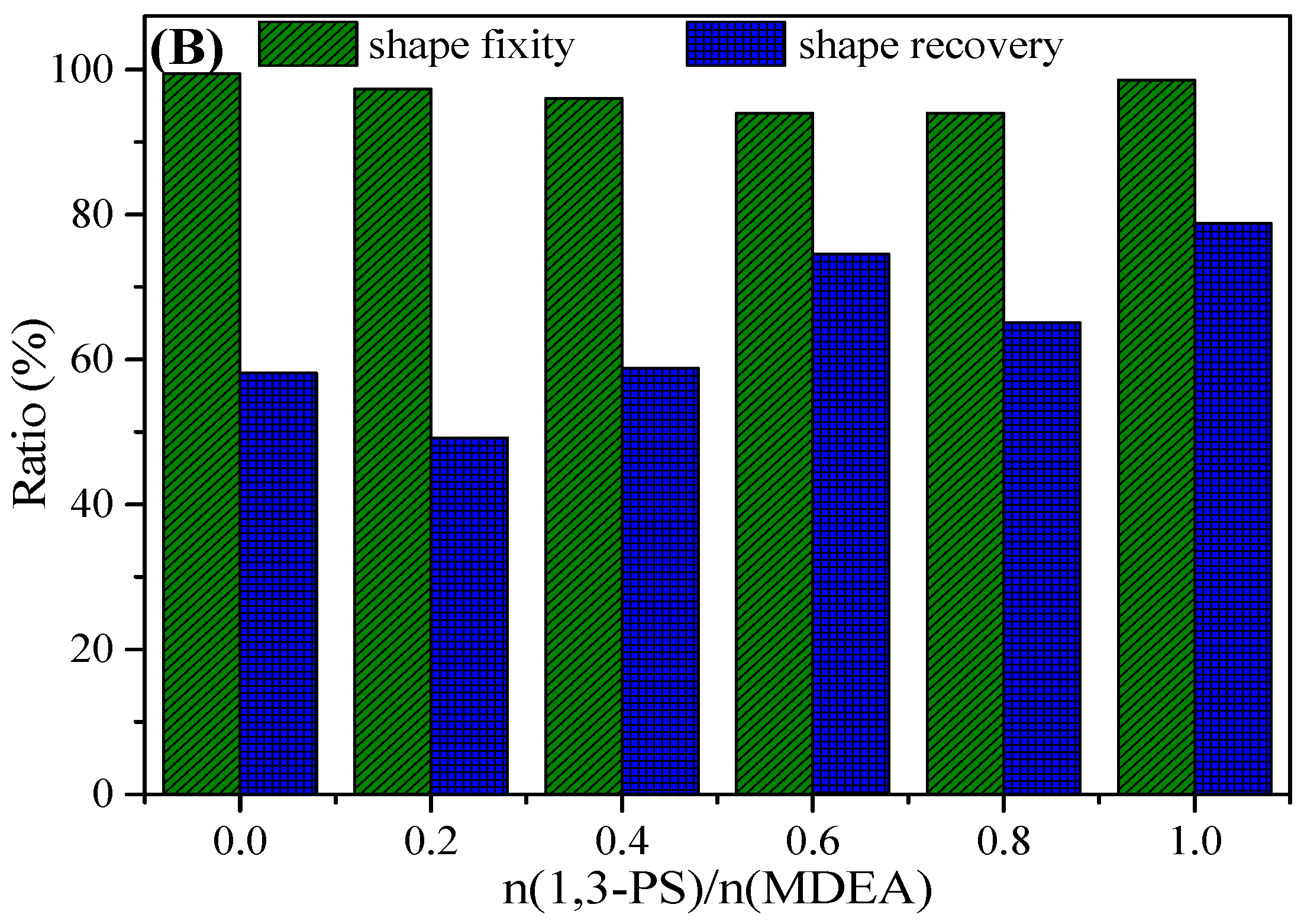
3.4. Shape Memory Properties of PEG-ZSMPUs
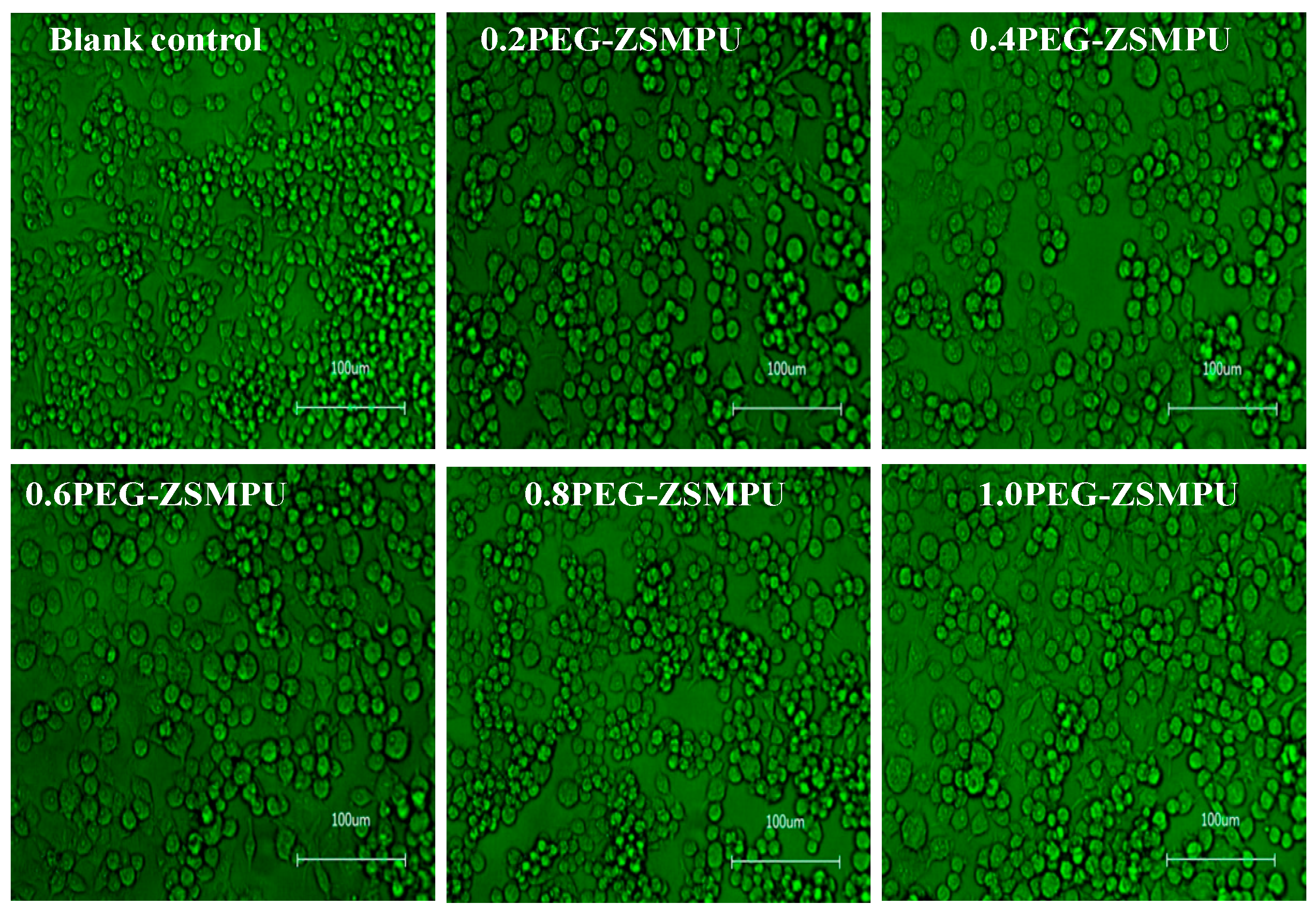
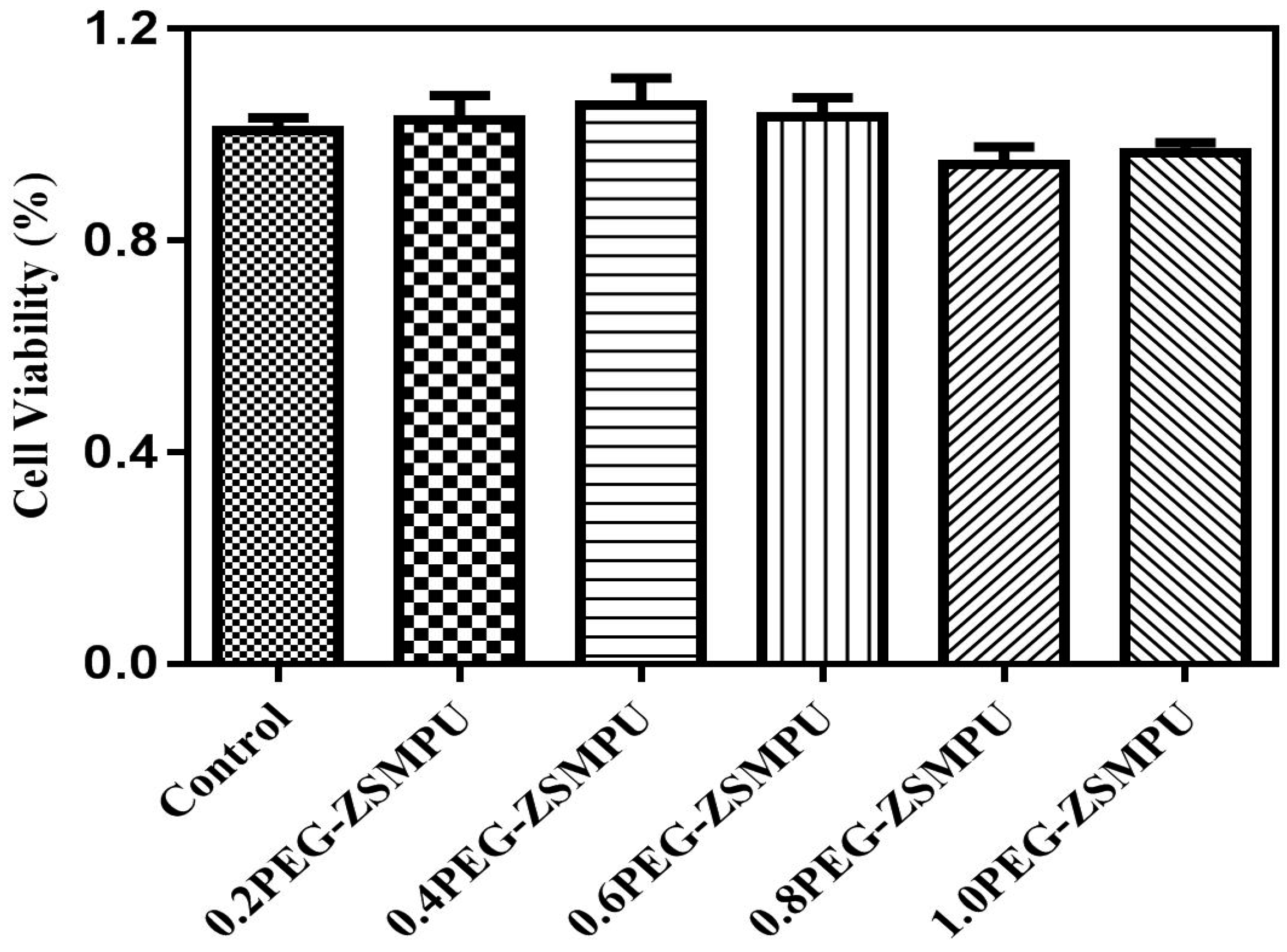
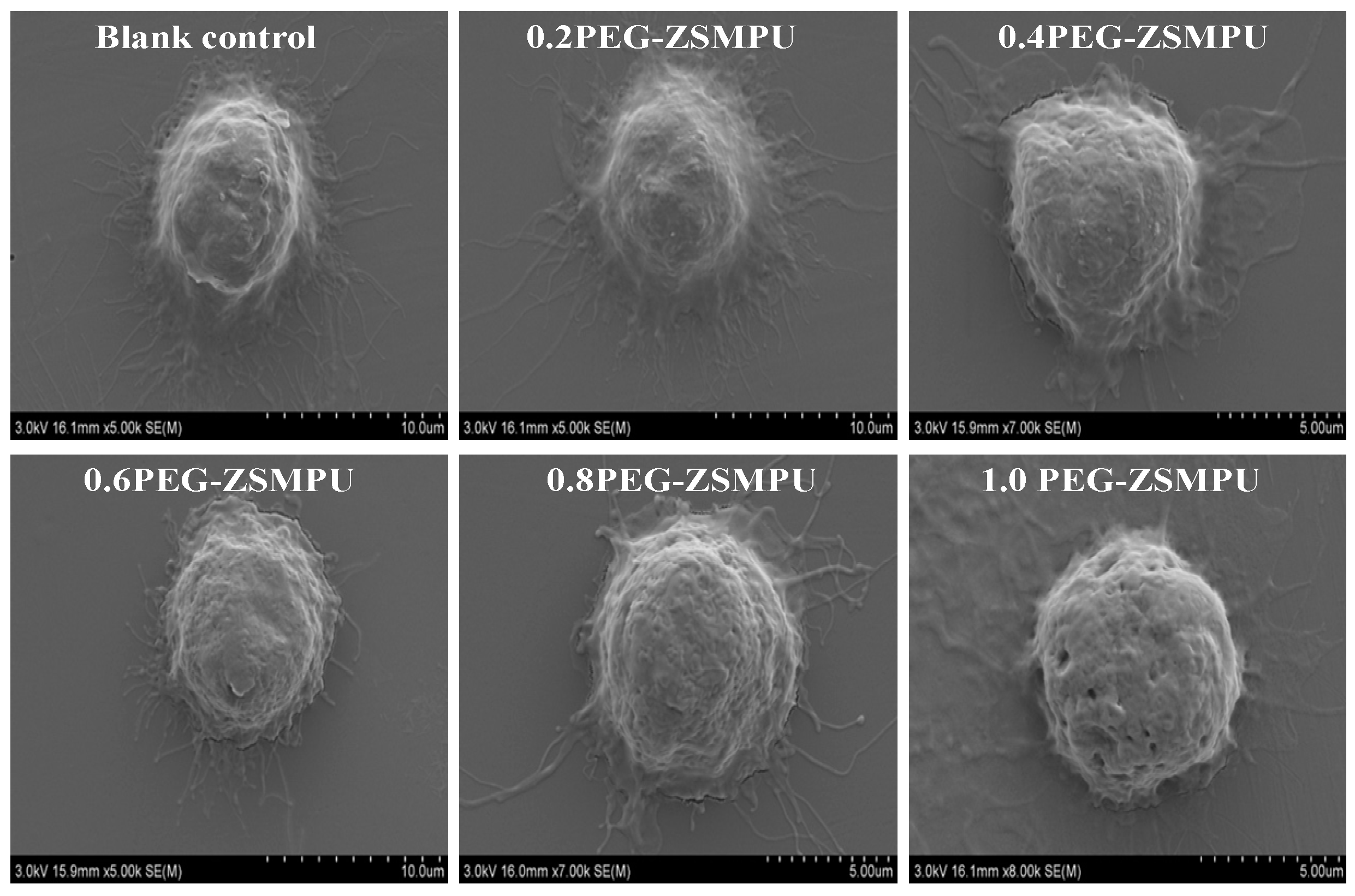
3.5. CytocompatibilityAnalysis of PEG-ZSMPUs
4. Conclusions
Supplementary Materials
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Atiqah, A.; Mastura, M.T.; Ali, B.A.A.; Jawaid, M.; Sapuan, S.M. A review on polyurethane and its polymer composites. Curr. Org. Synth. 2017, 14, 233–248. [Google Scholar] [CrossRef]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent advances in shape memory soft materials for biomedical applications. ACS Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49, 503–533. [Google Scholar] [CrossRef]
- Chen, S.J.; Hu, J.L.; Chen, S.G. Studies of the moisture-sensitive shape memory effect of pyridine-containing polyurethanes. Polym. Int. 2012, 61, 314–320. [Google Scholar] [CrossRef]
