Imidazole, a New Tunable Reagent for Producing Nanocellulose, Part I: Xylan-Coated CNCs and CNFs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
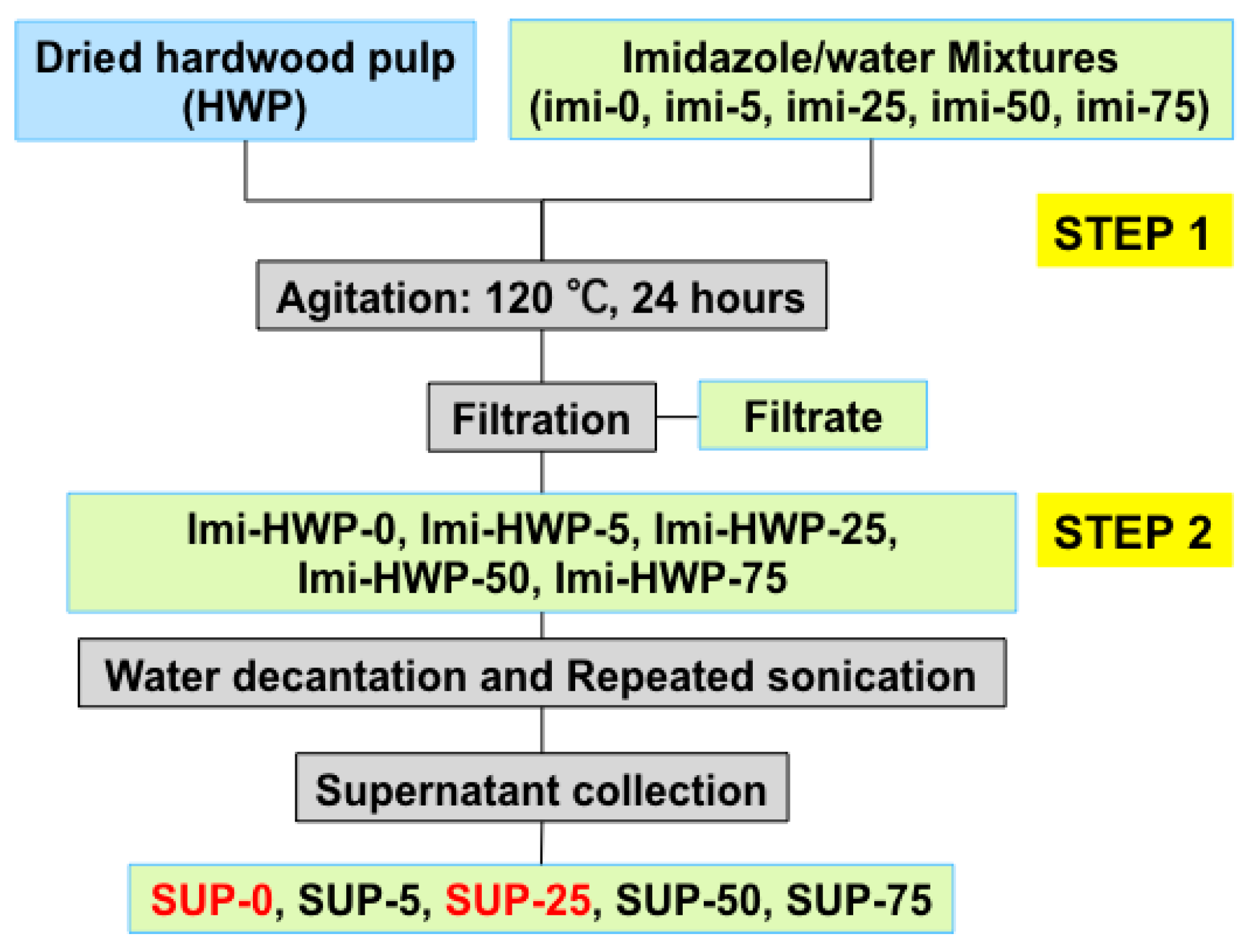
2.2. Treatment of Hardwood Pulp in Imidazole/Water Mixture
2.3. Characterization of the Original Pulp (HWP), Treated Pulps (Imi-HWP) and Collected Supernatants (SUP)
2.3.1. HWP and imi-HWP Characterization
2.3.2. SUP Characterization
3. Results and Discussion
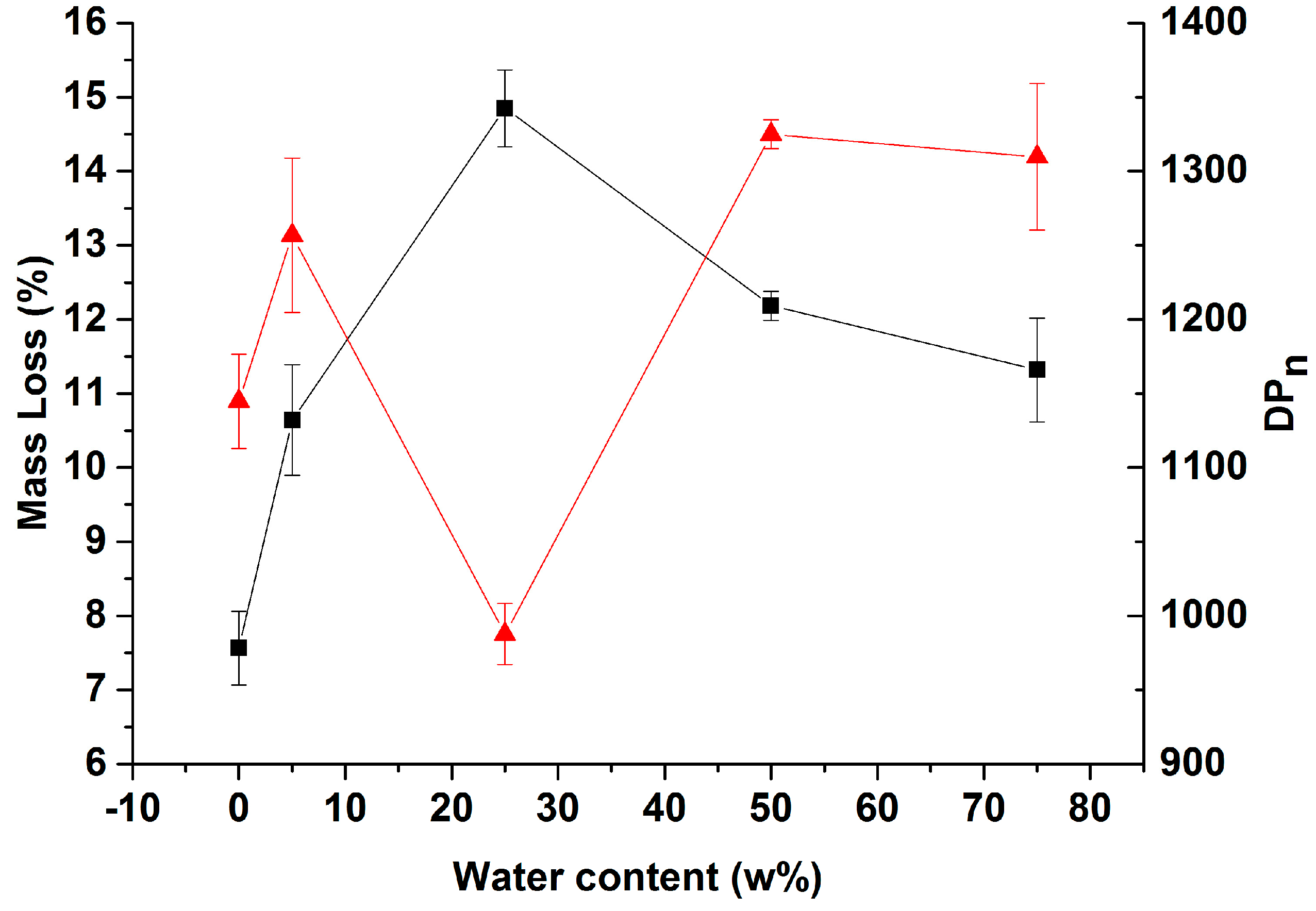
3.1. Process Characterization
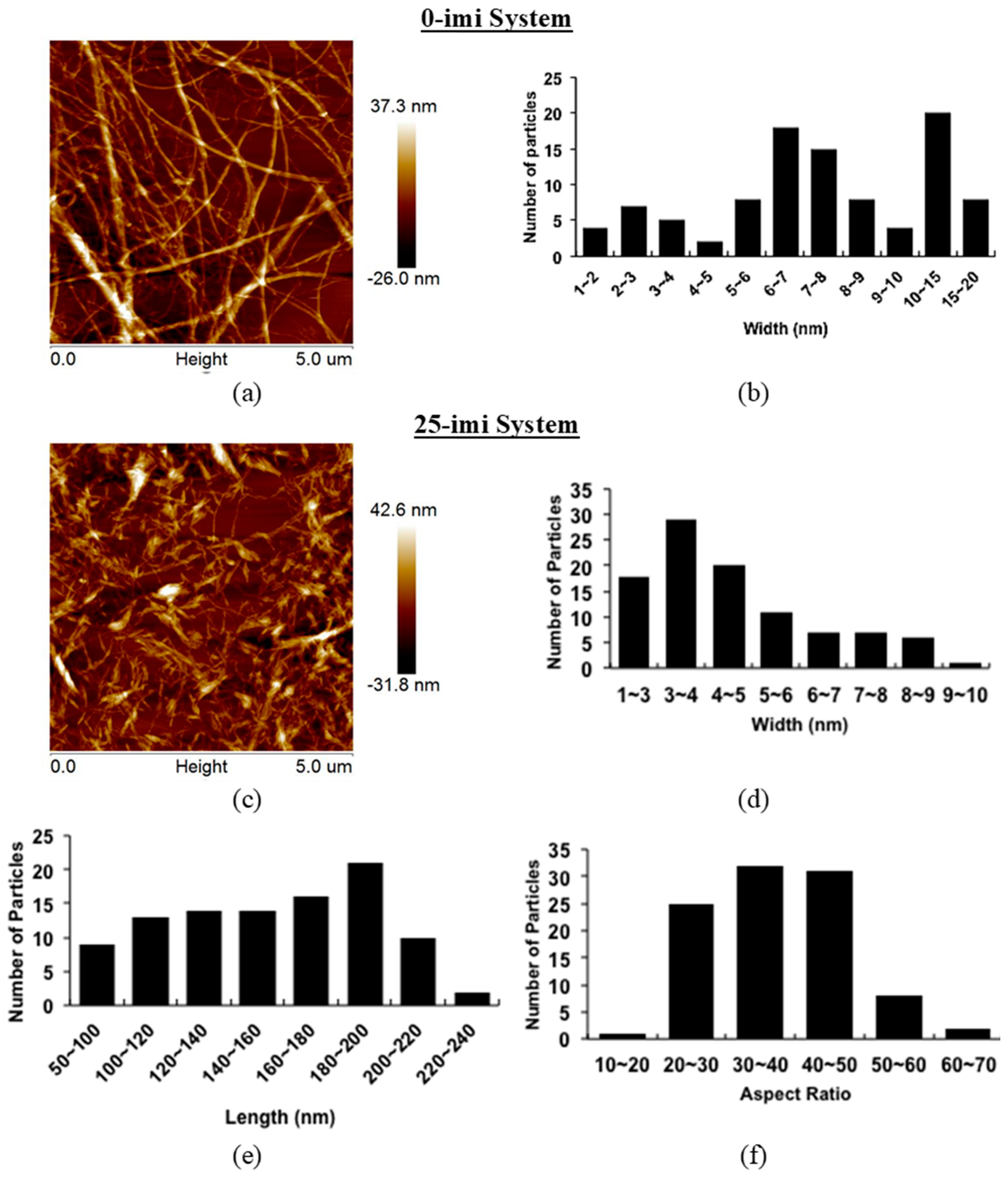
3.2. Product Morphological Characterization
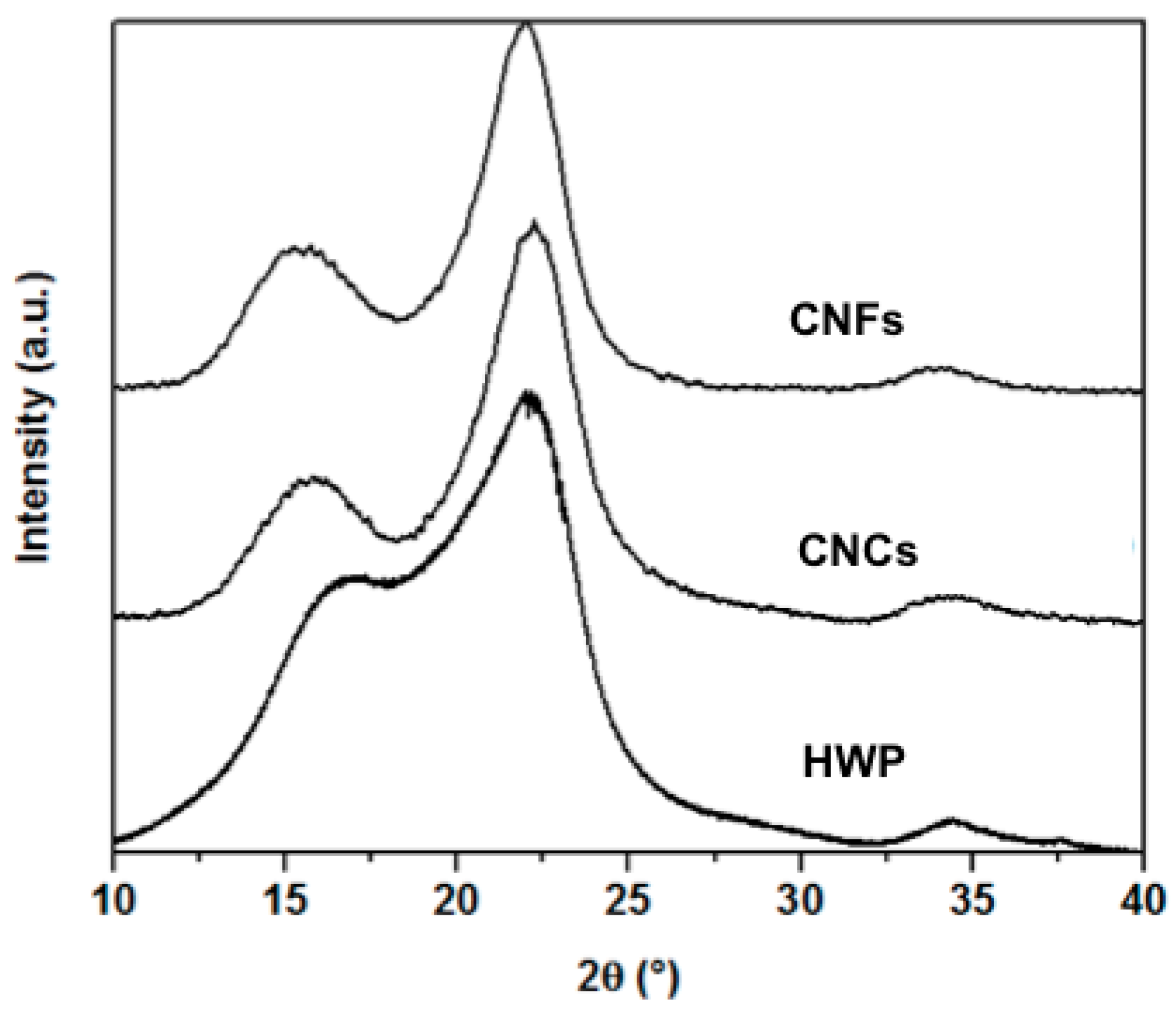
3.3. Product Composition and Surface Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–813. [Google Scholar]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surf. A 2006, 289, 219–225. [Google Scholar] [CrossRef]
- Rånby, B.G. Aqueous colloidal solutions of cellulose micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Roman, M.; Renneckar, S.; Gindl, W.; Veigel, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Mao, J.; Abushammala, H.; Brown, N.; Laborie, M.-P. (Eds.) Nano-Celluloses, Their Preparation, Properties, and Applications: Comparative Assesment of Methods for Producing Cellulose I Nanocrystals from Cellulosic Sources; American Chemical Society: Washington, DC, USA, 2017. [Google Scholar]
