The Chemistry and Physics of Bayfol® HX Film Holographic Photopolymer
Abstract
:1. Introduction
Volume Holographic Optical Elements and Their Applications
2. Photopolymers as Recording Media for vHOEs
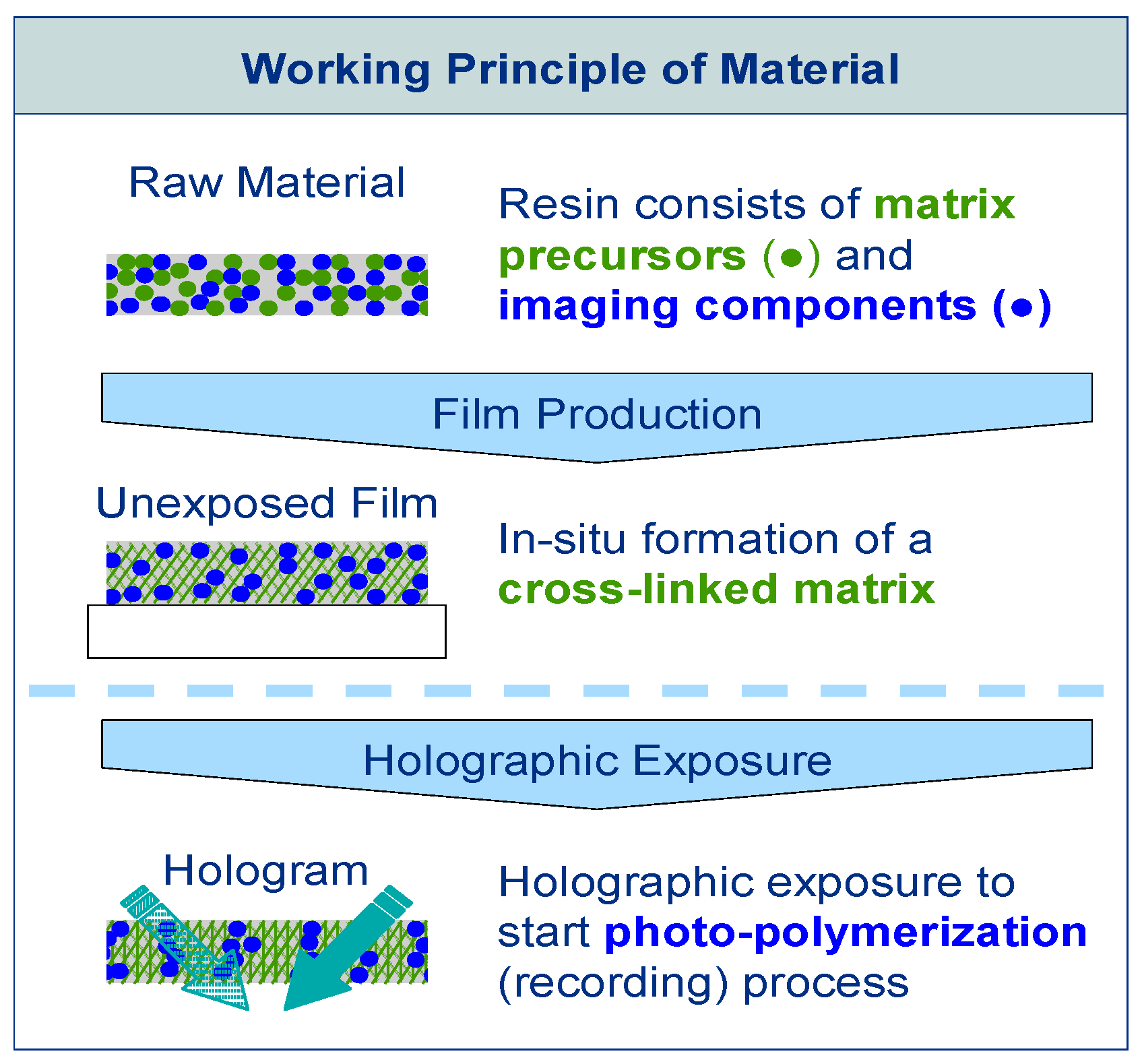
2.1. Working Principle of a 2-Chemistry Photopolymer Film
2.2. Web-Coating of Bayfol® HX Film
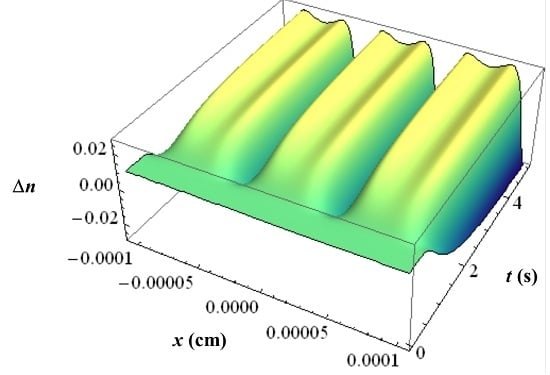
3. Modelling the Recording Mechanism of Bayfol® HX Film
Recording at the Low Spatial Frequency Cutoff
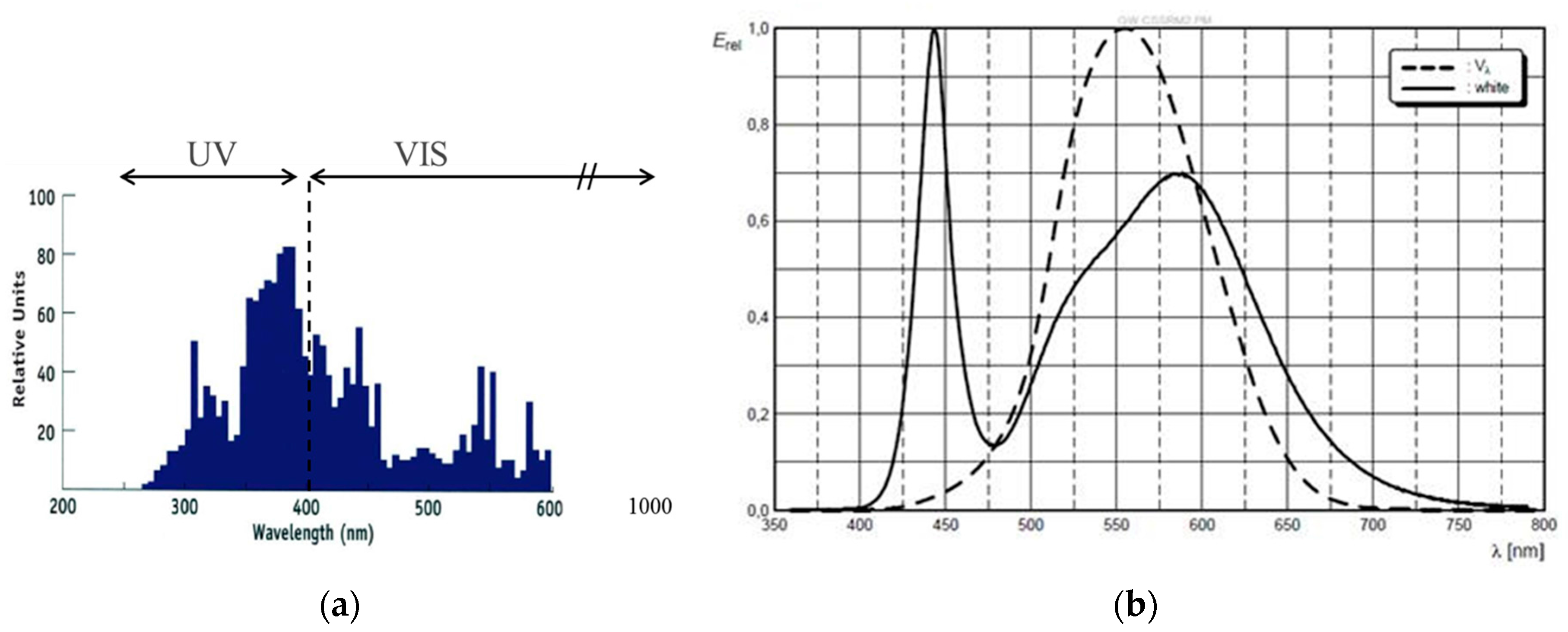
4. Optimization of the Photo-Initiator System for the Visible Spectrum
4.1. One-Component System
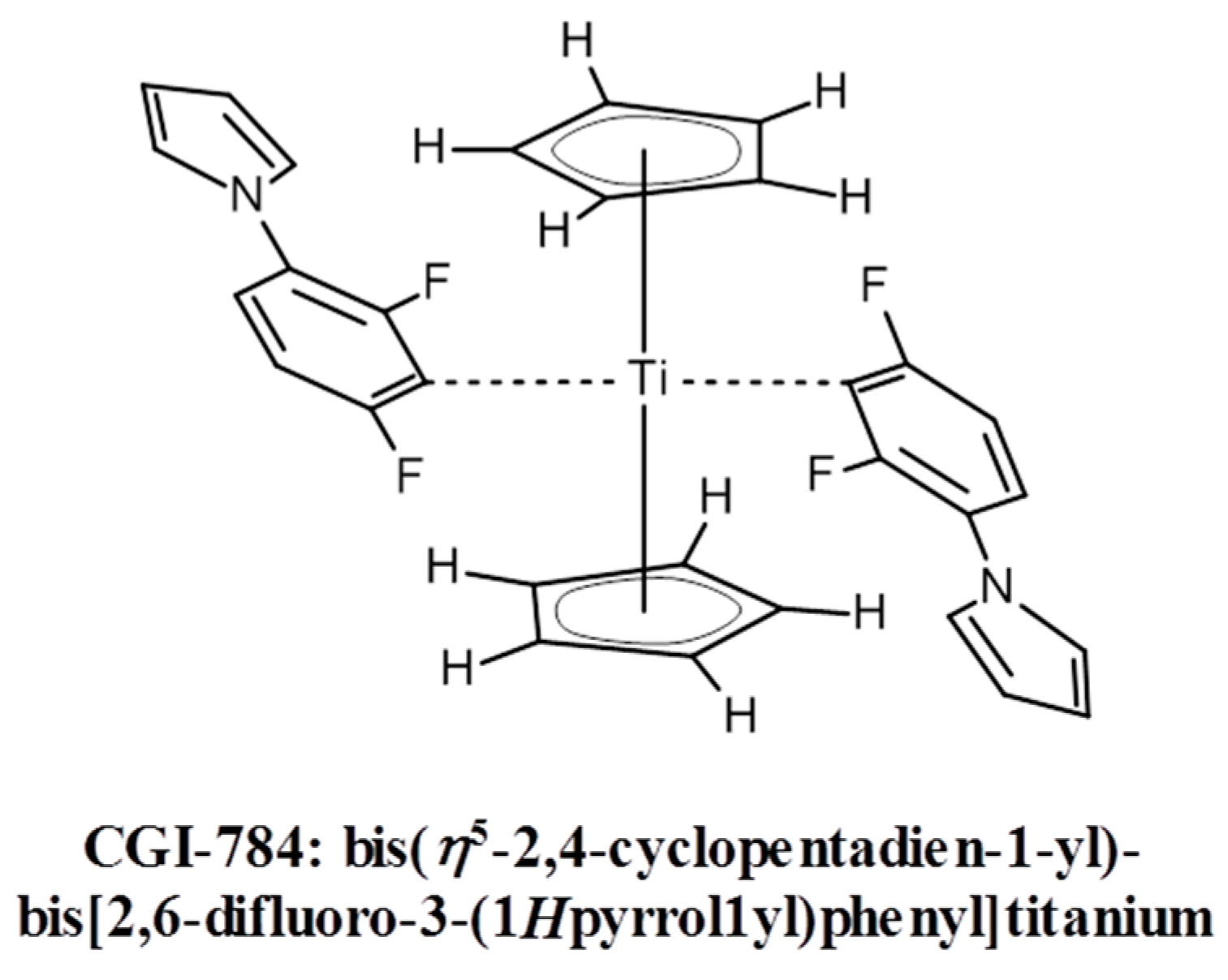
4.2. Two-Component System Dye/Organo-Borate Salt
4.3. Excited State Process
4.4. Holographic Recording
5. Full Non-Local Reaction Driven Diffusion Model for Bayfol® HX Film
