Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behavior. Part 2: Domain Formation by Self-Assembly in Lipid Bilayer Membranes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The GUV Model System
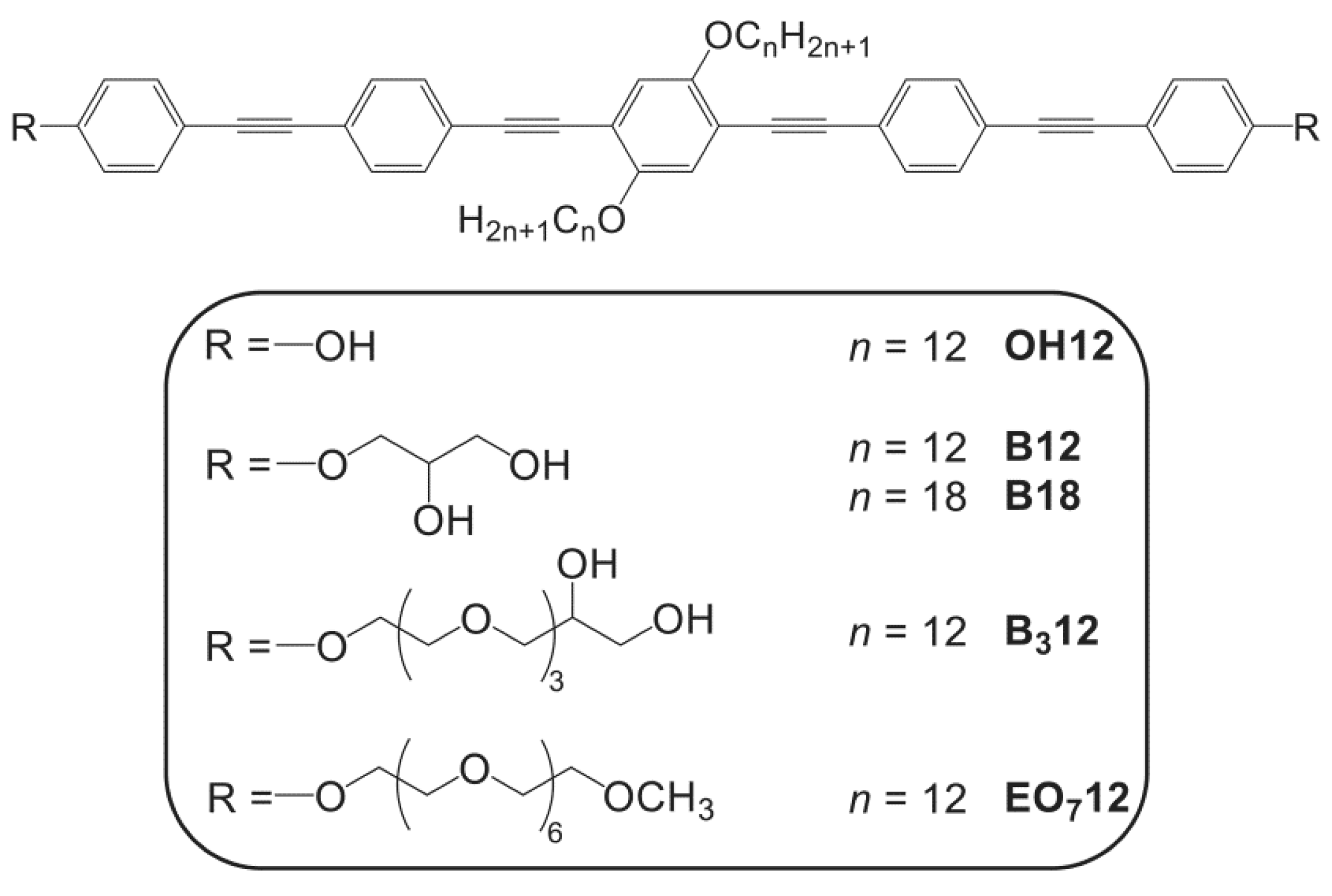
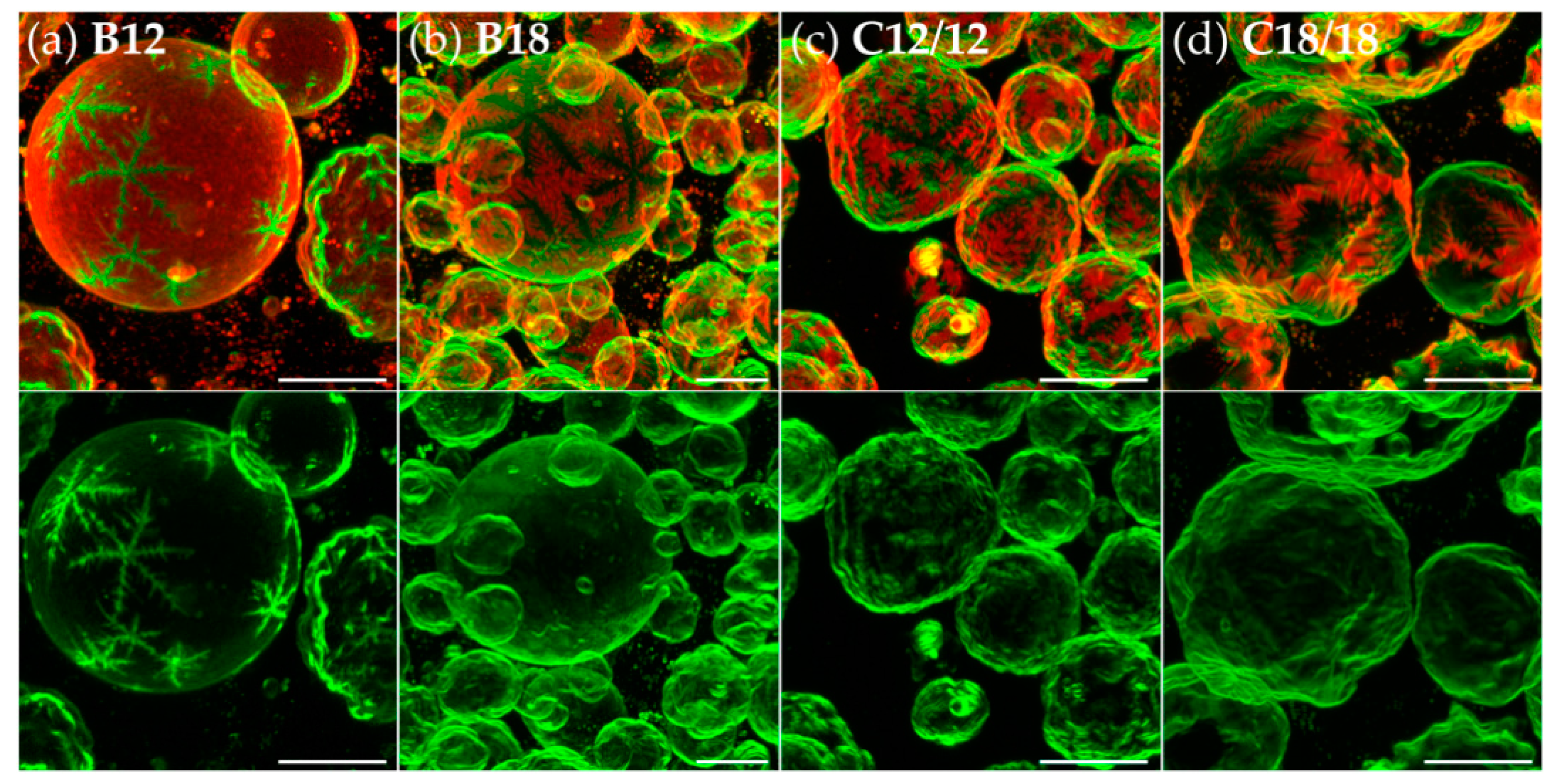
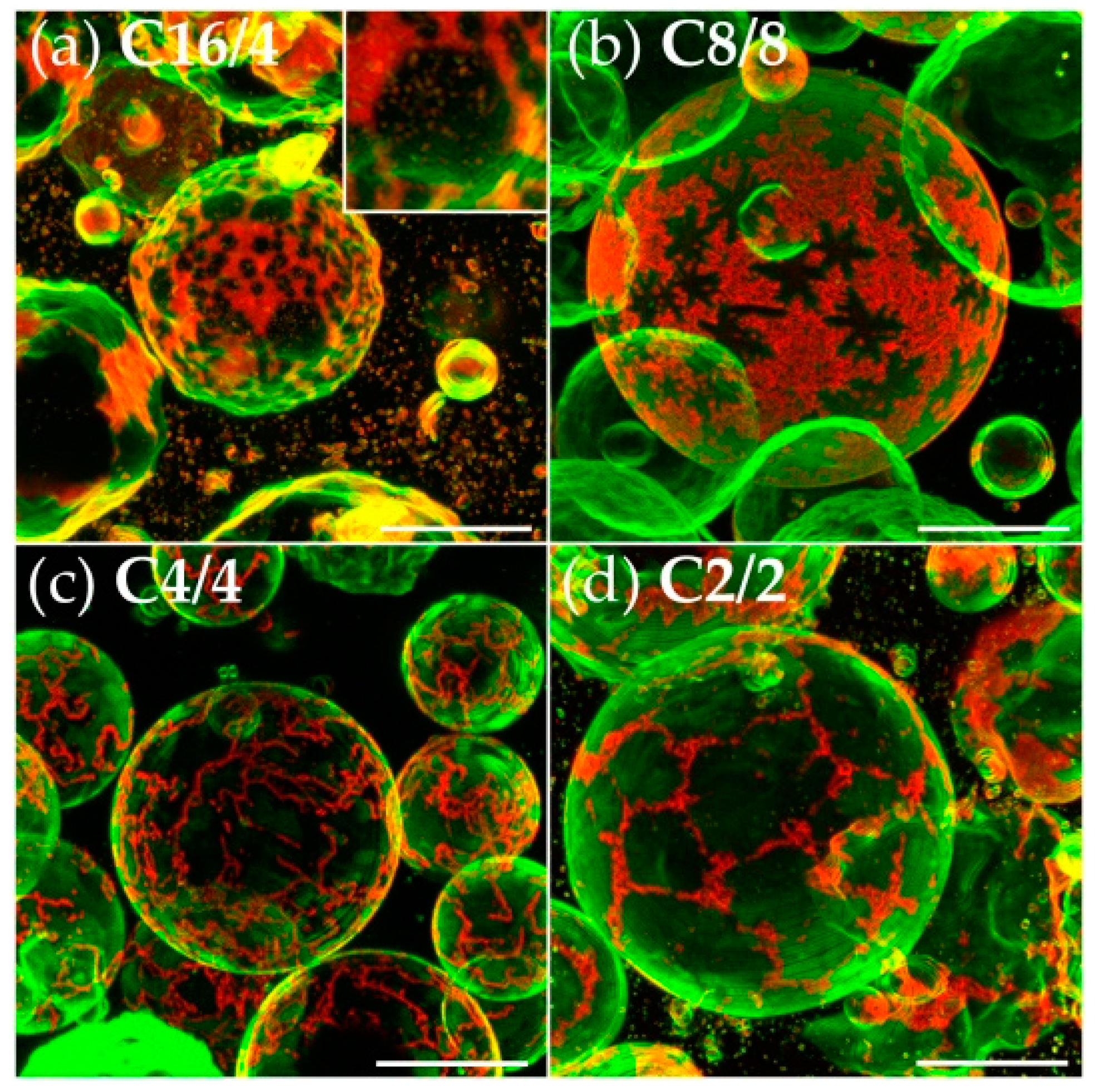
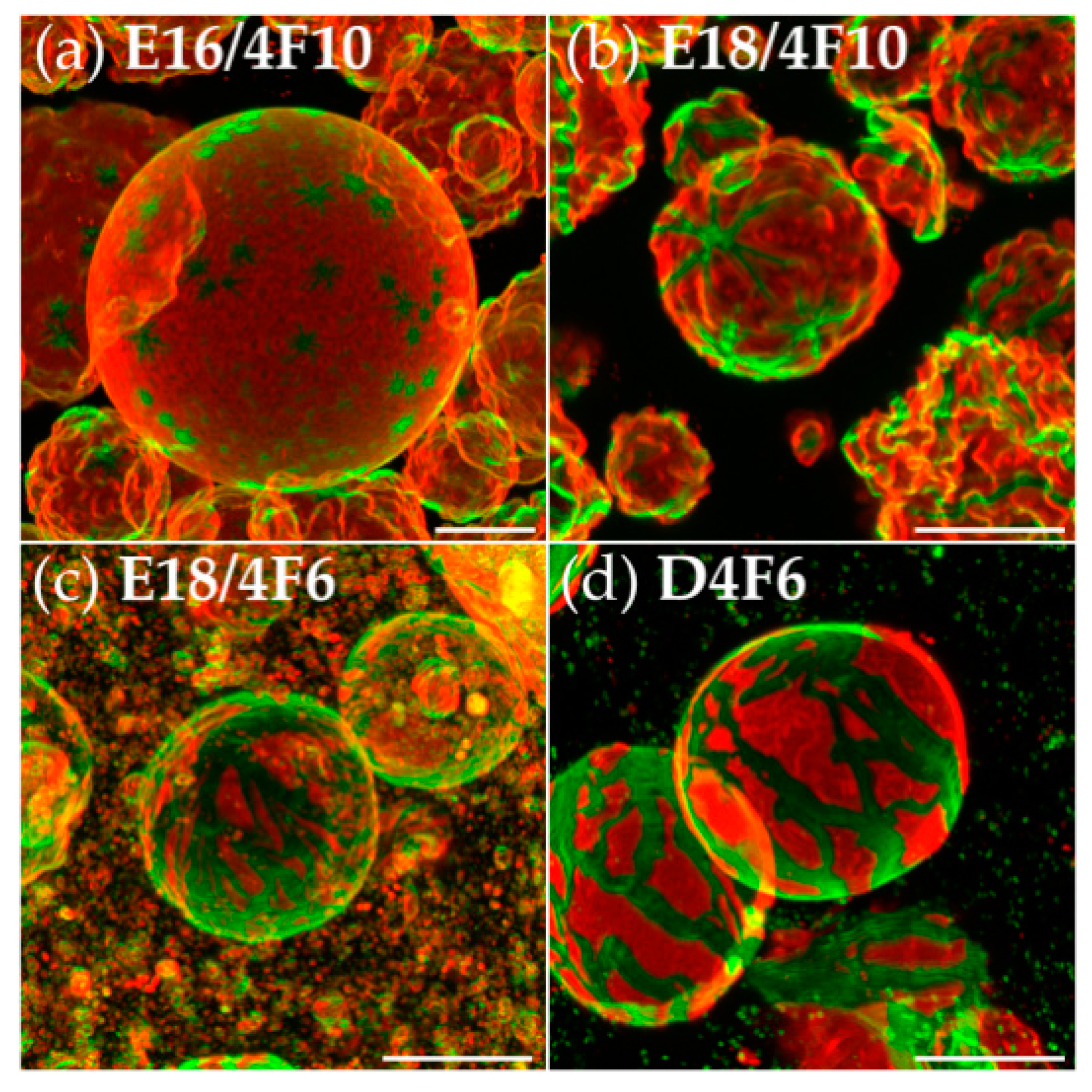
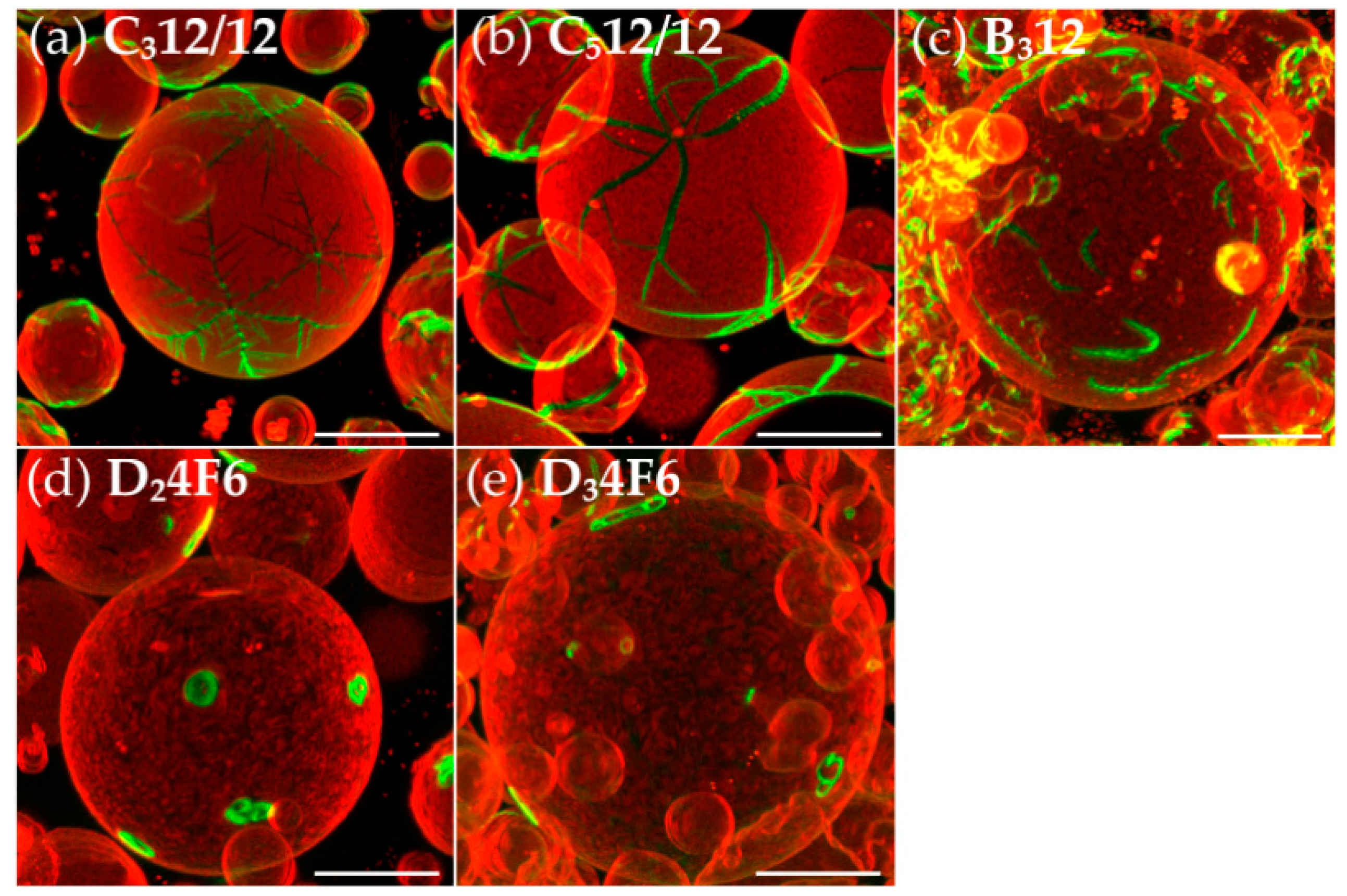
3.2. Effects of Variation of the Lipophilic Lateral Chains
3.3. Dendritic Growth under Non-Equilibrium Conditions
3.4. Semiperfluorination of the Lateral Chains
3.5. Oligo(Ethylene Oxide) Expanded Bolapolyphile Headgroups
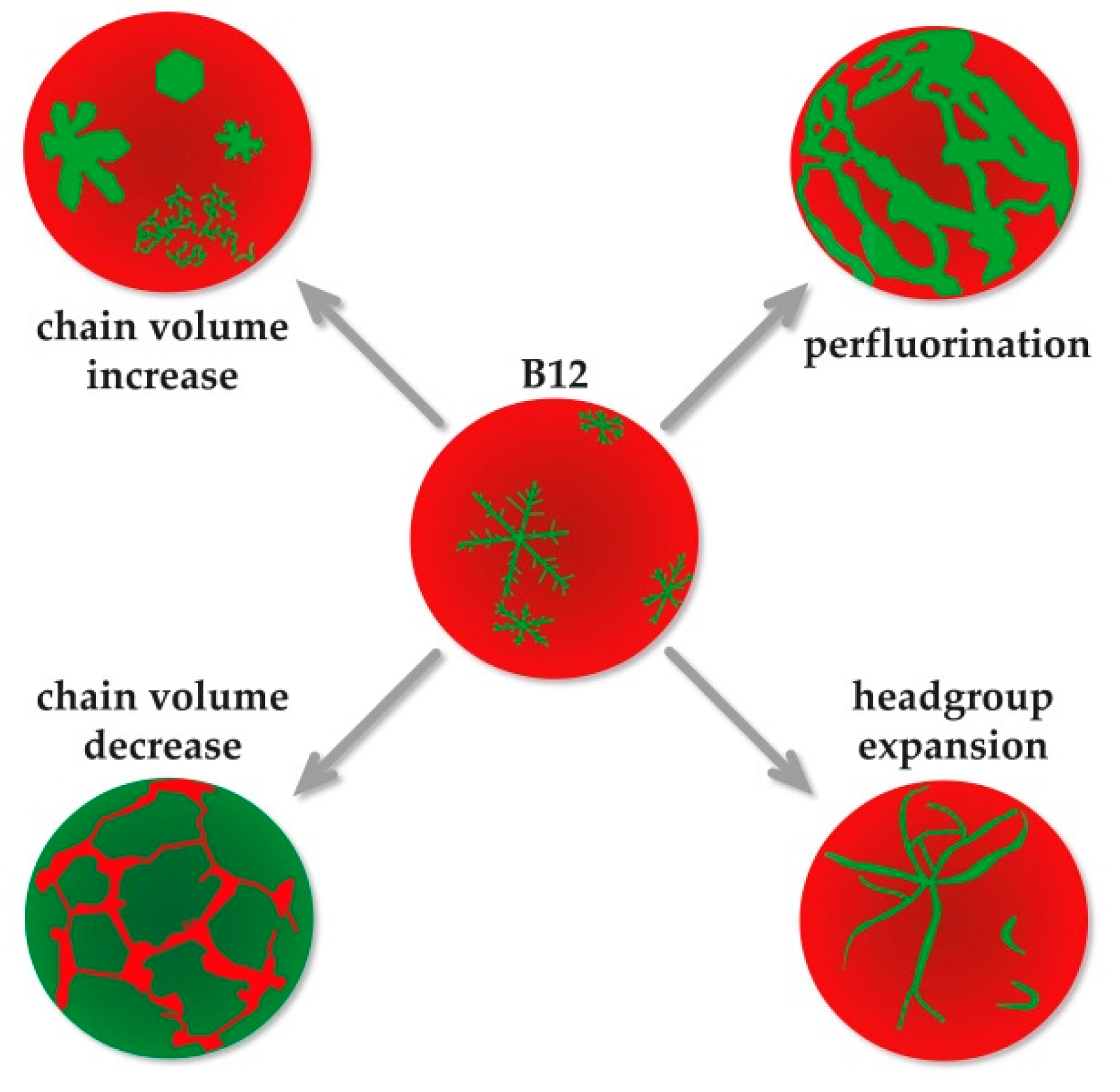
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Straub, F.B.; Feuer, G. Adenosinetriphosphate. The functional group of actin. Biochim. Biophys. Acta 1950, 4, 455–470. [Google Scholar] [CrossRef]
- Weisenberg, R.C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science 1972, 177, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Crowther, R.A.; Finch, J.T.; Pearse, B.M.F. On the structure of coated vesicles. J. Mol. Biol. 1976, 103, 785–798. [Google Scholar] [CrossRef]