- Tang, Y.; Yang, S.; Zhang, N.; Zhang, J. Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose 2014, 21, 335–346. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, X.; Chen, M. Cellulose nanowhiskers isolation and properties from acid hydrolysis combined with high pressure homogenization. BioResources 2013, 8, 933–943. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Chen, X.; Deng, X.; Shen, W.; Jiang, L. Controlled enzymolysis preparation of nanocrystalline cellulose from pretreated cotton fibers. BioResources 2012, 7, 4237–4248. [Google Scholar]
- Lazko, J.; Sénéchal, T.; Landercy, N.; Dangreau, L.; Raquez, J.M.; Dubois, P. Well defined thermostable cellulose nanocrystals via two-step ionic liquid swelling-hydrolysis extraction. Cellulose 2014, 21, 4195–4207. [Google Scholar] [CrossRef]
- Mao, J.; Abushammala, H.; Pereira, L.B.; Laborie, M.-P. Swelling and hydrolysis kinetics of Kraft pulp fibers in aqueous 1-butyl-3-methylimidazolium hydrogen sulfate solutions. Carbohydr. Polym. 2016, 153, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Muhammad, N.; Sarwono, A.; Bustam, M.A.; Kumar, M.V.; Rafiq, S. Preparation of cellulose nanocrystals using an ionic liquid. J. Polym. Environ. 2011, 19, 726–731. [Google Scholar] [CrossRef]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Mao, J.; Heck, B.; Reiter, G.; Laborie, M.-P. Cellulose nanocrystals’ production in near theoretical yields by 1-butyl-3-methylimidazolium hydrogen sulfate ([Bmim]HSO4)—Mediated hydrolysis. Carbohydr. Polym. 2015, 117, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zweckmair, T.; Hettegger, H.; Abushammala, H.; Bacher, M.; Potthast, A.; Laborie, M.P.; Rosenau, T. On the mechanism of the unwanted acetylation of polysaccharides by 1,3-dialkylimidazolium acetate ionic liquids: Part 1—Analysis, acetylating agent, influence of water, and mechanistic considerations. Cellulose 2015, 22, 3583–3596. [Google Scholar] [CrossRef]