5.1. Outline of the Fully Detailed Model
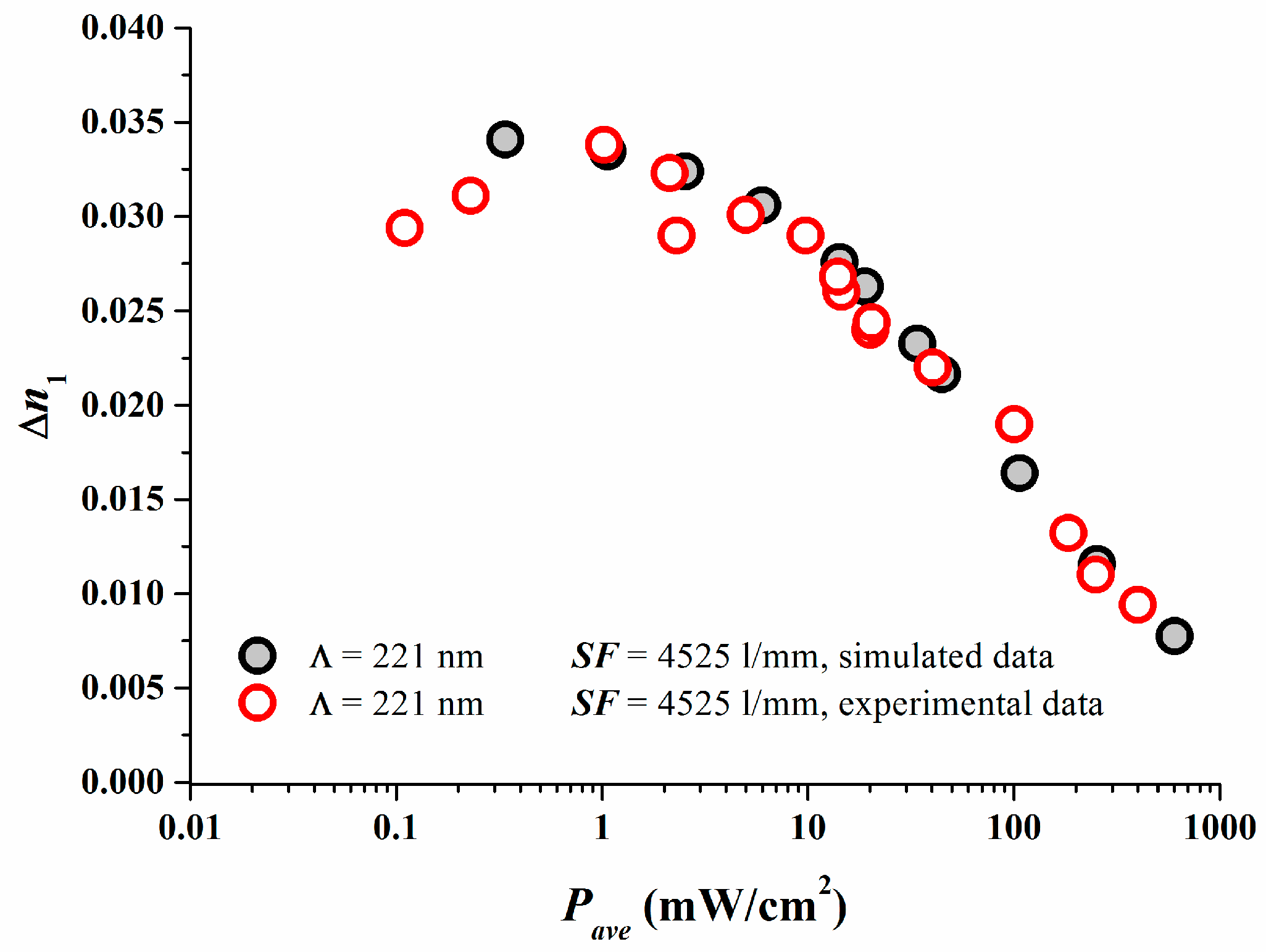
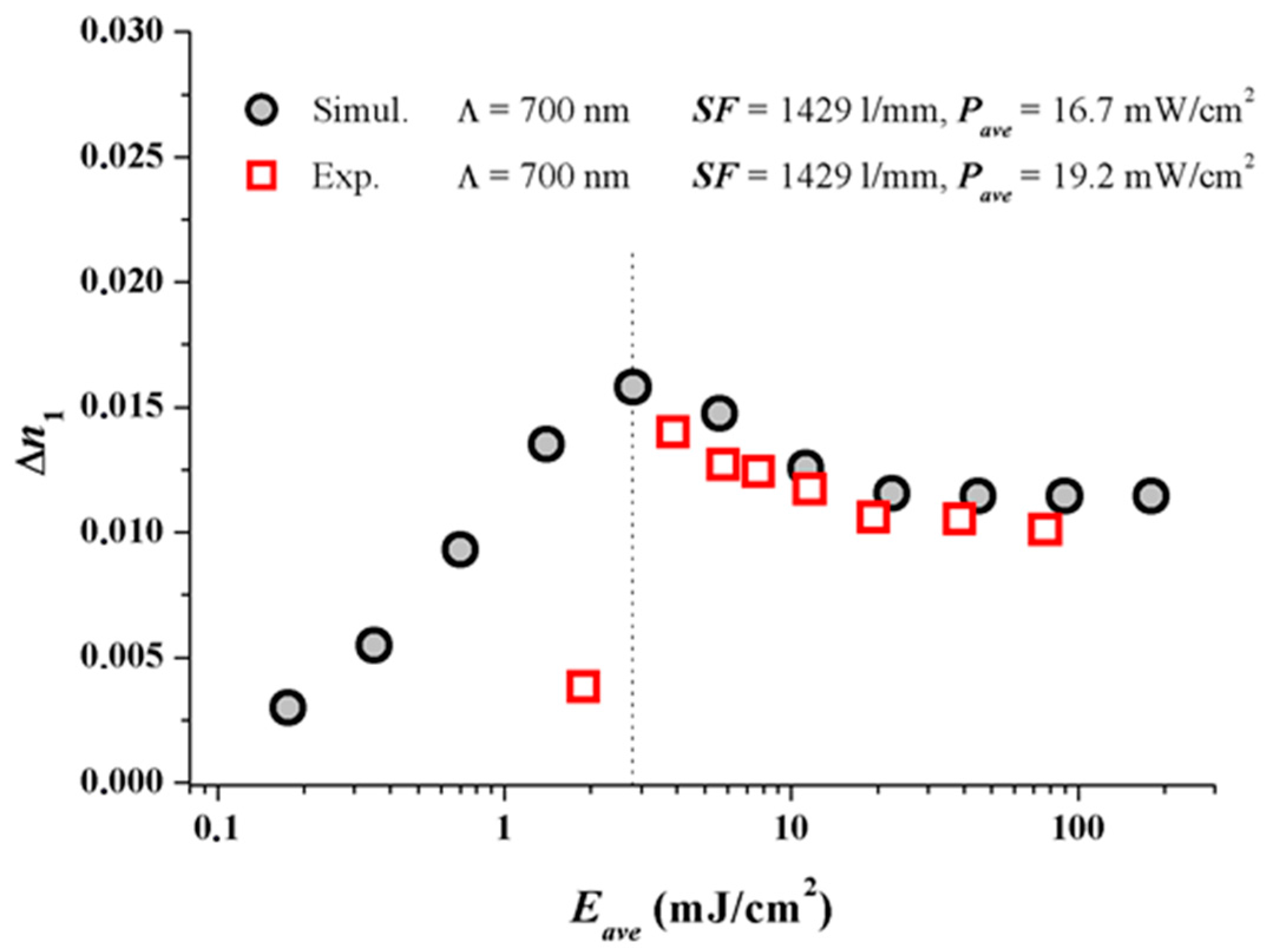
5.2. Estimation of the Model Parameters by Comparing Experimental and Simulated Dosage Response Curves of RGB Sensitive Bayfol® HX Film
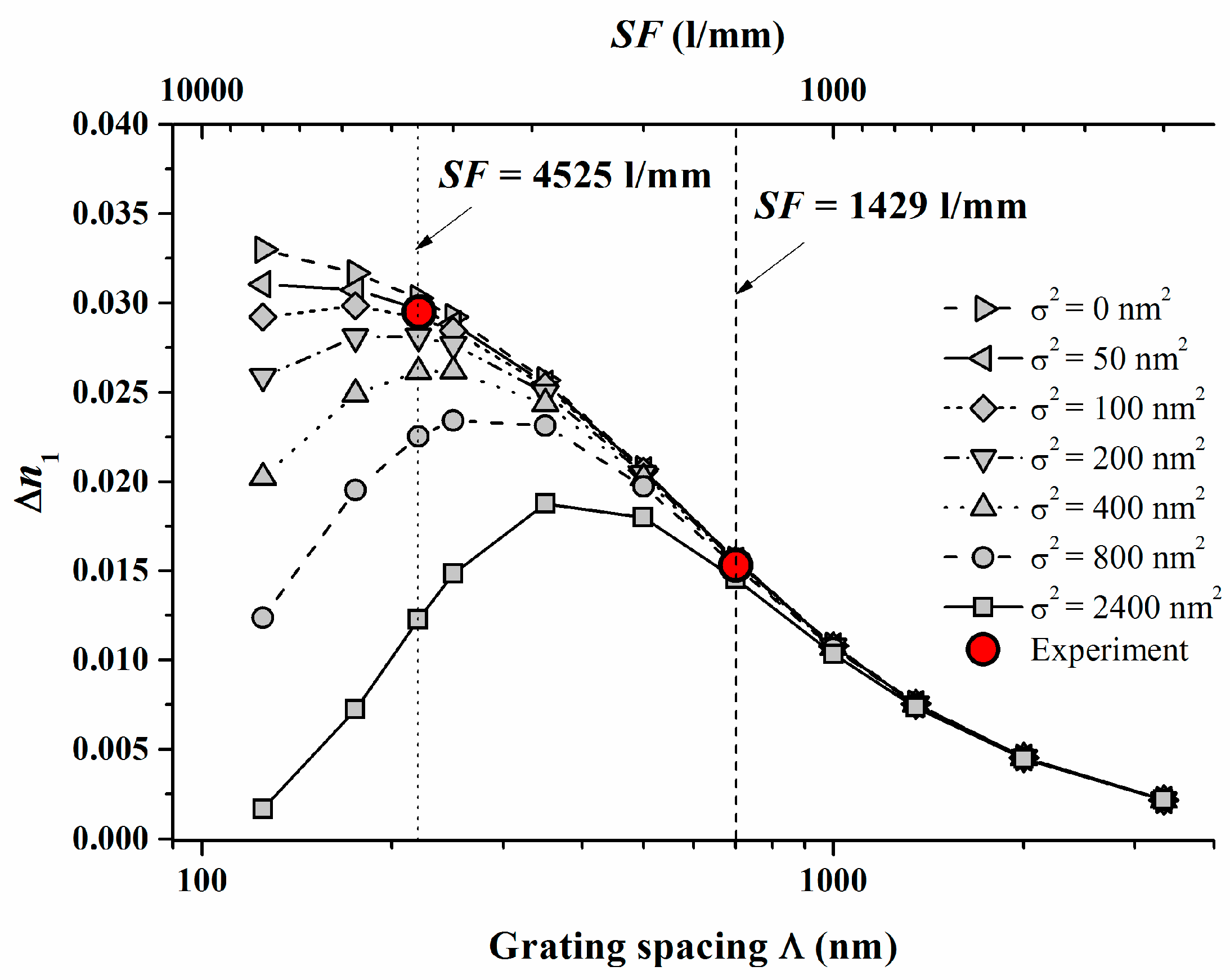
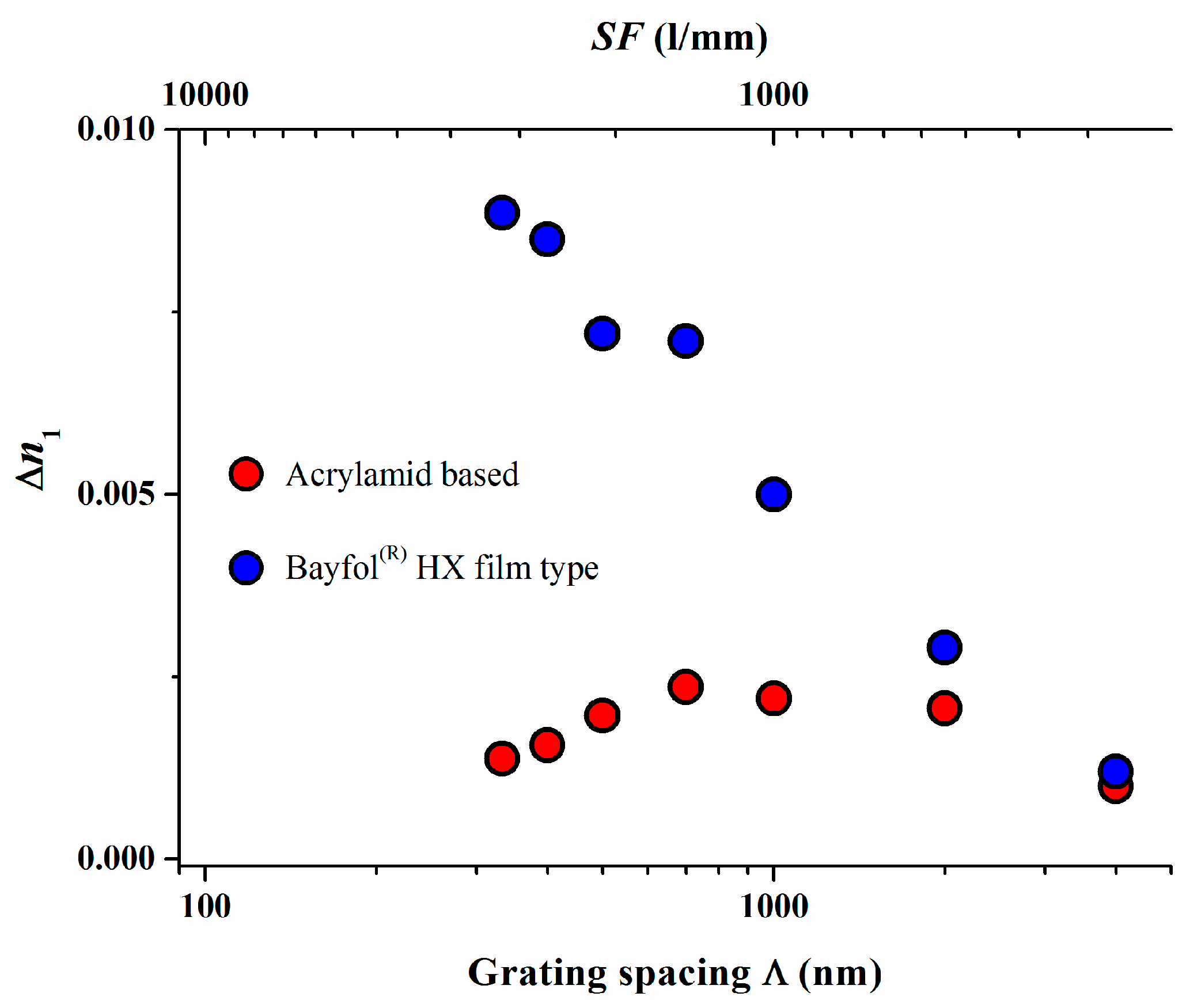
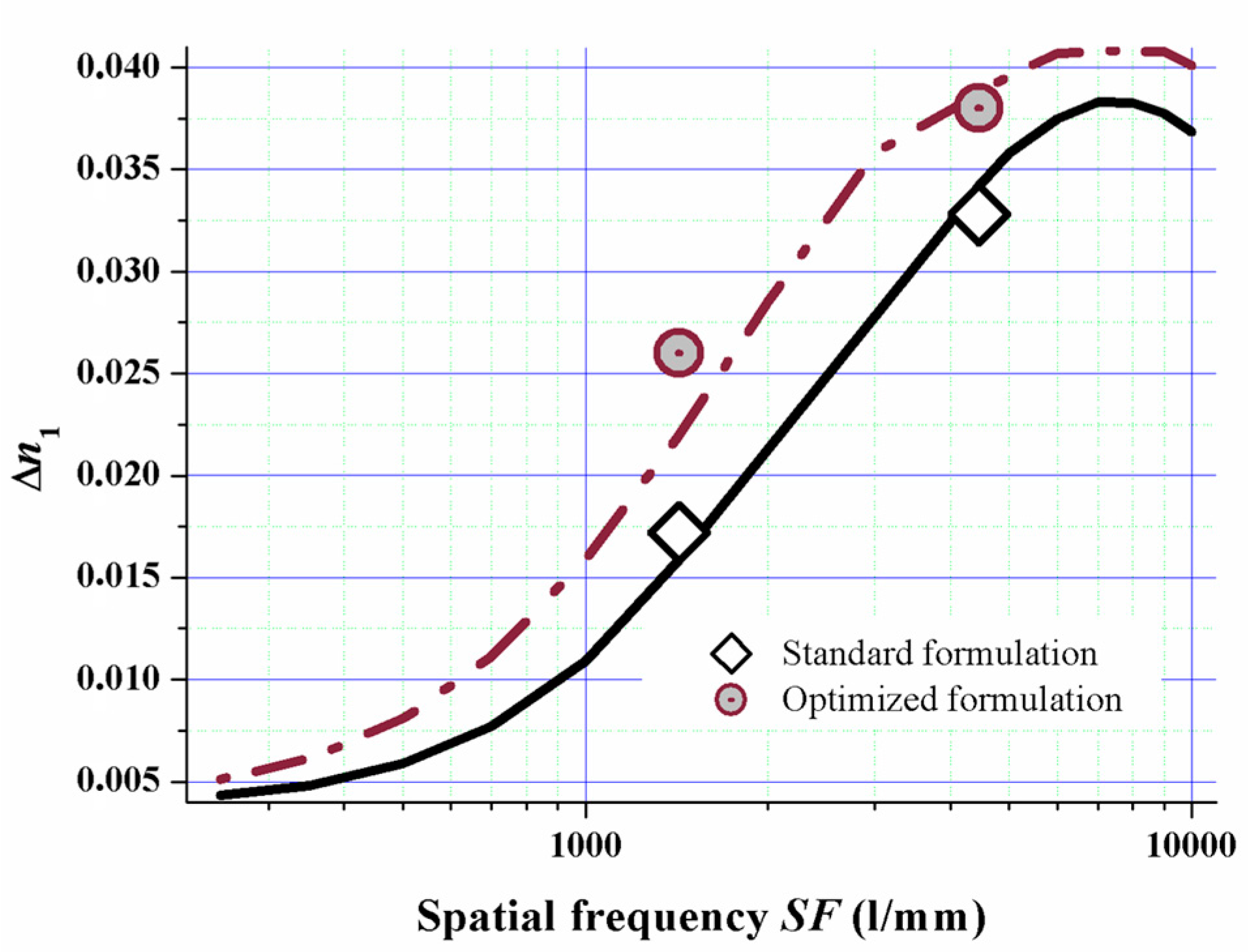
5.3. Improving the Spatial Frequency Response Based on the Fully Detailed Reaction-Diffusion Model
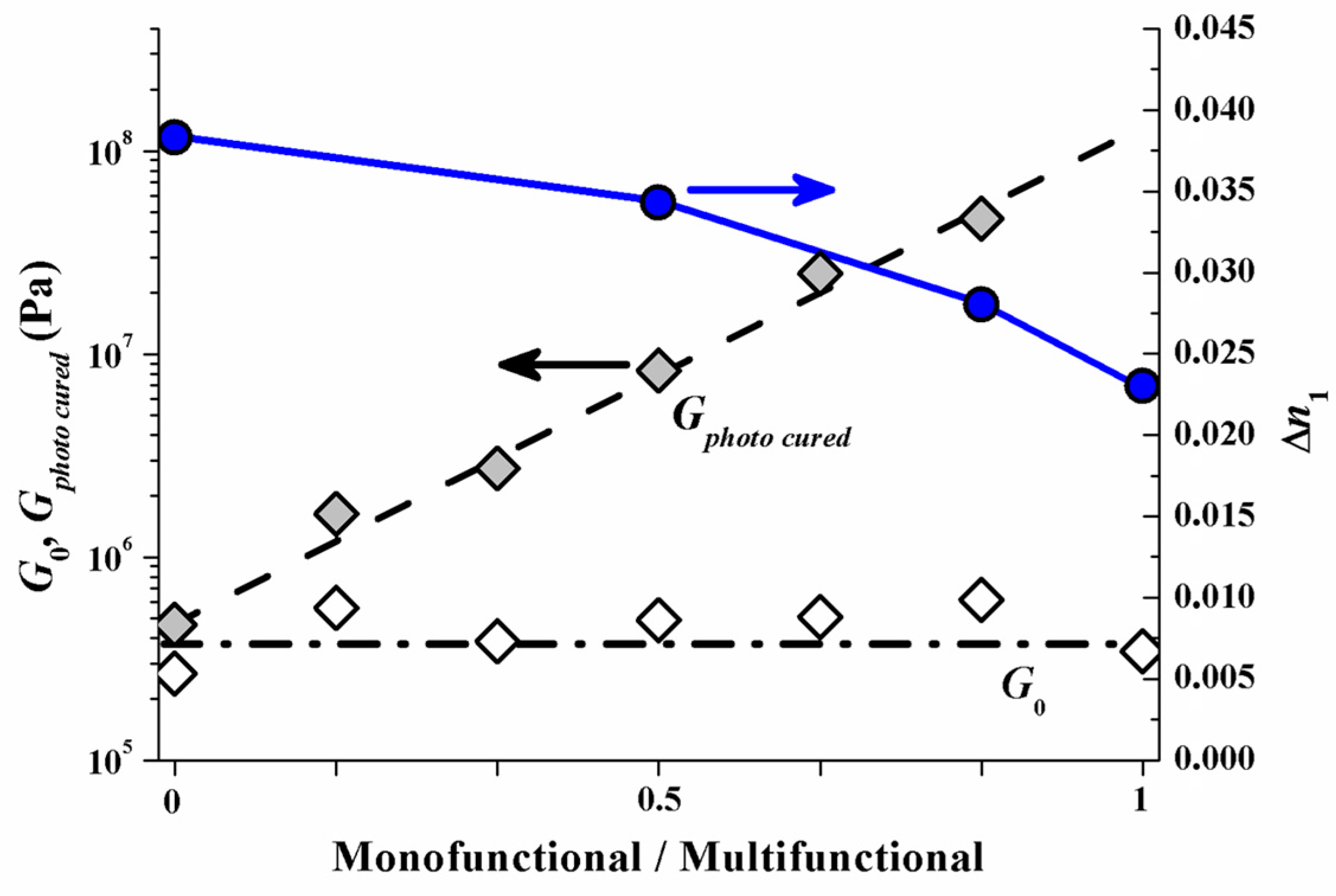
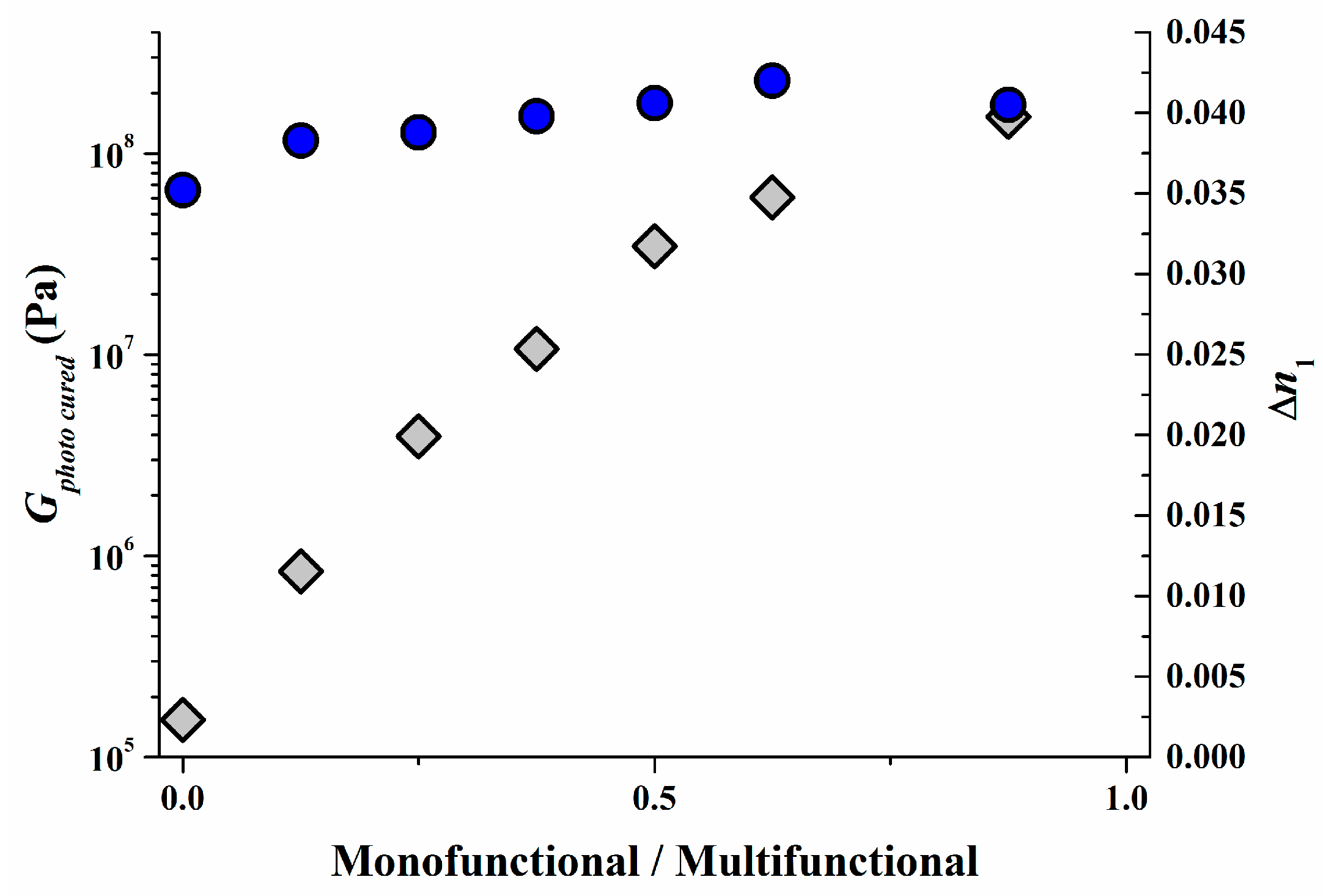
6. Mechanical Modulus from Soft Rubber to Hard Rubber
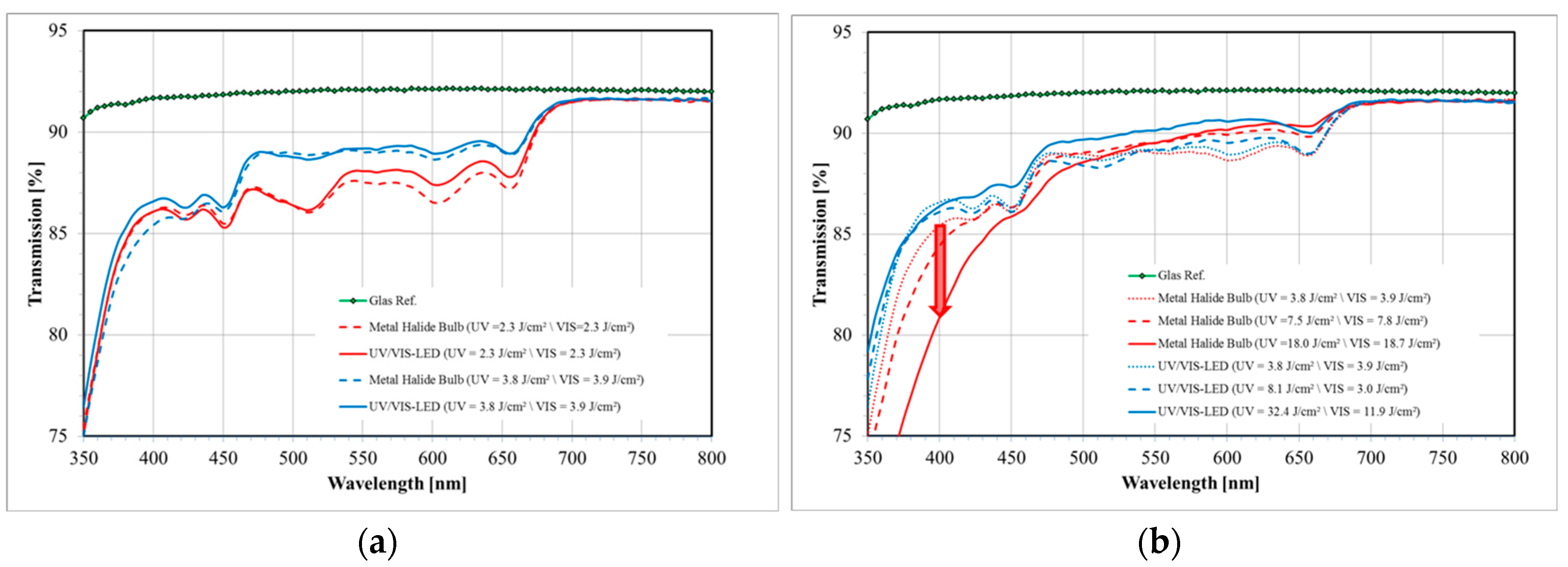
7. Bleaching Process
8. How to Mass Produce Volume Holographic Optical Elements with Bayfol® HX Film
8.1. Mastering-Overcoming Size and Laser Power Limitations