- Crick, F.H.C.; Watson, J.D. Structure of small viruses. Nature 1956, 177, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Olson, A.J. Structural symmetry and protein function. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 105–153. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.; Ipsen, J.H.; Simonson, A.C.; Mouritsen, O.G. An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res. 2010, 49, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Glatte, D.; Meister, A.; Scholtysek, P.; Kerth, A.; Blume, A.; Bacia, K.; Binder, W.H. Hybrid lipid/polymer giant unilamellar vesicles: Effects of incorporated biocompatible pib–peo block copolymers on vesicle properties. Soft Matter 2011, 7, 8100–8110. [Google Scholar] [CrossRef]
- Schulz, M.; Werner, S.; Bacia, K.; Binder, W.H. Controlling molecular recognition with lipid/polymer domains in vesicle membranes. Angew. Chem. Int. Ed. 2013, 52, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Wan, C.; Tamm, L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta 2009, 1788, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.-D.; Ebert, H.; Prehm, M.; Werner, S.; Meister, A.; Hause, G.; Beerlink, A.; Saalwächter, K.; Bacia, K.; Tschierske, C.; et al. Temperature-dependent in-plane structure formation of an X-shaped bolapolyphile within lipid bilayers. Langmuir 2015, 31, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Ebert, H.; Lechner, B.-D.; Lange, F.; Achilles, A.; Bärenwald, R.; Poppe, S.; Blume, A.; Saalwächter, K.; Tschierske, C.; et al. Dendritic domains with hexagonal symmetry formed by X-shaped bolapolyphiles in lipid membranes. Chem. Eur. J. 2015, 21, 8840–8850. [Google Scholar] [CrossRef] [PubMed]
- Achilles, A.; Bärenwald, R.; Lechner, B.-D.; Werner, S.; Ebert, H.; Tschierske, C.; Blume, A.; Bacia, K.; Saalwächter, K. Self-assembly of X-shaped bolapolyphiles in lipid membranes: Solid-state nmr investigations. Langmuir 2016, 32, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Marcelja, S.; Horn, R.G. Physical principles of membrane organization. Q. Rev. Biophys. 1980, 13, 121–200. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Gambacorta, A. The lipids of archaebacteria. Prog. Lipid Res. 1988, 27, 153–175. [Google Scholar] [CrossRef]