- Liebner, F.; Patel, I.; Ebner, G.; Becker, E.; Horix, M.; Potthast, A.; Rosenau, T. Thermal aging of 1-alkyl-3-methylimidazolium ionic liquids and its effect on dissolved cellulose. Holzforschung 2010, 64. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Peral, F.; Gallego, E. Self-association of imidazole and its methyl derivatives in aqueous solution. A study by ultraviolet spectroscopy. J. Mol. Struct. 1997, 415, 187–196. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect solvent for lignocellulosic biomass fractionation and delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Chen, L.; Sharifzadeh, M.; Mac Dowell, N.; Welton, T.; Shah, N.; Hallett, J.P. Inexpensive ionic liquids: [HSO4]‒-based solvent production at bulk scale. Green Chem. 2014, 16, 3098–3106. [Google Scholar] [CrossRef]
- Shalini, K.; Sharma, P.K.; Kumar, N. Imidazole and its biological activities: A review. Pelagia Res. Libr. 2010, 1, 36–47. [Google Scholar]
- Toscan, A.; Morais, A.R.C.; Paixão, S.M.; Alves, L.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. Effective extraction of lignin from elephant grass using imidazole and its effect on enzymatic saccharification to produce fermentable sugars. Ind. Eng. Chem. Res. 2017, 56, 5138–5145. [Google Scholar] [CrossRef]