- Hu, J.L.; Zhu, Y.; Huang, H.H.; Lu, J. Recent advances in shape-memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Song, J.J.; Chang, H.H.; Naguib, H.E. Design and characterization of biocompatible shape memory polymer (SMP) blend foams with a dynamic porous structure. Polymer 2015, 56, 82–92. [Google Scholar] [CrossRef]
- Chen, S.J.; Yuan, H.M.; Zhuo, H.T.; Chen, S.G.; Yang, H.P.; Ge, Z.C.; Liu, J.H. Development of liquid-crystalline shape-memory polyurethane composites based on polyurethane with semi-crystalline reversible phase and hexadecyloxybenzoic acid for self-healing applications. J. Mater. Chem. C 2014, 2, 4203–4212. [Google Scholar] [CrossRef]
- Dueramae, I.; Nishida, M.; Nakaji-Hirabayashi, T.; Matsumura, K.; Kitano, H. Biodegradable shape memory polymers functionalized with anti-biofouling interpenetrating polymer networks. J. Mater. Chem. B 2016, 4, 5394–5404. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Shams, S.S.; Wei, Y.P.; Liu, X.Q.; Ma, S.Q.; Zhang, R.Y.; Zhu, J. Origin of highly recoverable shape memory polyurethanes (SMPUs) with non-planar ring structures: A single molecule force spectroscopy investigation. J. Mater. Chem. A 2014, 2, 20010–20016. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Gong, X.L.; Kang, Y.; Jiang, Z.C.; Zhang, S.; Li, B.J. Light-, pH- and thermal-responsive hydrogels with the triple-shape memory effect. Chem. Commun. 2016, 52, 10609–10612. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.N.; Zhou, F.X.; Chen, S.J.; Yang, H.P.; Ge, Z.C.; Chen, S.G. Development of shape memory polyurethane based on polyethylene glycol and liquefied 4,4’-diphenylmethane diisocyanate using a bulk method for biomedical applications. Polym. Int. 2015, 64, 477–485. [Google Scholar] [CrossRef]
- Zhou, H.W.; Xue, C.G.; Weis, P.; Suzuki, Y.; Huang, S.L.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.J.; Wu, S. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, M.; Sirkecioglu, A.; Bingol-Ozakpinar, O.; Uras, F.; Guner, F.S. Castor Oil and PEG-Based Shape Memory Polyurethane Films for Biomedical Applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, X.M.; Wu, G.L.; Chen, J.T.; Wang, Y.N.; Gao, H.; Ma, J.B. Precise control of drug release from dually responsive poly(ether urethane) nanoparticles. RSC Adv. 2013, 3, 13859–13868. [Google Scholar] [CrossRef]
- Liu, R.W.; Chen, Y.; Fan, H.J. Design, Characterization, Dyeing Properties, and Application of Acid-Dyeable Polyurethane in the Manufacture of Microfiber Synthetic Leather. Fibers Polym. 2015, 16, 1970–1980. [Google Scholar] [CrossRef]
- Chen, K.; Liu, R.W.; Zou, C.; Shao, Q.Y.; Lan, Y.J.; Cai, X.Q.; Zhai, L.L. Linear polyurethane ionomers as solid-solid phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 130, 466–473. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S.Y. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.N.; Ren, H.H.; Chen, S.J.; Ge, Z.C. Novel zwitterionic polyurethanes with good biocompatibility and antibacterial activity. Mater. Lett. 2015, 145, 174–176. [Google Scholar] [CrossRef]
- Chen, S.J.; Mo, F.N.; Yang, Y.; Stadler, F.J.; Chen, S.G.; Yang, H.P.; Ge, Z.C. Development of zwitterionic polyurethanes with multi-shape memory effects and self-healing properties. J. Mater. Chem. A 2015, 3, 2924–2933. [Google Scholar] [CrossRef]