- Poppe, S.; Poppe, M.; Ebert, H.; Prehm, M.; Chen, C.; Liu, F.; Werner, S.; Bacia, K. Effects of lateral and terminal chains on the bulk self-assembly and membrane modification of X-shaped bolapolyphiles with oligo(phenylene enthynylene) cores. Part 1: Transition between amphiphilic and polyphilic LC self-assembly. Polymers 2017, 9, 471. [Google Scholar] [CrossRef]
- Mabrey, S.; Sturtevant, J.M. Investigation of phase transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA 1976, 73, 3862–3866. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Suter, R.; Knewtson, M.; Worthington, C.; Tristram-Nagle, S.; Zhang, R.; Nagle, J. Order and disorder in fully hydrated unoriented bilayers of gel-phase dipalmitoylphosphatidylcholine. Phys. Rev. E 1994, 49, 4665–4676. [Google Scholar] [CrossRef]
- Beattie, M.E.; Veatch, S.L.; Stottrup, B.L.; Keller, S.L. Sterol structure determines miscibility versus melting transitions in lipid vesicles. Biophys. J. 2005, 89, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Sankaram, M.B.; Thompson, T.E. Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. USA 1991, 88, 8686–8690. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kieffer, R.; Glettner, B.; Nürnberger, C.; Liu, F.; Pelz, K.; Prehm, M.; Baumeister, U.; Hahn, H.; Lang, H.; et al. Complex multicolor tilings and critical phenomena in