- Bohrn, R.; Potthast, A.; Schiehser, S.; Rosenau, T.; Sixta, H.; Kosma, P. The FDAM method: Determination of carboxyl profiles in cellulosic materials by combining group-selective fluorescence labeling with GPC. Biomacromolecules 2006, 7, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Röhrling, J.; Potthast, A.; Rosenau, T.; Lange, T.; Borgards, A.; Sixta, H.; Kosma, P. A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 2. Validation and applications. Biomacromolecules 2002, 3, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Röhrling, J.; Potthast, A.; Rosenau, T.; Lange, T.; Ebner, G.; Sixta, H.; Kosma, P. A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 1. Method development. Biomacromolecules 2002, 3, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Potthast, A.; Röhrling, J.; Rosenau, T.; Borgards, A.; Sixta, H.; Kosma, P. A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 3. Monitoring oxidative processes. Biomacromolecules 2003, 4, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Potthast, A.; Röhrling, J.; Rosenau, T.; Borgards, A.; Sixta, H.; Kosma, P. Comparison testing of methods for gel permeation chromatography of cellulose: Coming closer to a standard protocol. Cellulose 2015, 22, 1591–1613. [Google Scholar] [CrossRef]
- Sundberg, A.; Sundberg, K.; Lillandt, C.; Holmbom, B. Determination of hemicelluloses and pectins in wood and pulp fibres by acid methanolysis and gas chromatography. Nord. Pulp Pap. Res. J. 1996, 11, 216–219. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the Interpretation of X-ray Diffraction Powder Patterns in Terms of the Nanostructure of Cellulose I Fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between length and degree of polymerization of TEMPO-oxidized cellulose nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Pulp and Paper Chemistry and Technology: Wood Chemistry and Wood Biotechnology; DE Gruyter: Berlin, Germany, 2009. [Google Scholar]
