Polystyrene Chain Growth from Di-End-Functional Polyolefins for Polystyrene-Polyolefin-Polystyrene Block Copolymers
Abstract
:1. Introduction
2. Experimental Section
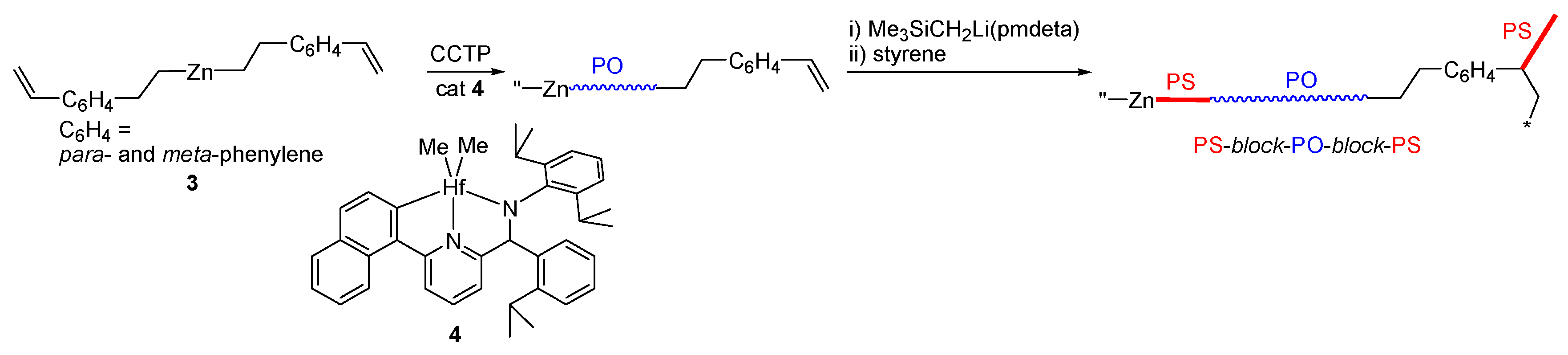
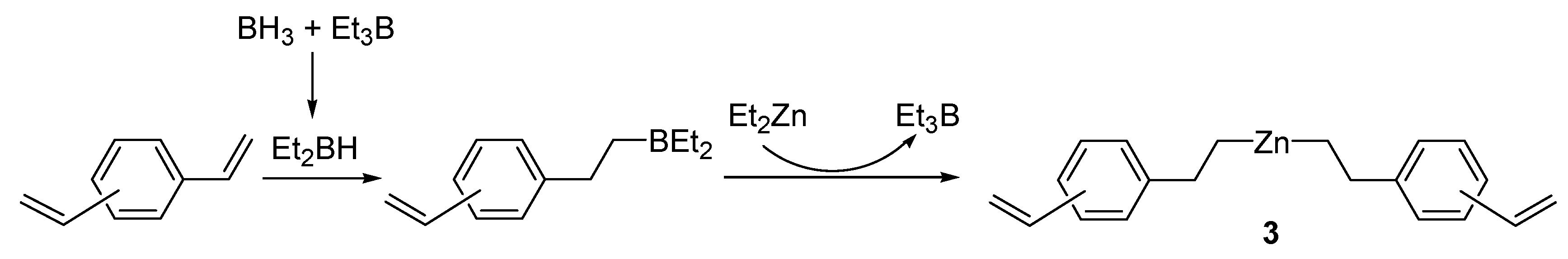
2.1. Preparation of (CH2=CHC6H4CH2CH2)2Zn (3)
2.2. Typical Procedure for the Synthesis of Block Copolymers (Entry 5 in Table 1)
2.3. High-Temperature GPC Studies
2.4. Sample Preparation for Transmission Electron Microscopy (TEM)
2.5. Tensile Tests
3. Results and Discussion
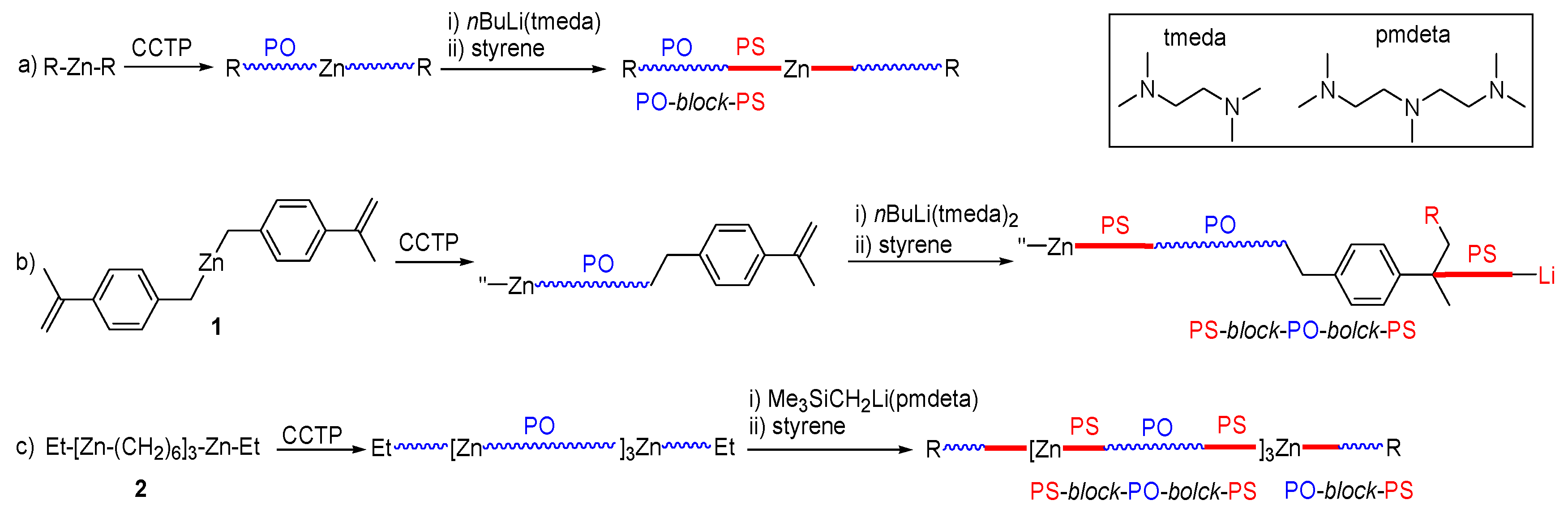
3.1. Strategy for the Preparation of PS-Block-PO-Block-PS
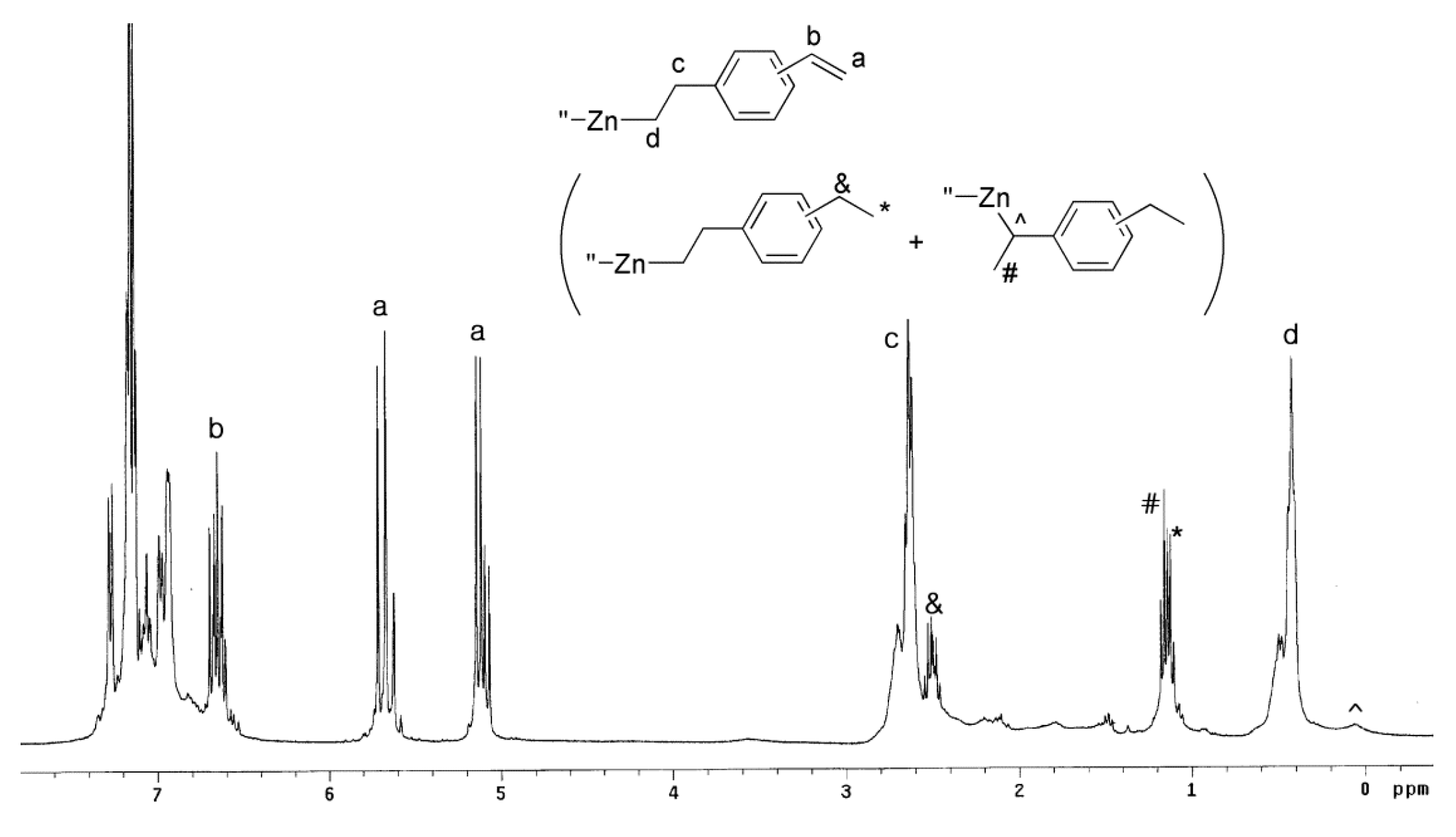
3.2. Synthesis of PS-Block-PO-Block-PS
3.3. Characterization of the Block Copolymers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eagan, J.M.; Xu, J.; Di Girolamo, R.; Thurber, C.M.; Macosko, C.W.; La Pointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yu, L.; Shiono, T.; Hasan, T.; Cai, Z. Synthesis of hydroxy-functionalized cyclic olefin copolymer and its block copolymers with semicrystalline polyolefin segments. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Mai, C.K.; Fredrickson, G.H.; Bazan, G.C. One-pot synthesis of semicrystalline/amorphous multiblock copolymers via divinyl-terminated telechelic polyolefins. Chem. Commun. 2016, 52, 2237–2240. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, H.; Deplace, F.; Vo, G.D.; Lapointe, A.M.; Shimizu, F.; Sugano, T.; Kramer, E.J.; Fredrickson, G.H.; Coates, G.W. Allyl-terminated polypropylene macromonomers: A route to polyolefin elastomers with excellent elastic behavior. Macromolecules 2015, 48, 7489–7494. [Google Scholar] [CrossRef]