tetraphilic liquid crystals. Science 2011, 331, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Chen, C.; Liu, F.; Prehm, M.; Poppe, S.; Tschierske, C. Emergence of tilt in square honeycomb liquid crystals. Soft Matter 2017, 13, 4676–4680. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.; Gratton, E.; Khan, T.K.; Chong, P.L.-G. Two-photon fluorescence microscopy studies of bipolar tetraether giant liposomes from thermoacidophilic archaebacteria sulfolobus acidocaldarius. Biophys. J. 2000, 79, 416–425. [Google Scholar] [CrossRef]
- Li, L.; Cheng, J.-X. Coexisting stripe- and patch-shaped domains in giant unilamellar vesicles. Biochemistry 2006, 45, 11819–11826. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.T.; Huang, J.; Feigenson, G.W.; Nagle, J.F. Effects of cholesterol and unsaturated dopc lipid on chain packing of saturated gel-phase dppc bilayers. Gen. Physiol. Biophys. 2009, 28, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C. Molecular self-organization of amphotropic liquid crystals. Prog. Polym. Sci. 1996, 21, 775–852. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, R.; Guo, C.; Cheng, X.; Gao, H.; Liu, F.; Bruckner, J.R.; Giesselmann, F.; Prehm, M.; Tschierske, C. Amphotropic azobenzene derivatives with oligooxyethylene and glycerol based polar groups. J. Mater. Chem. C 2015, 3, 11202–11211. [Google Scholar] [CrossRef]
- Ali, M.R.; Cheng, K.H.; Huang, J. Assess the nature of cholesterol–lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA 2007, 104, 5372–5377. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.; Brewer, J.; Simonson, A.C. Texture defects in lipid membrane domains. Soft Matter 2012, 8, 4894–4904. [Google Scholar] [CrossRef]
- Bacia, K.; Schwille, P.; Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 3272–3277. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, T.; Hunt, G.; Farkas, E.R.; Webb, W.W.; Feigenson, G.W. Fluorescence probe partitioning between lo/ld phases in lipid membranes. Biochim. Biophys. Acta 2007, 1768, 2182–2194. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R. Modeling and numerical simulations of dendritic crystal growth. Phys. D Nonlinear Phenom. 1993, 63, 410–423. [Google Scholar] [CrossRef]