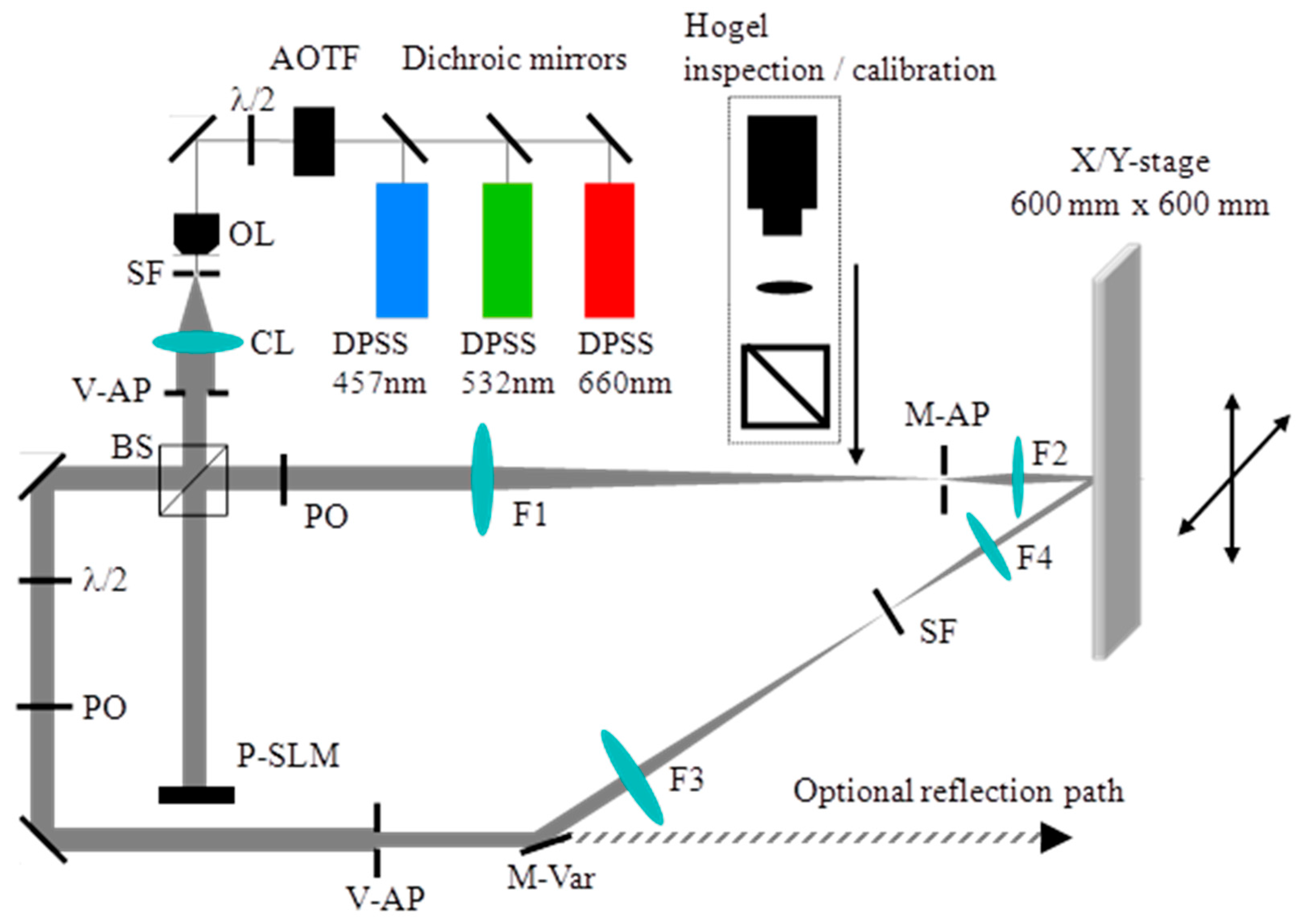
8.2. Experimental Setup of the HOE Printer
8.3. Application Example: Off-Axis vHOE Lens
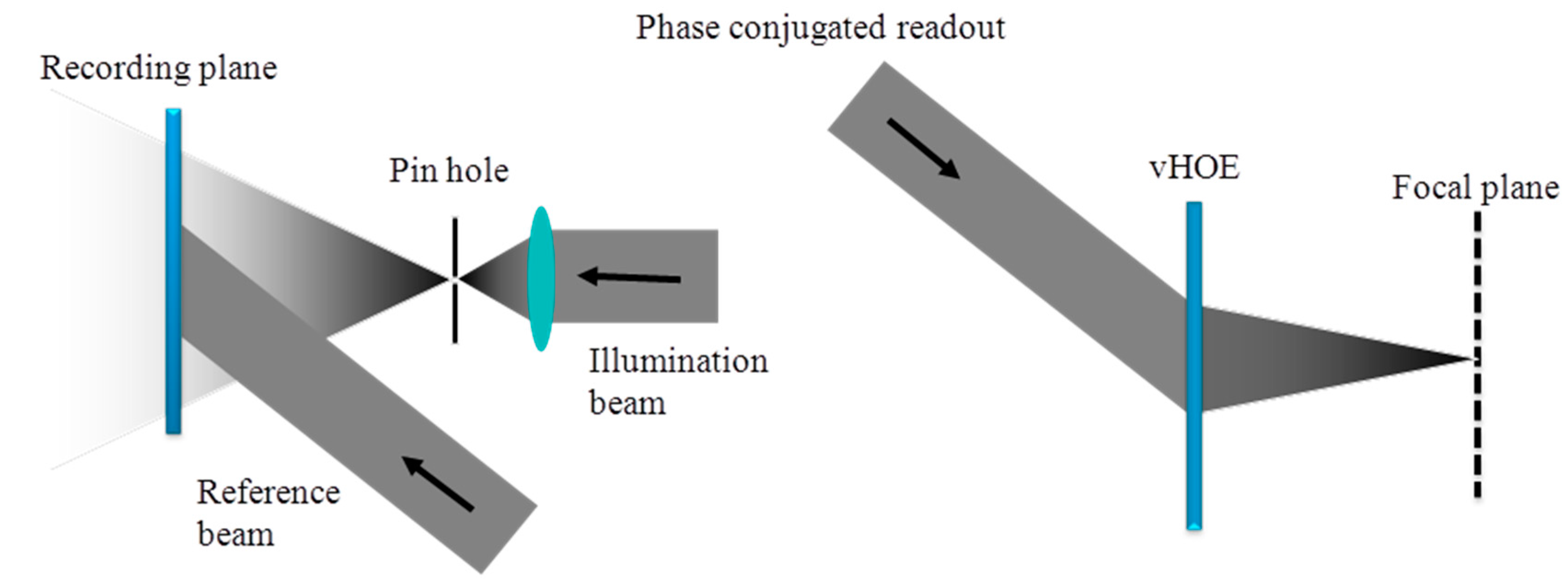
8.3.1. Recording Scheme
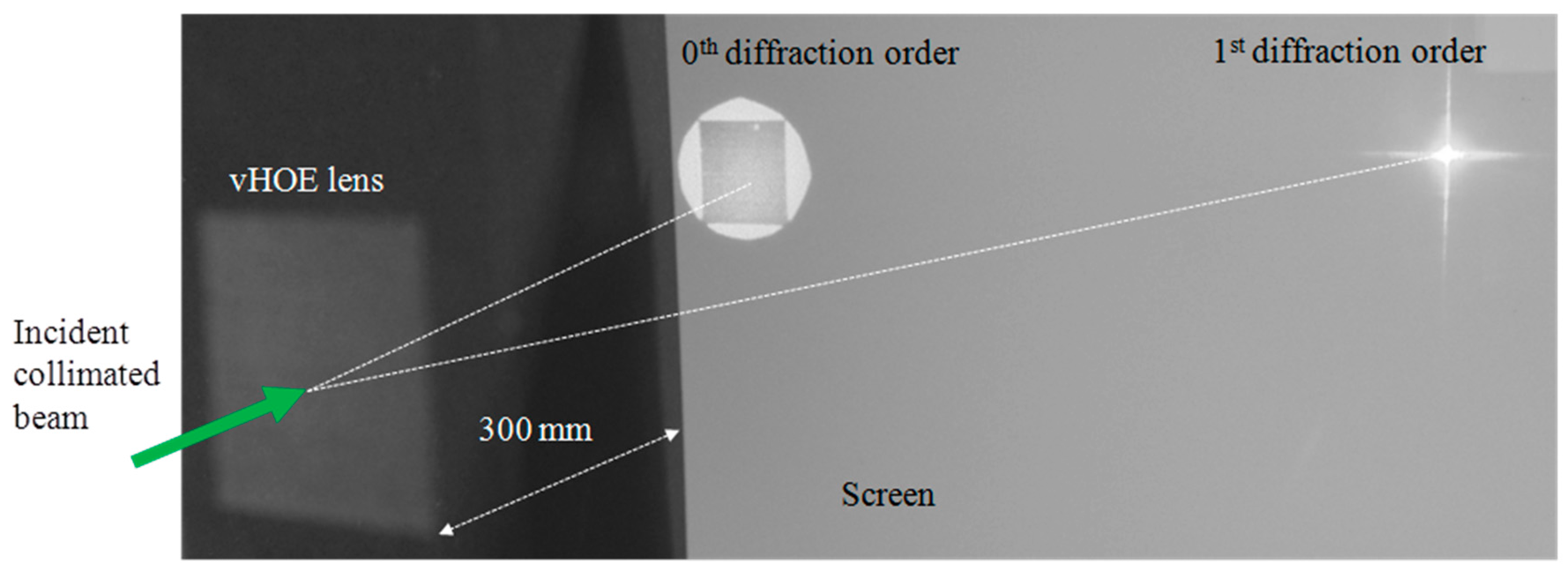
8.3.2. Experimental Verification
8.4. R2R Replication of Volume Holographic Optical Elements
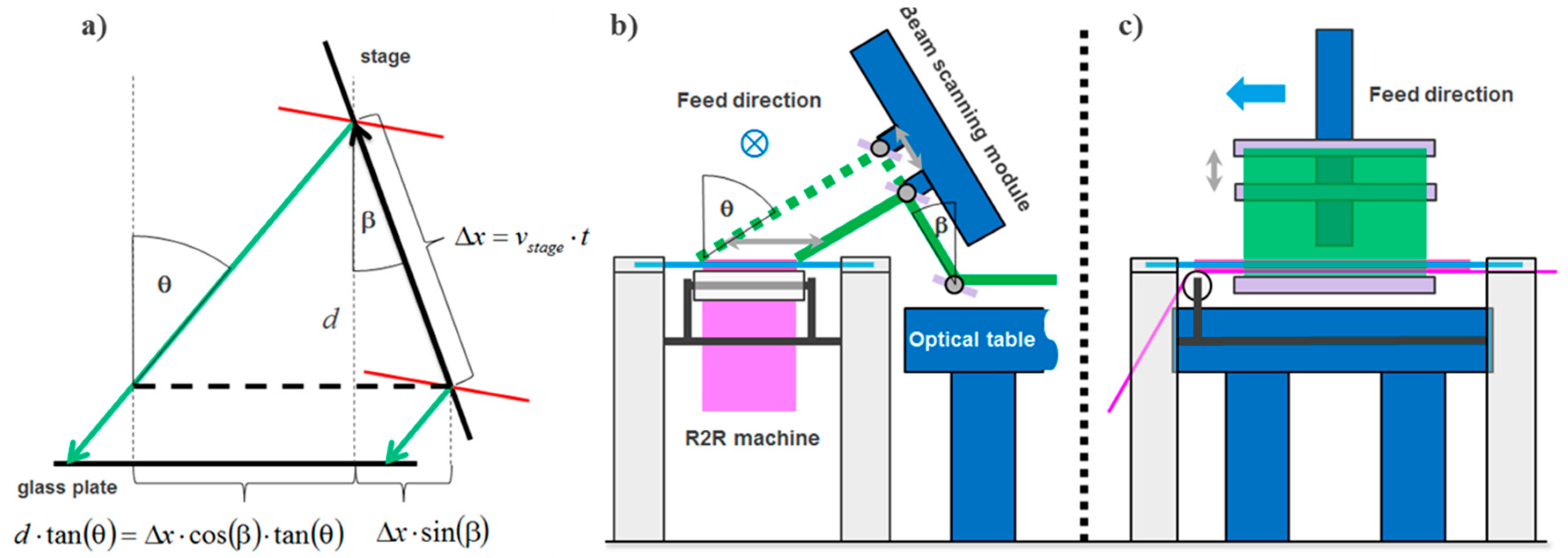
8.4.1. Roll-To-Roll (R2R) Mechanics
8.4.2. The Laser Scanning Module
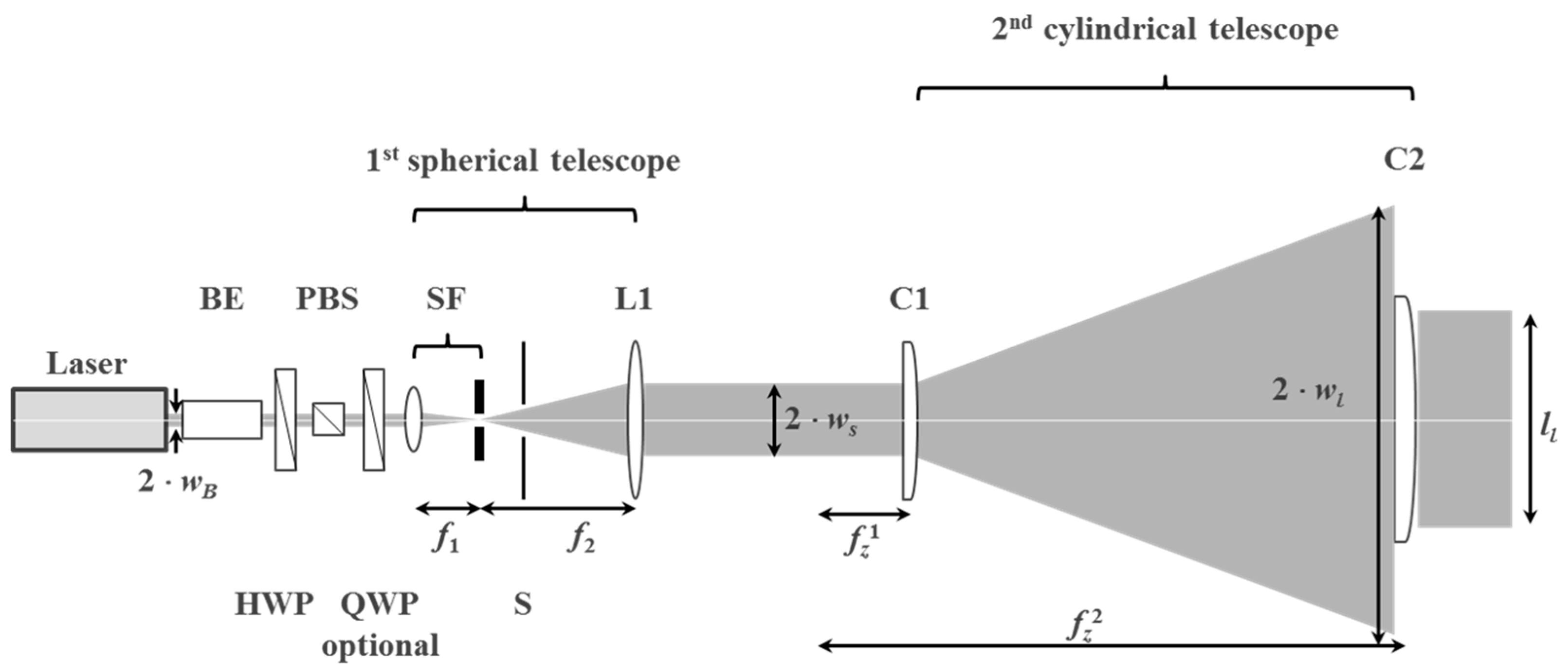
8.4.3. Optics for Beam Shaping and Wave Front Shaping
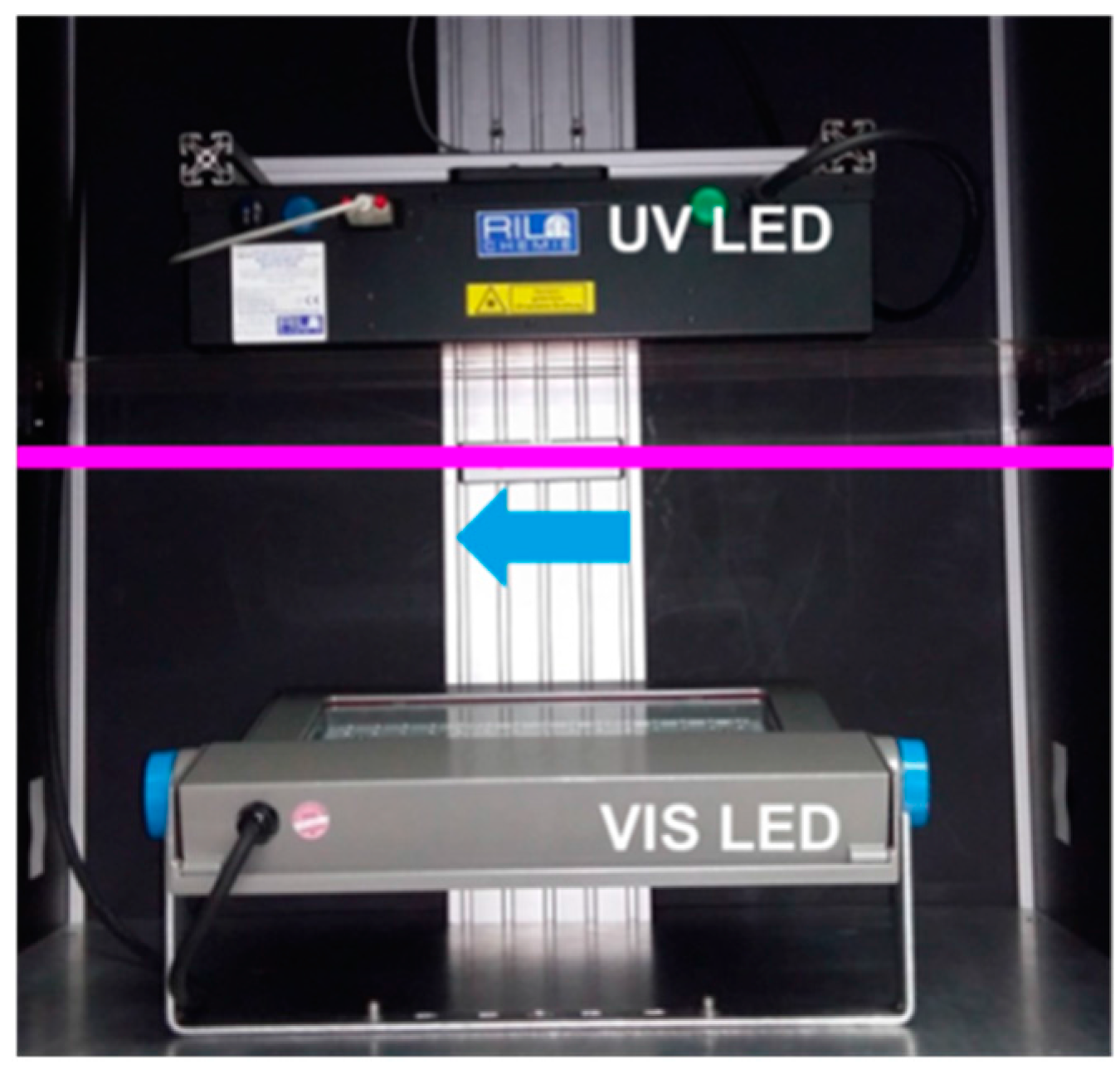
8.4.4. Bleaching Module
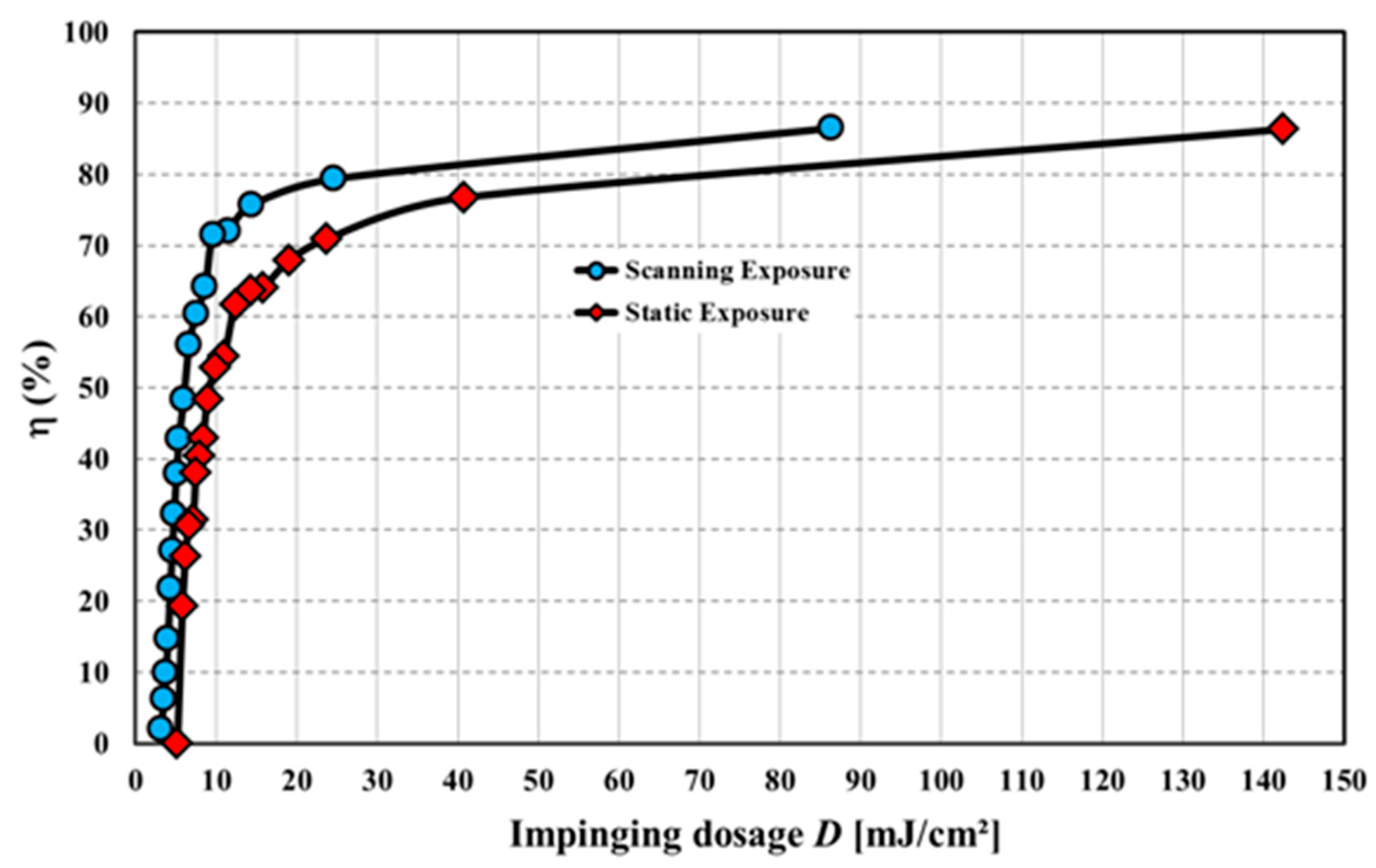
8.4.5. Dosage Response of the Contact Copy Process
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kogelnik, H. Coupled wave theory for thick hologram gratings. Bell Syst. Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Kress, B.C. Restocking the optical designer’s toolbox for next-generation wearable displays (Presentation Recording). Proc. SPIE 2015, 9579, 957903. [Google Scholar] [CrossRef]
- Luminit Transparent Holographic Components for Motorcycle Head-up Display. Available online: http://www.prnewswire.com/news-releases/sid-announces-2017-display-industry-award-winners-honorees-reflect-the-state-of-the-art-in-display-technology-300458321.html (accessed on 29 July 2017).
- HoloPro™. Available online: http://www.holopro.com/en/products.html (accessed on 29 July 2017).
- Zanutta, A.; Orselli, E.; Fäcke, T.; Bianco, A. Photopolymer based VPHGs: From materials to sky results. Proc. SPIE 2016, 9912, 99123B. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Bruder, F.K.; Fäcke, T.; Kim, S.C.; Walze, G.; Hagen, R. Time-sequential autostereoscopic 3-D display with a novel directional backlight system based on volume-holographic optical elements. Opt. Express 2014, 22, 9820. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.L.; Su, W.C.; Chen, C.Y.; Lin, B.S.; Ho, H.W. Full color image splitter based on holographic optical elements for stereogram application. J. Display Technol. 2013, 9, 607–612. [Google Scholar] [CrossRef]
- Metamaterial Holography. Available online: http://www.metamaterial.com/metamaterial-holography/ (accessed on 29 July 2017).
- Holographic Technology Revolutionizes Rear Lighting. Available online: http://www.hella.com/hella-com/en/Technology-Products-19-10-2016-12601.html (accessed on 29 July 2017).
- HOPS. Available online: http://www.visionoptics.de/index.php?id=18 (accessed on 29 July 2017).