- Jiang, B.; Shao, H.; Nie, H.; He, A. Sequential two-stage polymerization for synthesis of isotactic polypropylene/isotactic polybutene-1 alloys: Composition, morphology and granule growing mechanism. Polym. Chem. 2015, 6, 3315–3323. [Google Scholar] [CrossRef]
- Saeb, M.R.; Mohammadi, Y.; Kermaniyan, T.S.; Zinck, P.; Stadler, F.J. Unspoken aspects of chain shuttling reactions: Patterning the molecular landscape of olefin multi-block copolymers. Polymer 2017, 116, 55–75. [Google Scholar] [CrossRef]
- Hustad, P.O.; Kuhlman, R.L.; Arriola, D.J.; Carnahan, E.M.; Wenzel, T.T. Continuous production of ethylene-based diblock copolymers using coordinative chain transfer polymerization. Macromolecules 2007, 40, 7061–7064. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic production of olefin block copolymers via chain shuttling polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.C.; Dong, J.Y. A Novel Consecutive chain transfer reaction to p-methylstyrene and hydrogen during metallocene-mediated olefin polymerization. J. Am. Chem. Soc. 2001, 123, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ye, Z.; Subramanian, R. Synthesis of block copolymers of ethylene with styrene and n-butyl acrylate via a tandem strategy combining ethylene “living” polymerization catalyzed by a functionalized Pd−diimine catalyst with atom transfer radical polymerization. Macromolecules 2008, 41, 640–649. [Google Scholar] [CrossRef]
- Liu, R.; Li, Z.; Yuan, D.; Meng, C.; Wu, Q.; Zhu, F. Synthesis and self-assembly of miktoarm star copolymers of (polyethylene)2−(polystyrene)2. Polymer 2011, 52, 356–362. [Google Scholar] [CrossRef]
- Weiser, M.-S.; Mülhaupt, R. Formation of polyolefin-block-polystyrene block copolymers on phenoxyimine catalysts. Macromol. Rapid Commun. 2006, 27, 1009–1014. [Google Scholar] [CrossRef]