- Tanaka, R.; Saito, T.; Hanninen, T.; Ono, Y.; Hakalahti, M.; Tammelin, T.; Isogai, A. Viscoelastic properties of core-shell-structured, hemicellulose-rich nanofibrillated cellulose in dispersion and wet-film states. Biomacromolecules 2016, 17, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Pääkkönen, T.; Dimic-Misic, K.; Orelma, H.; Pönni, R.; Vuorinen, T.; Maloney, T. Effect of xylan in hardwood pulp on the reaction rate of TEMPO-mediated oxidation and the rheology of the final nanofibrillated cellulose gel. Cellulose 2016, 23, 277–293. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(ethylene glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Ebringerova, A.; Heinze, T. Xylan and xylan derivatives—Biopolymers with valuable properties, 1. Naturally occurring xylans structures, isolation procedures and properties. Macromol. Rapid Commun. 2000, 21, 543–556. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]

| Characterization | HWP | CNFs | CNCs | ||||
|---|---|---|---|---|---|---|---|
| -imi | -TEMPO [44,45,46] | -imi | -SA [47] | -IL [19] | |||
| Chemical Composition | Xylose (%) | 12.42 (2.02) | 10.22 (1.54) | n/a | 8.96 (2.45) | n/a | 6.40 (0.96) |
| Arabinose (%) | 0.11 (0.01) | 0.07 (0.01) | n/a | 0.09 (0.02) | n/a | n/a | |
| 4-OMe-GlcUA (%) | 0.85 (0.00) | 0 | n/a | 0 | n/a | n/a | |
| Mannose (%) | 0.11 (0.00) | 0 | n/a | 0 | n/a | 0.18 (0.02) | |
| Morphologies | W (nm) | 10,000~20,000 | 8 ± 4 | 3~5, 15 | 4 ± 2 | 4.8 ± 0.4 | 6 ± 2 |