- Akbari, H.; Naydenova, I.; Martin, S. Using acrylamide-based photopolymers for fabrication of holographic optical elements in solar energy applications. Appl. Opt. 2014, 53, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.E.; Russo, J.M.; Kostuk, R.K.; Rosenberg, G.A. Thermal effects of the extended holographic regions for holographic planar concentrator. J. Photonics Energy 2011, 1, 015504. [Google Scholar] [CrossRef]
- Bruder, F.K.; Hagen, R.; Rölle, T.; Weiser, M.S.; Fäcke, T. From the surface to volume: Concepts for the next generation of optical–holographic data-storage materials. Angew. Chem. Int. Ed. 2011, 50, 4552–4573. [Google Scholar] [CrossRef] [PubMed]
- Trentler, T.J.; Boyd, E.J.; Colvin, V.L. Epoxy-photopolymer composites: Thick recording media for holographic data storage. Proc. SPIE 2001, 4296, 259–261. [Google Scholar] [CrossRef]
- Trentler, T.J.; Boyd, E.J.; Colvin, V.L. Epoxy Resin−Photopolymer Composites for Volume Holography. Chem. Mater. 2000, 12, 1431–1438. [Google Scholar] [CrossRef]
- Weiser, M.-S.; Bruder, F.K.; Fäcke, T.; Hönel, D.; Jurbergs, D.; Rölle, T. Self-Processing, Diffusion-Based Photopolymers for Holographic Applications. Macromol. Symp. 2010, 296, 133–137. [Google Scholar] [CrossRef]
- Bruder, F.K.; Deuber, F.; Fäcke, T.; Hagen, R.; Hönel, D.; Jurbergs, D.; Rölle, T.; Weiser, M.S. Reaction-diffusion model applied to high resolution Bayfol® HX photopolymer. Proc. SPIE 2010, 7619, 76190I. [Google Scholar] [CrossRef]
- Gleeson, M.R.; Sheridan, J.T.; Bruder, F.K.; Rölle, T.; Berneth, H.; Weiser, M.S.; Fäcke, T. Comparison of a new self-developing photopolymer with AA/PVA based photopolymer utilizing the NPDD model. Opt. Express 2011, 19, 26325–26342. [Google Scholar] [CrossRef] [PubMed]
- Rölle, T.; Bruder, F.K.; Fäcke, T.; Weiser, M.S.; Hönel, D.; Stöckel, N. Photopolymerzusammensetzungen für Optische Elemente und Visuelle Darstellungen. European Patent EP 2 172 505 A1, 7 April 2010. [Google Scholar]
- Zhao, G.; Mouroulis, P. Diffusion model of hologram formation in dry photopolymer materials. J. Mod. Opt. 1994, 41, 1929–1939. [Google Scholar] [CrossRef]
- Noiret, N.; Meyer, C.; Lougnot, D.J. Photopolymers for holographic recording: V. Self-processing systems with near infrared sensitivity. Pure Appl. Opt. 1994, 3, 55–71. [Google Scholar] [CrossRef]
- Sheridan, J.T.; Lawrence, J.R. Nonlocal-response diffusion model of holographic recording in photopolymer. J. Opt. Soc. Am. A 2000, 17, 1108–1114. [Google Scholar] [CrossRef]
- O’Neill, F.T.; Lawrence, J.R.; Sheridan, J.T. Comparison of holographic photopolymer materials by use of analytical nonlocal diffusion models. Appl. Opt. 2002, 41, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.V.; O’Neill, F.T.; Sheridan, J.T.; Neipp, C.; Gallego, S.; Ortuno, M. Holographic photopolymer materials: Nonlocal polymerization-driven diffusion under nonideal kinetic conditions. J. Opt. Soc. Am. B 2005, 22, 407–416. [Google Scholar] [CrossRef]
- Gallego, S.; Ortuno, M.; Neipp, C.; Fernandez, E.; Belendez, A.; Pascual, I. Improved maximum uniformity and capacity of multiple holograms recorded in absorbent photopolymers. Opt. Express 2005, 15, 9308–9319. [Google Scholar] [CrossRef]
- Gleeson, M.R.; Sheridan, J.T. A review of the modeling of free-radical photo polymerization in the formation of holographic gratings. J. Opt. A Pure Appl. Opt. 2009, 11, 1–12. [Google Scholar] [CrossRef]
- Sheridan, J.T.; Gleeson, M.R.; Close, C.E.; Kelly, J.V. Optical response of photopolymer materials for holographic data storage applications. J. Nanosci. Nanotechnol. 2007, 7, 1–11. [Google Scholar] [CrossRef]
- Zanutta, A.; Orselli, E.; Fäcke, T.; Bianco, A. Photopolymeric films with highly tunable refractive index modulation for high precision diffractive optics. Opt. Mater. Express 2016, 6, 252–263. [Google Scholar] [CrossRef]
- Drobny, J.G. Radiation Technology for Polymers; CRC: Bocan Raton, FL, USA, 2010; ISBN 978-1-4200-9404-6. [Google Scholar]
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444529794. [Google Scholar]