- Kermagoret, A.; Debuigne, A.; Jérôme, C.; Detrembleur, C. Precision design of ethylene- and polar-monomer-based copolymers by organometallic-mediated radical polymerization. Nat. Chem. 2014, 6, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Dommanget, C.; D’Agosto, F.; Monteil, V. Polymerization of ethylene through reversible addition—Fragmentation chain transfer (RAFT). Angew. Chem. Int. Ed. 2014, 53, 6683–6686. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.R.; Hwang, W.; Sita, L.R. Dynamic sub-10-nm nanostructured ultrathin films of sugar–polyolefin conjugates thermoresponsive at physiological temperatures. J. Am. Chem. Soc. 2017, 139, 5281–5284. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Zych, A.; Przybysz, M.; Bouyahyi, M.; Sowinski, P.; Koevoets, R.; Haponiuk, J.; Graf, R.; Hansen, M.R.; Jasinska-Walc, L.; et al. Toward polyethylene—Polyester block and graft copolymers with tunable polarity. Macromolecules 2017, 50, 107–122. [Google Scholar] [CrossRef]
- Thomas, T.S.; Hwang, W.; Sita, L.R. End-group-functionalized poly(α-olefinates) as non-polar building blocks: Self-assembly of sugar-polyolefin hybrid conjugates. Angew. Chem. Int. Ed. 2016, 55, 4683–4687. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.; Léger, R.; Otazaghine, B.; Ienny, P. Hyperelastic behavior of modified sepiolite/SEBS thermoplastic elastomers. J. Mater. Sci. 2017, 52, 7591–7604. [Google Scholar] [CrossRef]
- Sahnoune, M.; Taguet, A.; Otazaghine, B.; Kaci, M.; Lopez-Cuesta, J.-M. Effects of functionalized halloysite on morphology and properties of polyamide-11/SEBS-g-MA blends. Eur. Polym. J. 2017, 90, 418–430. [Google Scholar] [CrossRef]
- Li, H.; Xie, X.M. Morphology development and superior mechanical properties of PP/PA6/SEBS ternary blends compatibilized by using a highly efficient multi-phase compatibilizer. Polymer 2017, 108, 1–10. [Google Scholar] [CrossRef]