- Ren, H.H.; Mei, Z.K.; Chen, S.J.; Zhuo, H.T.; Chen, S.G.; Yang, H.P.; Zuo, J.D.; Ge, Z.C. A new strategy for designing multifunctional shape memory polymers with amine-containing polyurethanes. J. Mater. Sci. 2016, 51, 9131–9144. [Google Scholar] [CrossRef]
- Xu, X.; Wu, X.T.; Wang, Q.Q.; Cai, N.; Zhang, H.X.; Jiang, Z.D.; Wan, M.; Oda, T. Immunomodulatory Effects of Alginate Oligosaccharides on Murine Macrophage RAW264.7 Cells and Their Structure-Activity Relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X.W.; Long, Y.C.; Liu, G.M.; Zhang, G.Z. Ion-Specific Conformational Behavior of Polyzwitterionic Brushes: Exploiting It for Protein Adsorption/Desorption Control. Langmuir 2013, 29, 6588–6596. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Goubran, H.; Seghatchian, J.; Burnouf, T. Smart blood cell and microvesicle-based Trojan horse drug delivery: Merging expertise in blood transfusion and biomedical engineering in the field of nanomedicine. Transfus. Apheresis Sci. 2016, 54, 309–318. [Google Scholar] [CrossRef] [PubMed]



| Samples | Soft segment | Hard segment | R # | ZHSC * (wt %) | |||