- Allonas, X.; Croutxe-Barghorn, C.; Fouassier, J.P.; Lalevée, J.; Malval, J.P.; Morlet-Savary, F. Lasers in Chemistry: Probing and Influencing Matter; Lackner, M., Ed.; Wiley-VCH: Weinheim, Germany, 2008; Volume II, pp. 1001–1027. ISBN 978-3-527-31997-8. [Google Scholar]
- Allonas, X.; Croutxé-Barghorn, C.; Bögl, K.W.; Helle, N.; GSchreiber, G.A. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527306732. [Google Scholar]
- Dietliker, K. A Compilation of Photo-Initiators Commercially Available for UV Today; SITA Technology Limited: London, UK, 2002; ISBN 0947798676, 9780947798673. [Google Scholar]
- Fouassier, J.P.; Allonas, X.; Lalevée, J.; Dietlin, C. Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 351–419. ISBN 978-0-470-13796-3. [Google Scholar]
- Ibrahim, A.; Allonas, X.; Ley, C.; Kawamura, K.; Berneth, H.; Bruder, F.K.; Fäcke, T.; Hagen, R.; Hönel, D.; Rölle, T.; et al. High Performance Photoinitiating Systems for Holography Recording: Need for a Full Control of Primary Processes. Chem. A Eur. J. 2014, 20, 15102–15107. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.; Carré, C.; Ibrahim, A.; Allonas, X. Holographic Materials and Systems: Application of High Performance Photoinitiating Systems for Holographic Grating Recording; Naydenova, I., Ed.; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Schilling, M.L.; Colvin, V.L.; Dhar, L.; Harris, A.L.; Schilling, F.C.; Katz, H.E.; Wysocki, T.; Hale, A.; Blyler, L.L.; Boyd, C. Acrylate oligomer-based photopolymers for optical storage applications. Chem. Mater. 1999, 11, 247–254. [Google Scholar] [CrossRef]
- Davidenko, N.; Garcia, O.; Sastre, R. The efficiency of titanocene as photo-initiator in the polymerization of dental formulations. J. Biomater. Sci. Polym. Ed. 2003, 14, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Fouassier, J.P.; Morlet-Savary, F. Photopolymers for laser imaging and holographic recording: Design and reactivity of photosensitizers. Opt. Eng. 1996, 35, 304–312. [Google Scholar] [CrossRef]
- Adair, P.C.; Gottschalk, P. Photo-Initiators and Photosensitive Compositions Containing Such Photo-Initiators. European Patent EP 0 368 629 A2, 8 November 1988. [Google Scholar]
- Davies, A.G.; Roberts, B.P. Bimolecular homolytic substitution by tert-butoxy radicals at metal atoms. Organomet. Chem. 1969, 19, 17–18. [Google Scholar] [CrossRef]
- Murphy, S.T.; Zou, C.; Miers, J.B.; Ballew, R.M.; Dlott, D.D.; Schuster, G.B. Tetraarylborates {[Ar]4B-}: Estimation of oxidation potentials and reorganization energies from electron-transfer rates. J. Phys. Chem. 1993, 97, 13152–13157. [Google Scholar] [CrossRef]
- Ermoshkin, A.A.; Nikolaeva, E.S.; Neckers, D.C.; Fedorov, A.V. New tetraalkylborate initiators for remote polymerization. Macromolecules 2008, 41, 9063–9066. [Google Scholar] [CrossRef]
- Polykarpov, A.Y.; Hassoon, S.; Neckers, D.C. Tetramethylammonium tetraorganylborates as coinitiators with 5,7-diiodo-3-butoxy-6-fluorone in visible light polymerization of acrylates. Macromolecules 1996, 29, 8274–8276. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Neumann, M.G.; Pastre, I.A.; Previtali, C.M. Comparison of photoinduced electron transfer to singlet and triplet states of safranine T. J. Photochem. Photobiol. A 1991, 61, 91–98. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.A.; Rabek, J.F. Camphorquinone–amines photo-initating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Dossot, M.; Allonas, X.; Jacques, P. Unexpected correlation between electronic coupling and excited state redox properties in PET. Phys. Chem. Chem. Phys. 2002, 4, 2989–2993. [Google Scholar] [CrossRef]
- Lambert, C.R.; Kochevar, I.E. Electron transfer quenching of the Rose Bengal triplet state. Photochem. Photobiol. 1997, 66, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Allonas, X.; Jacques, P. Factors affecting adiabaticity in bimolecular photoinduced electron transfer reaction between anthracene derivatives and organic donors. Chem. Phys. 1997, 215, 371–378. [Google Scholar] [CrossRef]