- Tomacheski, D.; Pittol, M.; Ermel, C.E.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Influence of processing conditions on the mechanical properties of SEBS/PP/oil blends. Polym. Bull. 2017, 1–15. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, W.; Ren, Q.; Feng, J. Regulation of physical networks and mechanical properties of triblock thermoplastic elastomer through introduction of midblock similar crystalline polymer with multiblock architecture. Macromolecules 2016, 49, 7379–7386. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, H.; Jia, J.; Hao, J.; Xie, F.; Chi, J.; Qin, B.; Fu, L.; Song, W.; Shao, Z. High performance anion exchange ionomer for anion exchange membrane fuel cells. RSC Adv. 2017, 7, 19153–19161. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.H.; Xu, R.W.; Zhang, S.; Wu, Y.X. Cross-linked quaternized poly(styrene-b-(ethylene-co-butylene)-b-styrene) for anion exchange membrane: Synthesis, characterization and properties. ACS Appl. Mater. Interf. 2016, 8, 20329–20341. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Luong, A.J.; Zhang, W.; Lin, L.; Ritchie, R.O.; Alivisatos, A.P. Cavitation-induced stiffness reductions in quantum dot-polymer nanocomposites. Chem. Mater. 2016, 28, 2540–2549. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, W.; Feng, J. A facile strategy to fabricate multishape memory polymers with controllable mechanical properties. Macromol. Rapid Commun. 2016, 37, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Quiles-Díaz, S.; Enrique-Jimenez, P.; Martínez, G.; Ania, F.; Flores, A.; Gómez-Fatou, M.A. Development of advanced elastomeric conductive nanocomposites by selective chemical affinity of modified Graphene. Macromolecules 2016, 49, 4948–4956. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Ryu, C.Y.; Kim, Y.S.; Bae, C. Stable elastomeric anion exchange membranes based on quaternary ammonium-tethered polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene triblock copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Lin, F.; Wu, C.; Cui, D. Synthesis and characterization of crystalline styrene-b-(ethylene-co-butylene)-b-styrene triblock copolymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1243–1249. [Google Scholar] [CrossRef]
- Wong, D.T.; Wang, C.; Pople, J.A.; Balsara, N.P. Effect of nonsolvent exposure on morphology of mesoporous semicrystalline block copolymer films. Macromolecules 2013, 46, 4411–4417. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Park, S.H.; Kim, D.H.; Park, S.S.; Park, G.H.; Lee, B.Y. Synthesis of polyolefin-block-polystyrene through sequential coordination and anionic polymerizations. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3110–3118. [Google Scholar] [CrossRef]
- Van Meurs, M.; Britovsek, G.J.P.; Gibson, V.C.; Cohen, S.A. Polyethylene chain growth on zinc catalyzed by olefin polymerization catalysts: A comparative investigation of highly active catalyst systems across the transition series. J. Am. Chem. Soc. 2005, 127, 9913–9923. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Dyer, H.E.; El Kinani, Y.; Dietz, C.; Roussel, P.; Bria, M.; Visseaux, M.; Zinck, P.; Mountford, P. Bis(phenolate)amine-supported lanthanide borohydride complexes for styrene and trans-1,4-isoprene (co-)polymerisations. Dalton Trans. 2015, 44, 12312–12325. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dong, B.; Liu, H.; Guo, J.; Zheng, W.; Zhang, C.; Zhao, L.; Bai, C.; Hu, Y.; Zhang, X. Synthesis of block copolymers containing polybutadiene segments by combination of coordinative chain transfer polymerization, ring-opening polymerization, and atom transfer radical polymerization. Macromol. Chem. Phys. 2015, 216, 321–328. [Google Scholar] [CrossRef]