|---|---|---|---|---|---|---|---|
| HDI (g) | PEG6000 (g) | HDI (g) | MDEA (g) | 1,3-PS (g) | |||
| 0PEG-ZSMPU | 0.33 | 11.67 | 4.68 | 3.32 | 0 | 0 | 40 |
| 0.2PEG-ZSMPU | 0.33 | 11.67 | 4.68 | 3.32 | 0.68 | 0.2 | 42 |
| 0.4PEG-ZSMPU | 0.33 | 11.67 | 4.68 | 3.32 | 1.36 | 0.4 | 44 |
| 0.6PEG-ZSMPU | 0.33 | 11.67 | 4.68 | 3.32 | 2.04 | 0.6 | 46 |
| 0.8PEG-ZSMPU | 0.33 | 11.67 | 4.68 | 3.32 | 2.72 | 0.8 | 47 |
| 1.0PEG-ZSMPU | 0.33 | 11.67 | 4.68 | 3.32 | 3.40 | 1.0 | 49 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.; Ren, H.; Ge, Z.; Zhuo, H.; Chen, S. Shape Memory Polyurethanes Based on Zwitterionic Hard Segments. Polymers 2017, 9, 465. https://doi.org/10.3390/polym9100465
Fu S, Ren H, Ge Z, Zhuo H, Chen S. Shape Memory Polyurethanes Based on Zwitterionic Hard Segments. Polymers. 2017; 9(10):465. https://doi.org/10.3390/polym9100465
Chicago/Turabian StyleFu, Shuqin, Huanhuan Ren, Zaochuan Ge, Haitao Zhuo, and Shaojun Chen. 2017. "Shape Memory Polyurethanes Based on Zwitterionic Hard Segments" Polymers 9, no. 10: 465. https://doi.org/10.3390/polym9100465