- Berneth, H.; Bruder, F.K.; Fäcke, T.; Hagen, R.; Hönel, H.; Jurbergs, D.; Rölle, T.; Weiser, M.S. Holographic recording aspects of high-resolution Bayfol® HX photopolymer. Proc. SPIE 2011, 7957, 79570H. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Bennet, F.; Schneider-Baumann, M.; Voll, D.; Rölle, T.; Fäcke, T.; Weiser, M.S.; Bruder, F.K.; Junkers, T. Detailed investigation of the propagation rate of urethane acrylates. Polym. Chem. 2010, 1, 470–479. [Google Scholar] [CrossRef]
- Weiser, M.S.; Rölle, T.; Bruder, F.K.; Fäcke, T.; Hönel, D. Method for Producing a Holographic Film. U.S. Patent #14/284,488, 3 November 2009. [Google Scholar]
- Weiser, M.S.; Rölle, T.; Bruder, F.K.; Fäcke, T.; Hönel, D. Photopolymer Formulations Having the Adjustable Mechanical Modulus GUV. U.S. Patent #14/543,963, 3 November 2009. [Google Scholar]
- Bruder, F.K.; Fäcke, T.; Grote, F.; Hagen, R.; Hönel, D.; Koch, E.; Rewitz, C.; Walze, G.; Wewer, B. Mass production of volume holographic optical elements (vHOEs) using Bayfol® HX photopolymer film in a roll-to-roll copy process. Proc. SPIE 2017, 10127, 101270A. [Google Scholar] [CrossRef]
- Bruder, F.K.; Fäcke, T.; Grote, F.; Hagen, R.; Hönel, D.; Koch, E.; Rewitz, C.; Walze, G.; Wewer, B. Performance optimization in mass production of volume holographic optical elements (vHOEs) using Bayfol® HX photopolymer film. Proc. SPIE 2017, 10233, 102330G. [Google Scholar] [CrossRef]
- Bruder, F.K.; Fäcke, T.; Hagen, R.; Hönel, D.; Kleinschmidt, T.P.; Orselli, E.; Rewitz, C.; Rölle, T.; Walze, G. Diffractive optics in large sizes: Computer-generated holograms (CGH) based on Bayfol® HX photopolymer. Proc. SPIE 2015, 93850, 93850C. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Koyama, T.; Endoh, H.; Ohyama, N.; Takahashi, S.; Iwata, F. Development of a prototype full-parallax holoprinter. Proc. SPIE 1995, 2406, 50. [Google Scholar] [CrossRef]
- Shirakura, A.; Kihara, N.; Baba, S. Instant holographic portrait printing system. Proc. SPIE 1998, 3293, 246. [Google Scholar] [CrossRef]
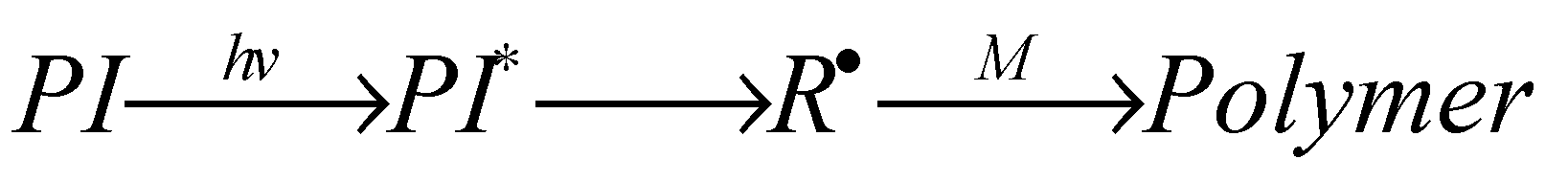
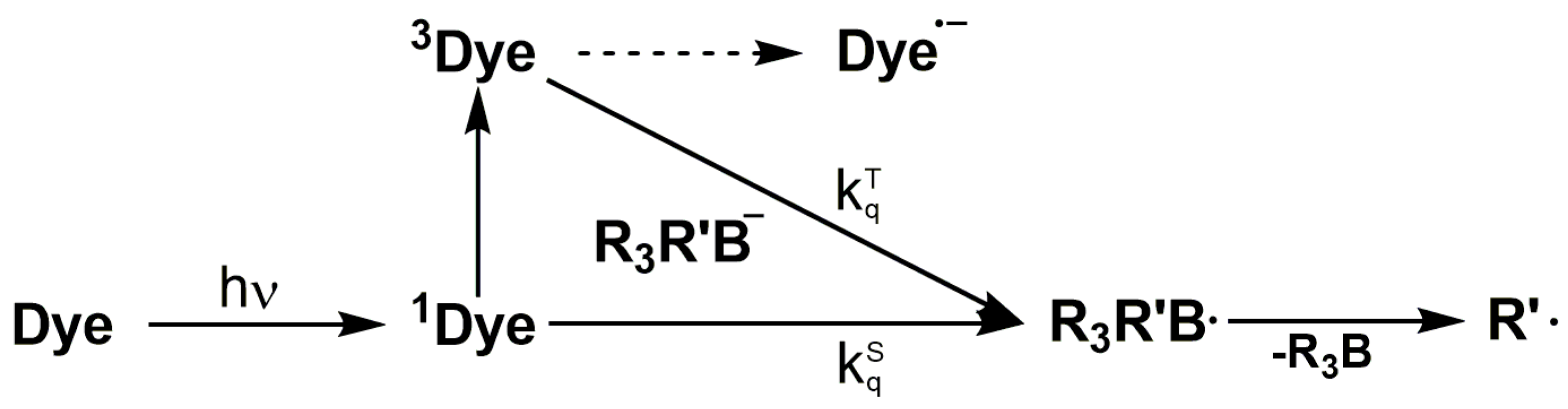

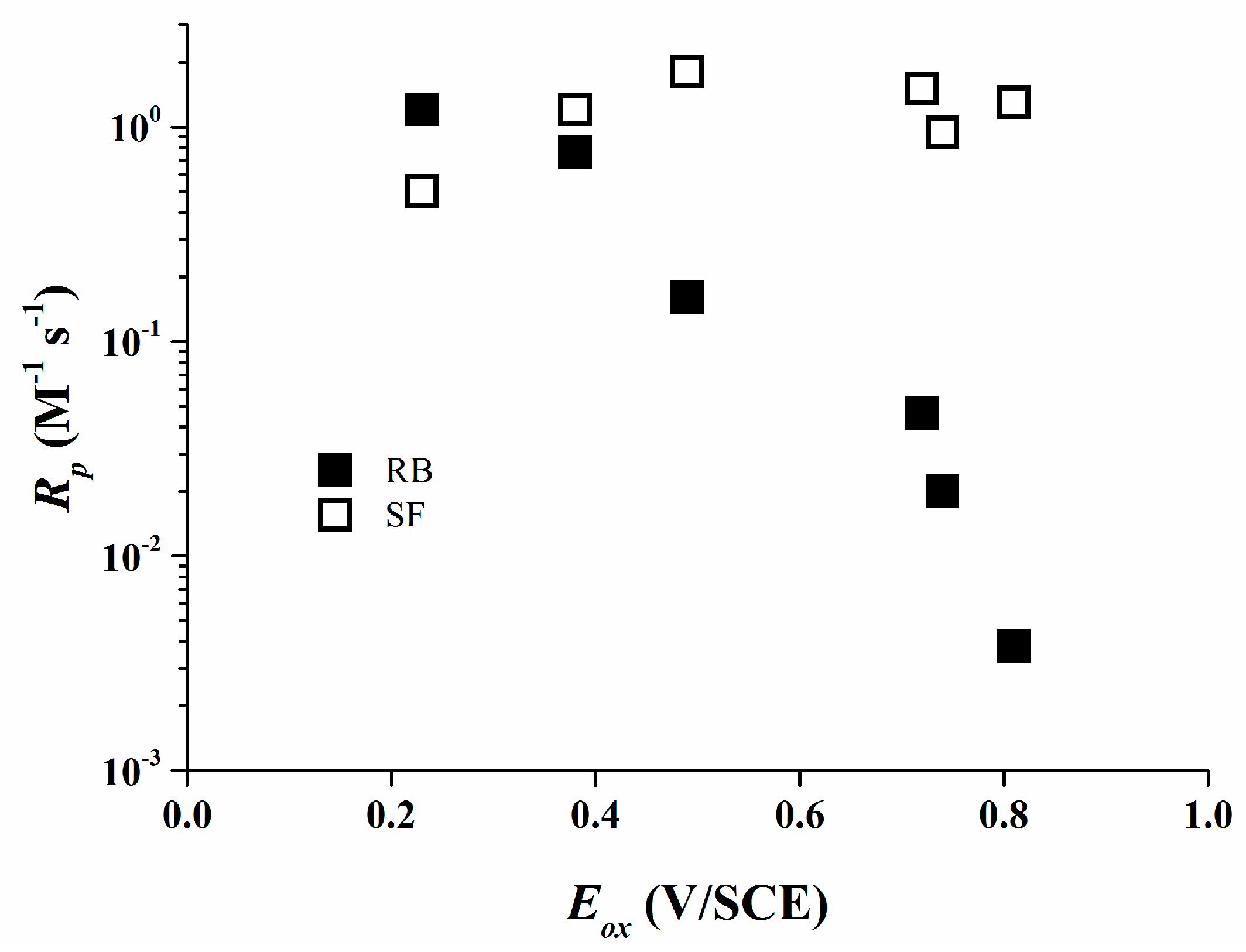
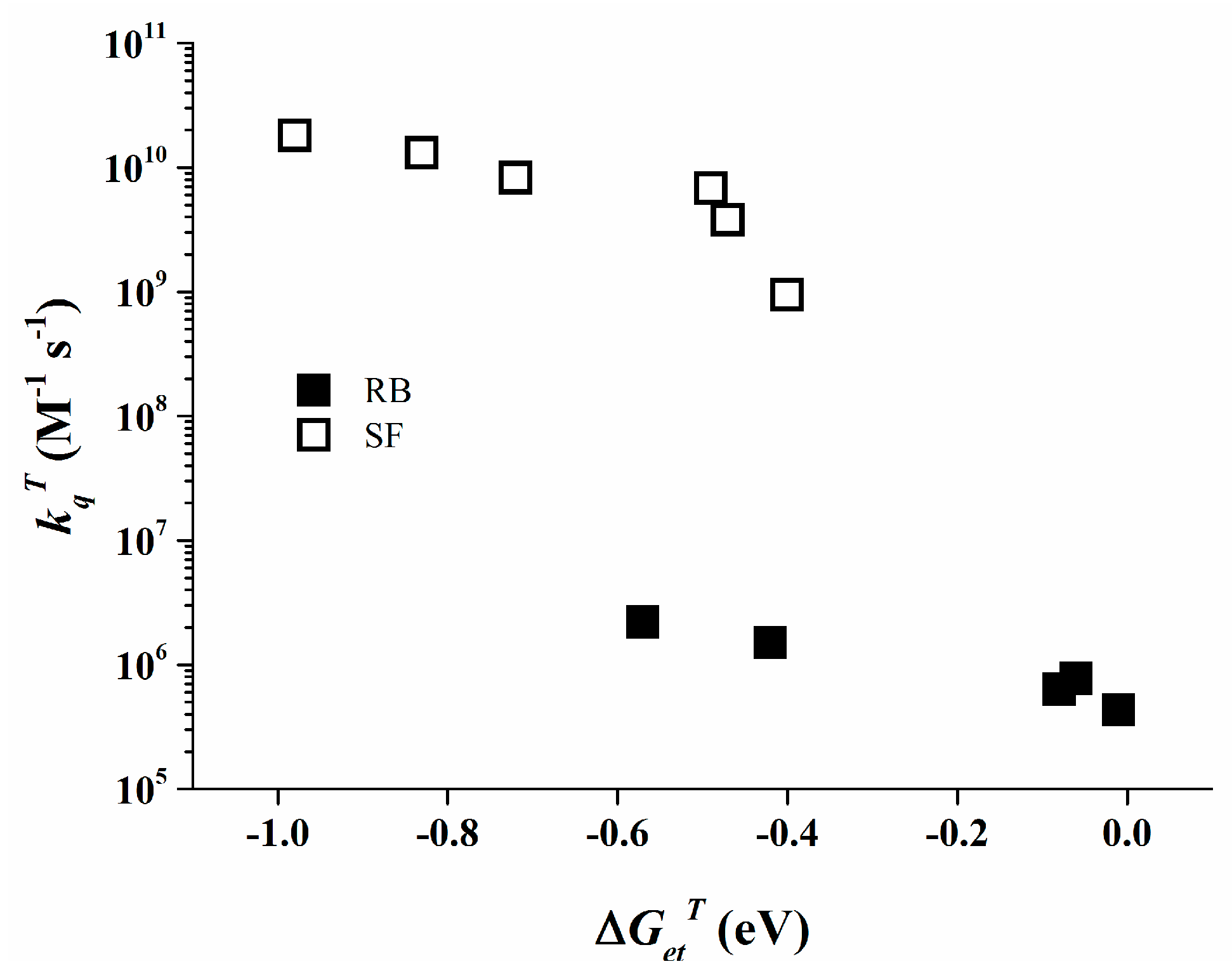
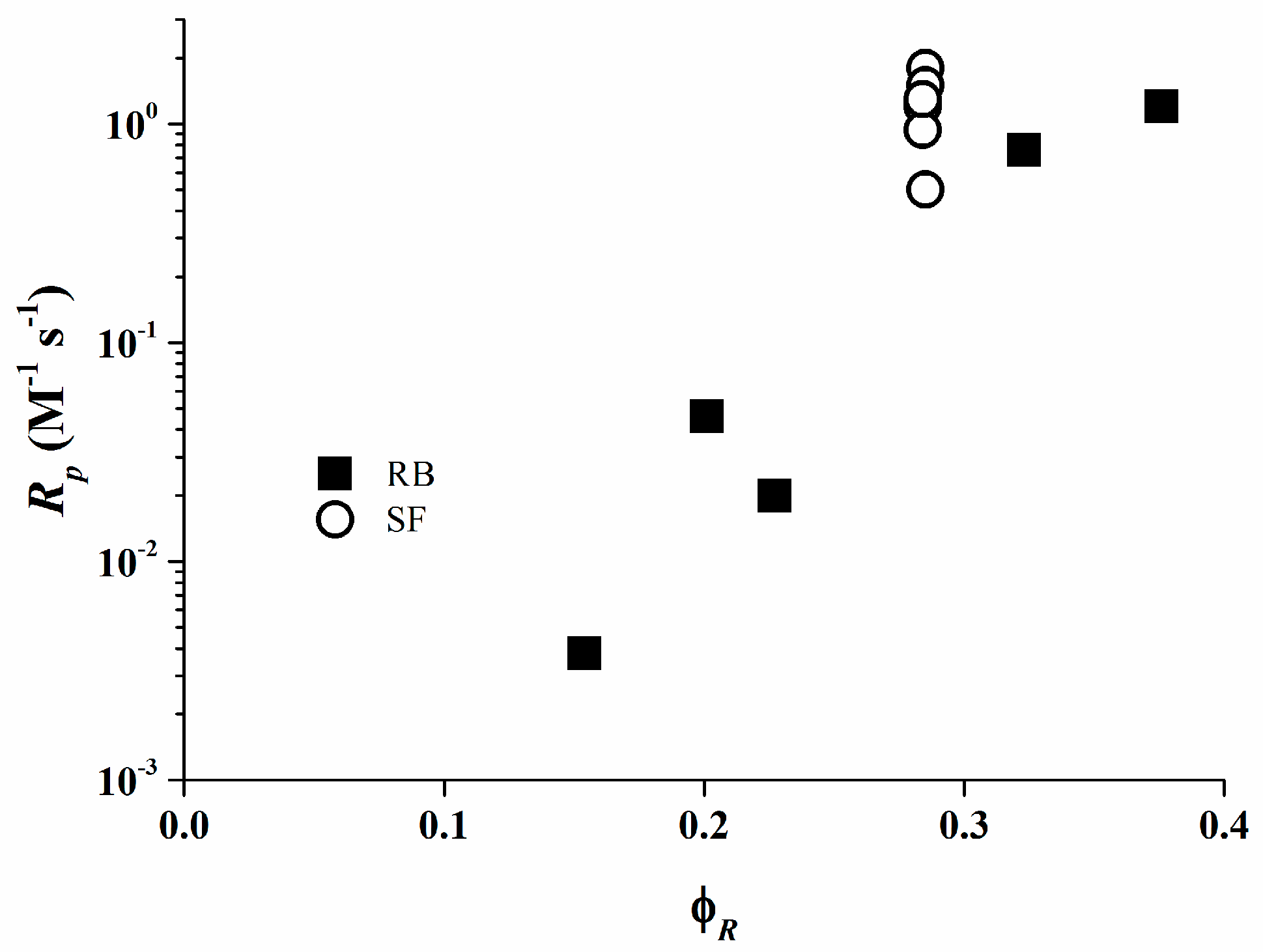
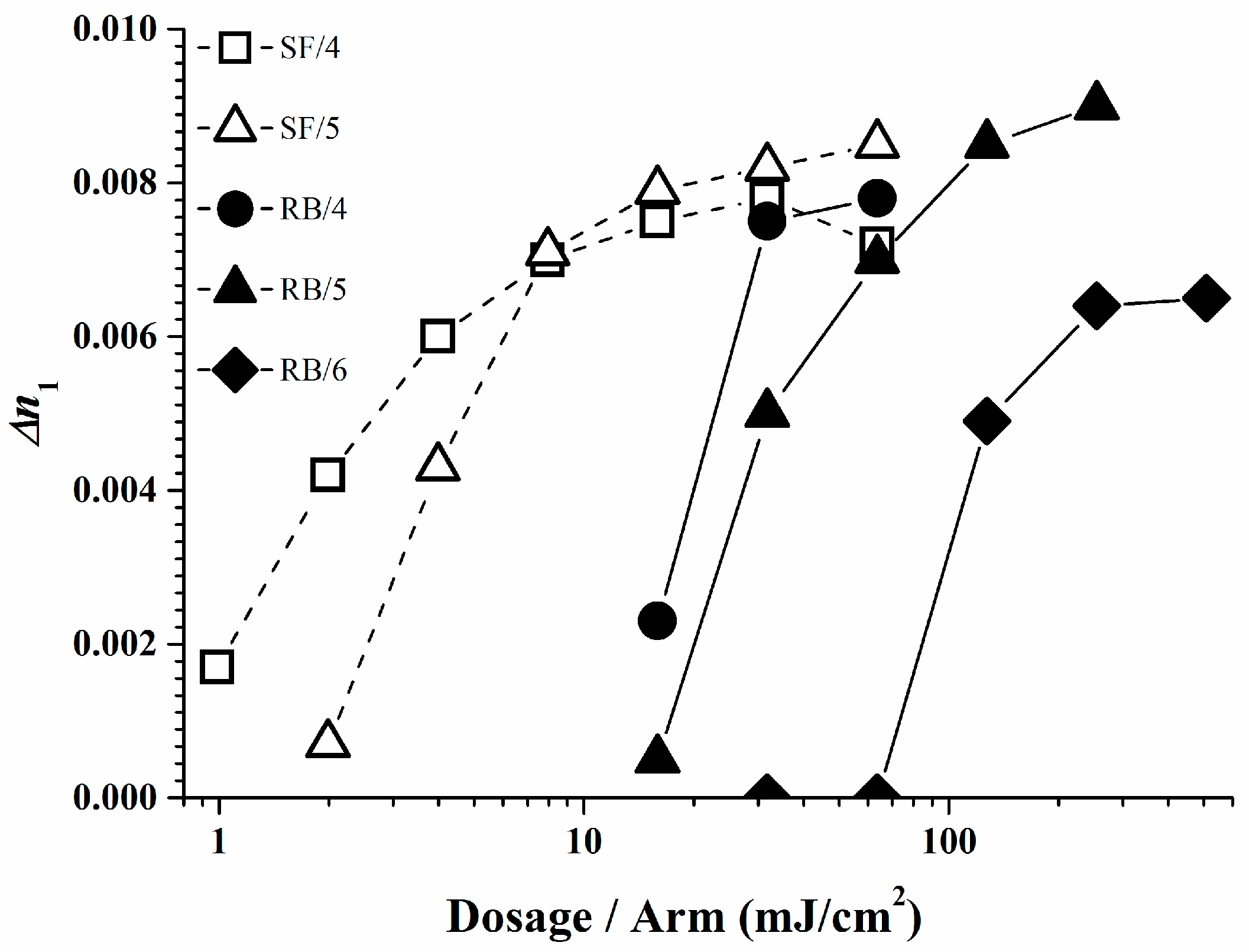
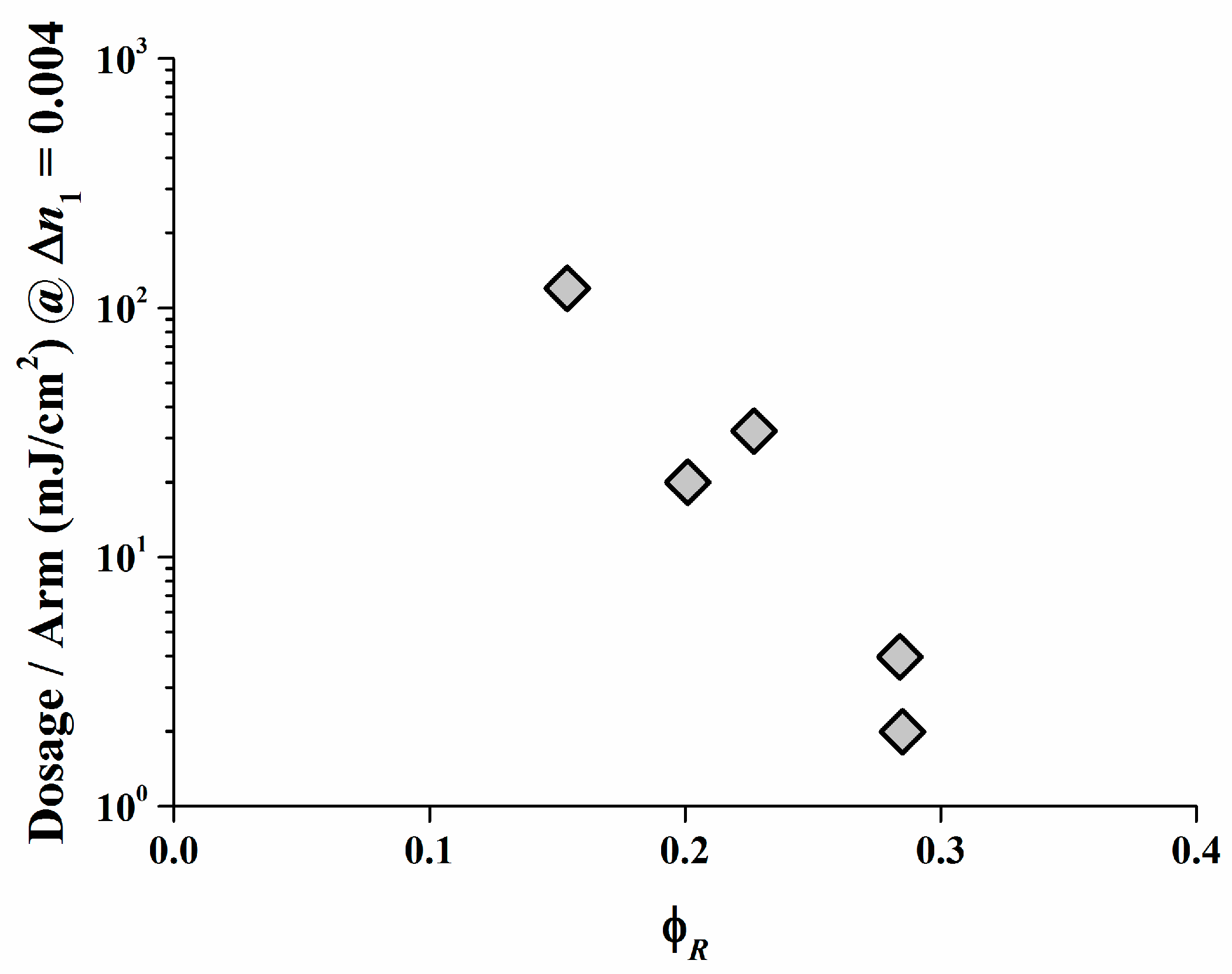
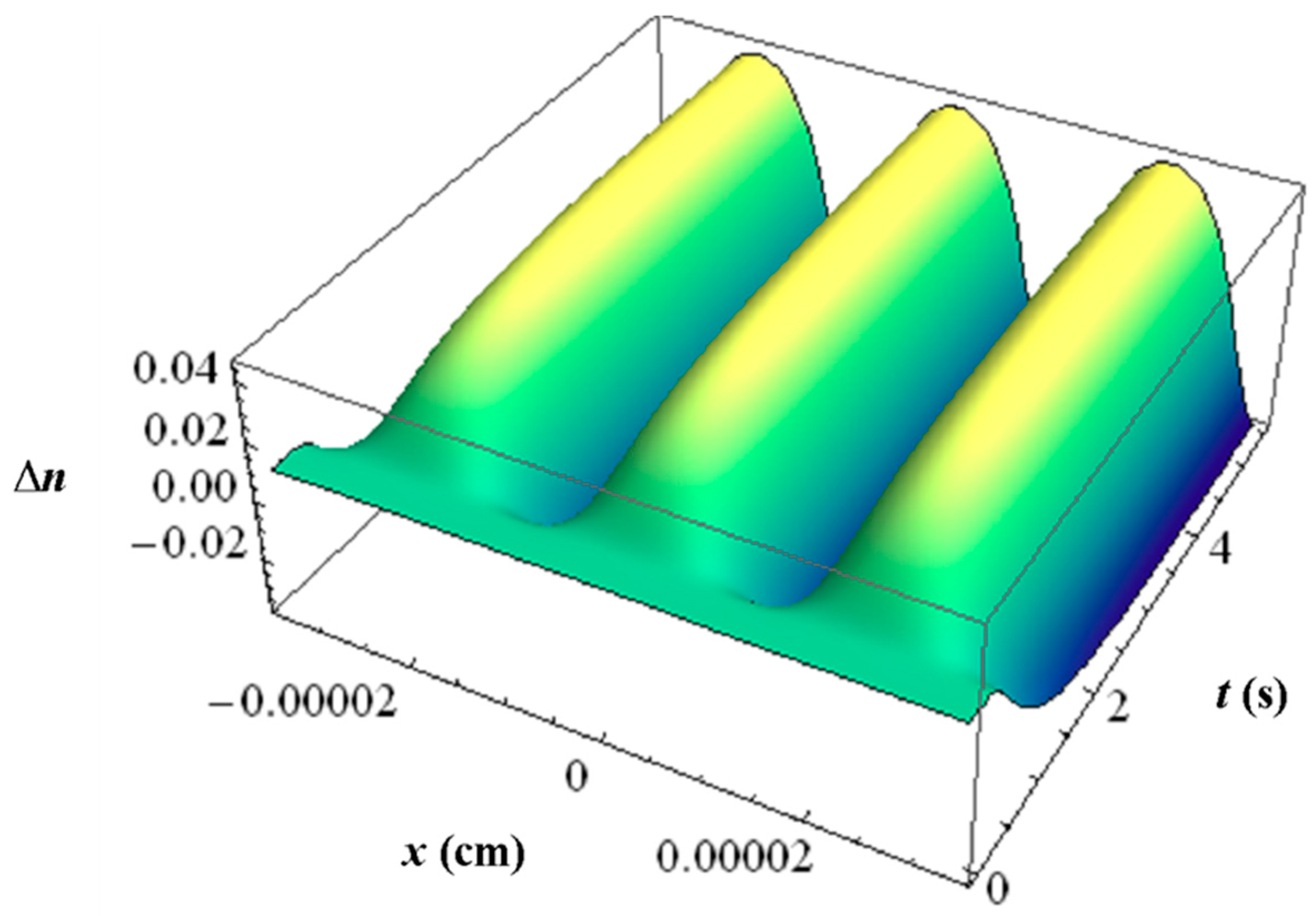
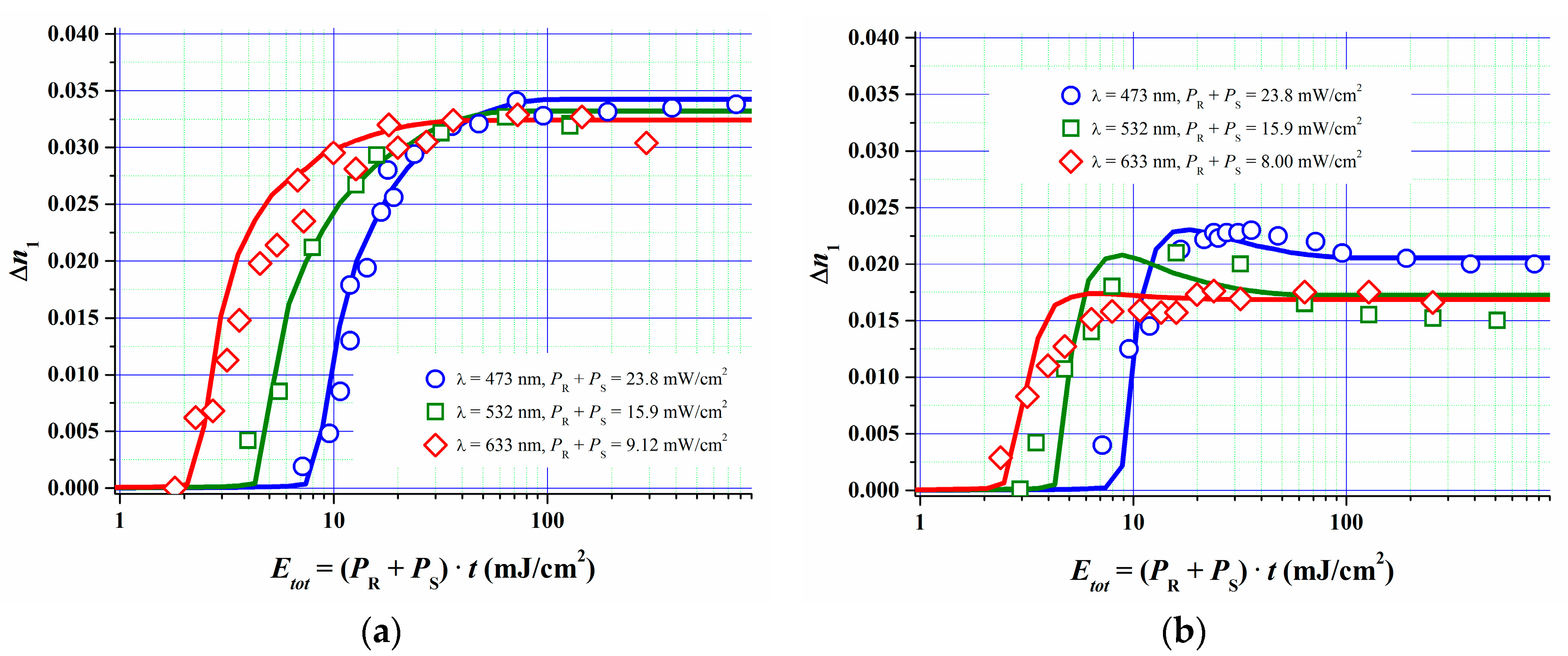




| Borate | Eox (V/SCE) | RB Rp (M−1∙s−1) | C (%) | SF Rp (M−1∙s−1) | C (%) |
|---|---|---|---|---|---|
| 1 | 0.23 | 1.20 | 80 | 0.50 | 61 |
| 2 | 0.38 | 0.76 | 77 | 1.2 | 67 |
| 3 | 0.49 | 0.16 | 72 | 1.8 | 74 |
| 4 | 0.72 | 0.046 | 63 | 1.5 | 72 |
| 5 | 0.74 | 0.020 | 49 | 0.94 | 70 |
| 6 | 0.81 | 0.0038 | 15 | 1.3 | 71 |
| Borate | kqS/∆GetS 1RB | kqT/∆GetT 3RB | kqS/∆GetS 1SF | kqT/∆GetT 3SF |
|---|---|---|---|---|
| 1 | 1.8 × 109/−0.94 | 2.2 × 106/−0.57 | 1.8 × 1010/−1.56 | 1.8 × 1010/−0.98 |
| 2 | 1.6 × 109/−0.79 | 1.5 × 106/−0.42 | 1.8 × 1010/−1.41 | 1.3 × 1010/−0.83 |
| 3 | a/−0.68 | a/−0.31 | 1.2 × 1010/−1.30 | 8.2 × 109/−0.72 |
| 4 | 8.0 × 108/−0.45 | 6.3 × 105/−0.08 | 2.0 × 1010/−1.07 | 6.8 × 109/−0.49 |
| 5 | 7.3 × 108/−0.43 | 7.7 × 105/−0.06 | 1.4 × 1010/−1.05 | 3.8 × 109/−0.47 |
| 6 | 4.8 × 108/−0.36 | 4.3 × 105/−0.01 | 1.6 × 1010/−0.98 | 9.4 × 108/−0.40 |
| Borate | RB φR | SF φR |
|---|---|---|
| 1 | 0.376 | 0.285 |
| 2 | 0.323 | 0.284 |
| 3 | - | 0.285 |
| 4 | 0.201 (0.0078) | 0.285 (0.0078) |
| 5 | 0.227 (0.0090) | 0.284 (0.0085) |
| 6 | 0.154 (0.0065) | 0.284 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruder, F.-K.; Fäcke, T.; Rölle, T. The Chemistry and Physics of Bayfol® HX Film Holographic Photopolymer. Polymers 2017, 9, 472. https://doi.org/10.3390/polym9100472
Bruder F-K, Fäcke T, Rölle T. The Chemistry and Physics of Bayfol® HX Film Holographic Photopolymer. Polymers. 2017; 9(10):472. https://doi.org/10.3390/polym9100472
Chicago/Turabian StyleBruder, Friedrich-Karl, Thomas Fäcke, and Thomas Rölle. 2017. "The Chemistry and Physics of Bayfol® HX Film Holographic Photopolymer" Polymers 9, no. 10: 472. https://doi.org/10.3390/polym9100472