- Zinck, P. Unexpected reactivities in chain shuttling copolymerizations. Polym. Int. 2016, 65, 11–15. [Google Scholar] [CrossRef]
- Ota, Y.; Murayama, T.; Nozaki, K. One-step catalytic asymmetric synthesis of all-syn deoxypropionate motif from propylene: Total synthesis of (2R,4R,6R,8R)-2,4,6,8-tetramethyldecanoic acid. Proc. Nat. Acad. Sci. USA 2016, 113, 2857–2861. [Google Scholar] [CrossRef] [PubMed]
- Briquel, R.; Mazzolini, J.; Le Bris, T.; Boyron, O.; Boisson, F.; Delolme, F.; D’Agosto, F.; Boisson, C.; Spitz, R. Polyethylene building blocks by catalyzed chain growth and efficient end functionalization strategies, including click chemistry. Angew. Chem. Int. Ed. 2008, 47, 9311–9313. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, J.; Espinosa, E.; D’Agosto, F.; Boisson, C. Catalyzed chain growth (CCG) on a main group metal: An efficient tool to functionalize polyethylene. Polym. Chem. 2010, 1, 793–800. [Google Scholar] [CrossRef]
- German, I.; Kelhifi, W.; Norsic, S.; Boisson, C.; D’Agosto, F. Telechelic polyethylene from catalyzed chain-growth polymerization. Angew. Chem. Int. Ed. 2013, 52, 3438–3441. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, S.S.; Park, S.H.; Jeon, J.Y.; Kim, H.B.; Lee, B.Y. Preparation of polystyrene-polyolefin multiblock copolymers by sequential coordination and anionic polymerization. RSC Adv. 2017, 7, 5948–5956. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, C.S.; Kim, S.D.; Kwon, S.J.; Lee, H.M.; Kim, T.H.; Jeon, J.Y.; Lee, B.Y. Biaxial chain growth of polyolefin and polystyrene from 1,6-hexanediylzinc species for triblock copolymers. Macromolecules 2017, 50, 6606–6616. [Google Scholar] [CrossRef]