- Nittmann, J.; Stanley, H.E. Tip splitting without interfacial tension and dendritic growth patterns arising from molecular anisotropy. Nature 1986, 321, 663–668. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wang, Z.; Yang, X.; Qian, Y. Hierarchical growth and shape evolution of hgs dendrites. Cryst. Growth Des. 2005, 5, 347–350. [Google Scholar] [CrossRef]
- He, K.; Xu, C.-Y.; Zhen, L.; Shao, W.-Z. Fractal growth of single-crystal α-fe2o3: From dendritic micro-pines to hexagonal micro-snowflakes. Mater. Lett. 2008, 62, 739–742. [Google Scholar] [CrossRef]
- Kotova, S.L.; Timofeeva, V.A.; Aksenova, N.A.; Sister, V.G.; Solov’eva, A.B. Peculiarities of pluronic crystallization in thin films. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2013, 7, 959–966. [Google Scholar] [CrossRef]
- Moran-Mirabal, J.M.; Aubrecht, D.M.; Craighead, H.G. Phase separation and fractal domain formation in phospholipid/diacetylene-supported lipid bilayers. Langmuir 2007, 23, 10661–10671. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.F.M.; Joly, E. Crystallization around solid-like nanosized docks can explain the specificity, diversity, and stability of membrane microdomains. Front. Plant Sci. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, K.C. Physical dynamics of ice crystal growth. Annu. Rev. Mater. Res. 2017, 47, 271–295. [Google Scholar] [CrossRef]