| L (nm) | >10,000 | Several microns | Several microns | 156 ± 37 | 147 ± 7 | 227 ± 74 | |
| AR | ~100 | >100 | >100 | 38 ± 10 | 20~30 | 43 ± 21 | |
| Surface Properties | * Zeta potential (mV) | ‒10 | ‒26 ± 3 | ‒33 | ‒27 ± 5 | ‒50~‒60 | ‒20~‒30 |
| COOH (μmol/g) | 50.0 (1.8) | 40.3 (1.1) | 0.1 × 103~1.5 × 103 | 44.5 (0.0) | n/a | n/a | |
| C=O (μmol/g) | 12.9 (0.0) | 30.6 (0.6) | 10~100 | 32.1 (0.0) | n/a | n/a | |
| Crystallite Properties | CrI (%) | 54 | 67 | 65~95 | 73 | 70~90 | 77 ± 1 |
| Τ (nm) | 4.2 | 3.3 | 4 | 2.9 | 4~6 | 4.4 ± 0.2 | |
| B (nm) | 5 | 5 | 6 | 5 | 6~30 | 6 ± 1 | |
| Preparation Route | Imidazole, filtration, sonication | TEMPO/NaBr/ NaClO, filtration | Imidazole/H2O, filtration, sonication | H2SO4, centrifugation, dialysis | [Bmim]HSO4, centrifugation, dialysis | ||
| Yield (wt %) | 10 | ~90 | 20 | 20–30 | ~57 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Abushammala, H.; Hettegger, H.; Rosenau, T.; Laborie, M.-P. Imidazole, a New Tunable Reagent for Producing Nanocellulose, Part I: Xylan-Coated CNCs and CNFs. Polymers 2017, 9, 473. https://doi.org/10.3390/polym9100473
Mao J, Abushammala H, Hettegger H, Rosenau T, Laborie M-P. Imidazole, a New Tunable Reagent for Producing Nanocellulose, Part I: Xylan-Coated CNCs and CNFs. Polymers. 2017; 9(10):473. https://doi.org/10.3390/polym9100473
Chicago/Turabian StyleMao, Jia, Hatem Abushammala, Hubert Hettegger, Thomas Rosenau, and Marie-Pierre Laborie. 2017. "Imidazole, a New Tunable Reagent for Producing Nanocellulose, Part I: Xylan-Coated CNCs and CNFs" Polymers 9, no. 10: 473. https://doi.org/10.3390/polym9100473