- Frazier, K.A.; Froese, R.D.; He, Y.; Klosin, J.; Theriault, C.N.; Vosejpka, P.C.; Zhou, Z.; Abboud, K.A. Pyridylamido hafnium and zirconium complexes: Synthesis, dynamic behavior, and rthylene/1-octene and propylene polymerization reactions. Organometallics 2011, 30, 3318–3329. [Google Scholar] [CrossRef]
- Scholte, T.G.; Meijerink, N.L.J.; Schoffeleers, H.M.; Brands, A.M.G. Mark–Houwink equation and GPC calibration for linear short-chain branched polyolefines, including polypropylene and ethylene—Propylene copolymers. J. Appl. Polym. Sci. 1984, 29, 3763–3782. [Google Scholar] [CrossRef]
- Dong, J.Y.; Chung, T.C. Synthesis of polyethylene containing a terminal p-methylstyrene group: Metallocene-mediated ethylene polymerization with a consecutive chain transfer reaction to p-methylstyrene and hydrogen. Macromolecules 2002, 35, 1622–1631. [Google Scholar] [CrossRef]
- Rocchigiani, L.; Busico, V.; Pastore, A.; Macchioni, A. Comparative NMR study on the reactions of Hf(IV) organometallic complexes with Al/Zn alkyls. Organometallics 2016, 35, 1241–1250. [Google Scholar] [CrossRef]
- Zuccaccia, C.; Macchioni, A.; Busico, V.; Cipullo, R.; Talarico, G.; Alfano, F.; Boone, H.W.; Frazier, K.A.; Hustad, P.D.; Stevens, J.C.; et al. Intra- and intermolecular NMR studies on the activation of arylcyclometallated hafnium pyridyl-amido olefin polymerization precatalysts. J. Am. Chem. Soc. 2008, 130, 10354–10368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Clark, T.P.; Jazdzewski, B.A.; Klamo, S.B.; Wenzel, T.T. Synthesis of bis(7-octenyl)zinc via heterogeneous Ni catalysts. Polyhedron 2014, 84, 32–36. [Google Scholar] [CrossRef]