- Sodt, A.J.; Pastor, R.W.; Lyman, E. Hexagonal substructure and hydrogen bonding in liquid-ordered phases containing palmitoyl sphingomyelin. Biophys. J. 2015, 109, 948–955. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, S.; Ebenhan, J.; Poppe, M.; Poppe, S.; Ebert, H.; Tschierske, C.; Bacia, K. Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behavior. Part 2: Domain Formation by Self-Assembly in Lipid Bilayer Membranes. Polymers 2017, 9, 476. https://doi.org/10.3390/polym9100476
Werner S, Ebenhan J, Poppe M, Poppe S, Ebert H, Tschierske C, Bacia K. Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behavior. Part 2: Domain Formation by Self-Assembly in Lipid Bilayer Membranes. Polymers. 2017; 9(10):476. https://doi.org/10.3390/polym9100476
Chicago/Turabian StyleWerner, Stefan, Jan Ebenhan, Marco Poppe, Silvio Poppe, Helgard Ebert, Carsten Tschierske, and Kirsten Bacia. 2017. "Effects of Lateral and Terminal Chains of X-Shaped Bolapolyphiles with Oligo(phenylene ethynylene) Cores on Self-Assembly Behavior. Part 2: Domain Formation by Self-Assembly in Lipid Bilayer Membranes" Polymers 9, no. 10: 476. https://doi.org/10.3390/polym9100476