- Weissig, V.; Beckhaus, R.; Banasiak, U.; Thiele, K.H. Koordinationschemische untersuchungen an zinkdialkylen. XV. darstellung, reinheit und eigenschaften von zinkdibenzylen. Z. Anorg. Allg. Chem. 1980, 467, 61–67. [Google Scholar] [CrossRef]
- Roberts, A.J.; Kennedy, A.R.; McLellan, R.; Robertson, S.D.; Hevia, E. Synthesis, structure and solution studies on mixed aryl/alkyl lithium zincates. Eur. J. Inorg. Chem. 2016, 2016, 4752–4760. [Google Scholar] [CrossRef]

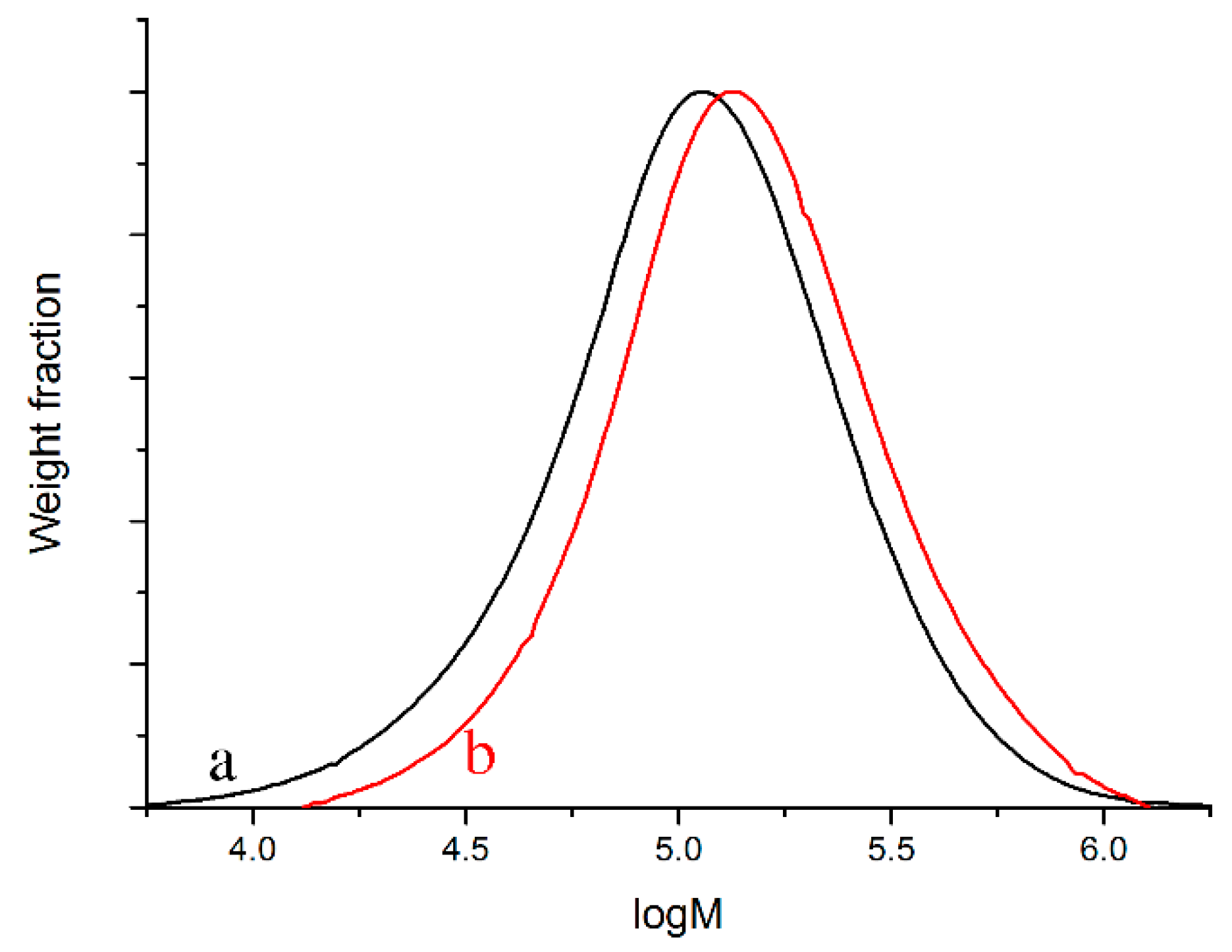

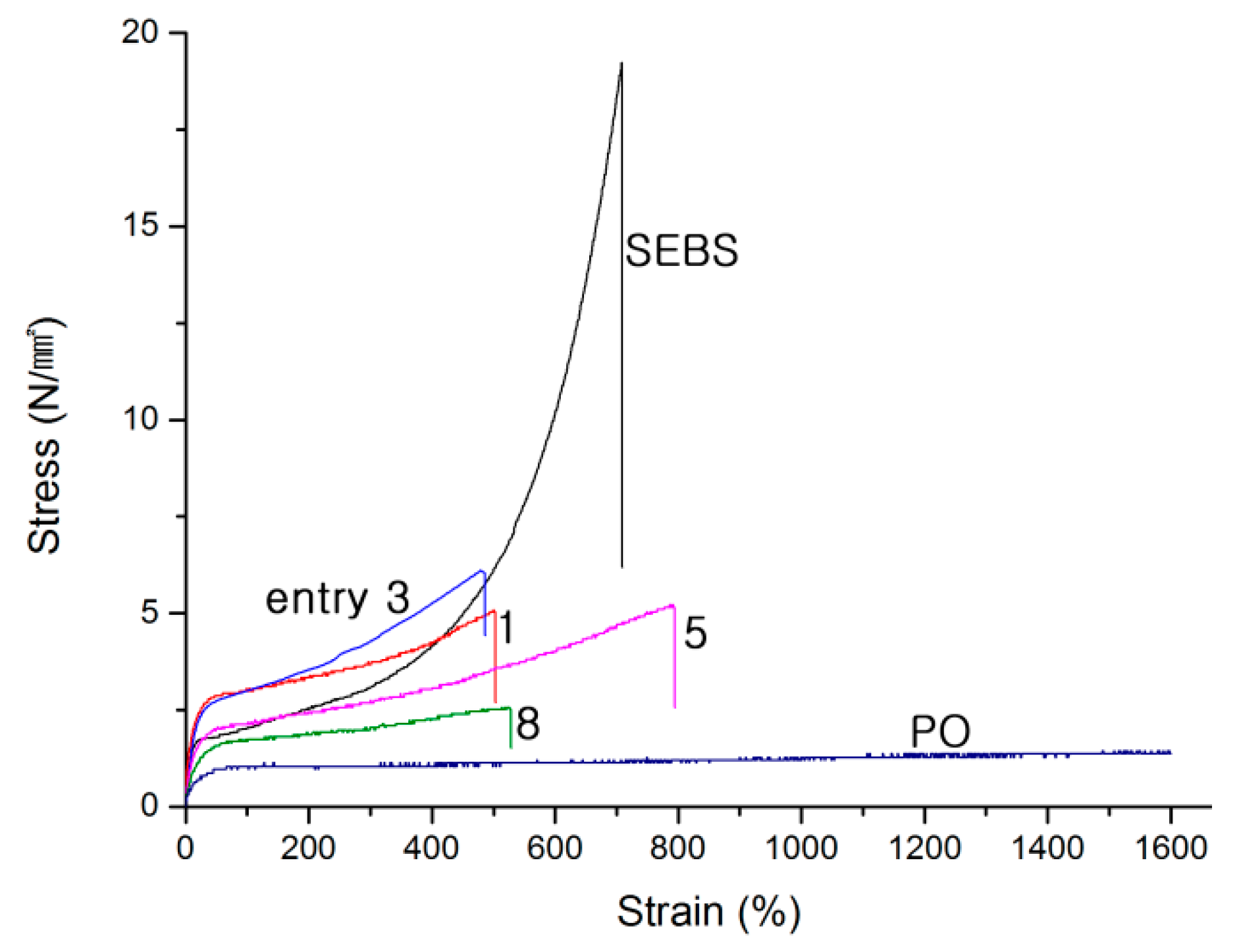
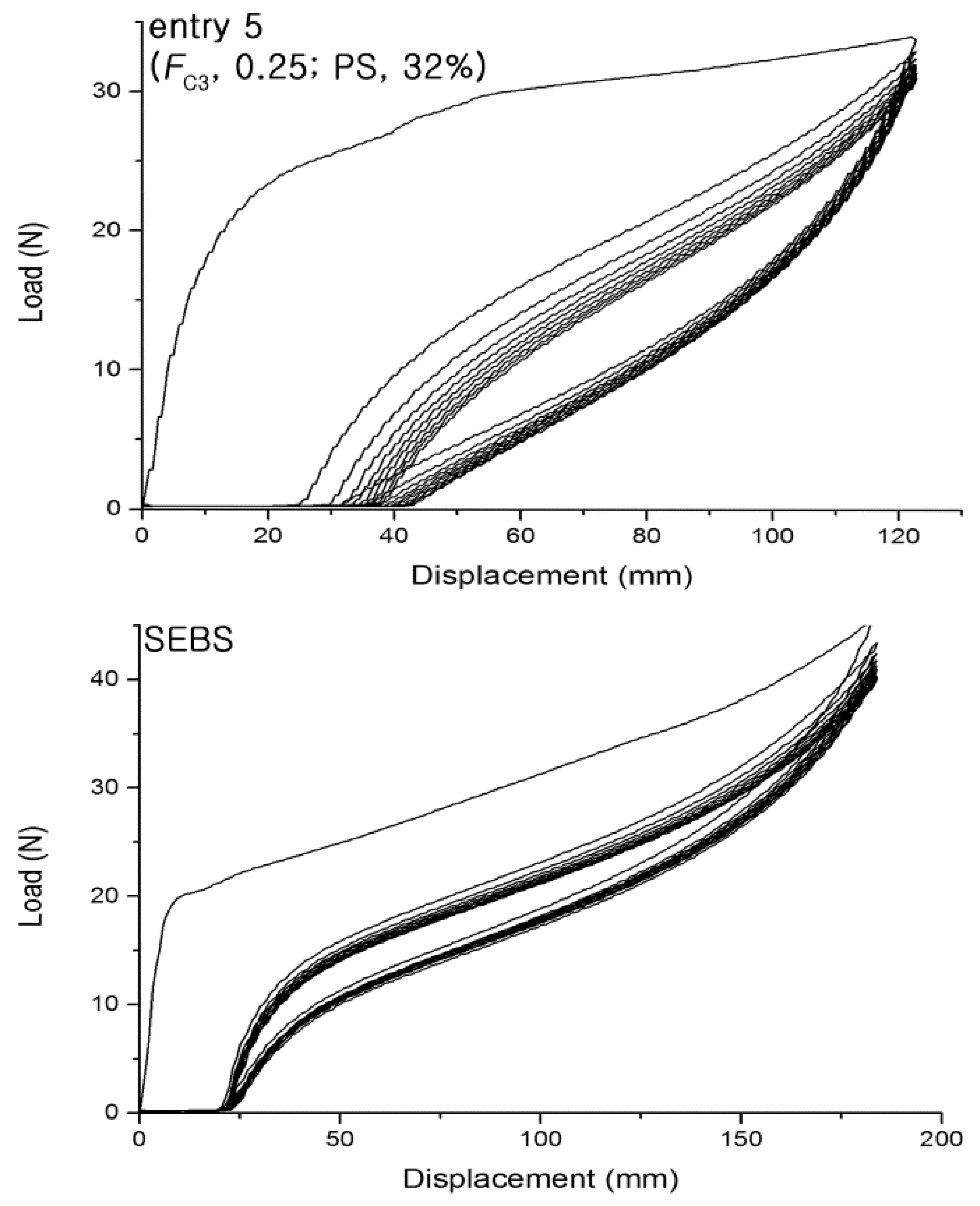
| Entry | Yield (g) | FC3 b | (PS, g)/(Total, g) | (Homo-PS, g)/(PS, g) | Homo-PS Mn (kDa); PDI c | PO Mn (kDa); PDI d | Block Copolymer Mn (kDa); PDI d | ||
|---|---|---|---|---|---|---|---|---|---|
| Expected e | PO Equiv f | PS Equiv d | |||||||
| 1 | 21.3 | 0.22 | 0.37 | 0.17 | 16; 1.20 | 45 | 52; 1.92 | 90; 1.95 | 123; 1.73 |
| 2 | 23.9 | 0.22 | 0.33 | 0.24 | 23; 2.01 | 54 | 48; 1.88 | 84; 1.90 | 120; 1.84 |
| 3 | 18.7 | 0.24 | 0.42 | 0.17 | 15; 1.13 | 36 | 47; 1.89 | 84; 1.92 | 111; 1.71 |
| 4 | 20.1 | 0.27 | 0.38 | 0.20 | 17; 1.17 | 41 | 54; 1.92 | 88; 1.95 | 125; 1.72 |
| 5 | 24.1 | 0.25 | 0.32 | 0.23 | 18; 1.46 | 54 | 47; 1.87 | 79; 1.88 | 109; 1.69 |
| 6 | 20.0 | 0.30 | 0.39 | 0.22 | 17; 1.15 | 41 | 51; 1.98 | 80; 2.00 | 134; 1.67 |
| 7 | 22.9 | 0.29 | 0.34 | 0.18 | 17; 1.25 | 50 | 50; 1.89 | 79; 1.91 | 106; 1.76 |
| 8 | 24.7 | 0.28 | 0.32 | 0.19 | 18; 1.25 | 56 | 50; 1.96 | 80; 2.00 | 110; 1.75 |
| 9 | 22.8 | 0.30 | 0.34 | 0.18 | 16; 1.17 | 50 | 49; 1.88 | 79; 1.91 | 94; 1.74 |
| 10 | 21.9 | 0.28 | 0.36 | 0.24 | 19; 1.55 | 47 | 50 ;1.87 | 80; 1.90 | 105; 1.79 |
| 11 g | 23.8 | 0.29 | 0.33 | 0.33 | 25; 1.68 | 53 | 51; 1.81 | 82; 1.83 | 114; 1.63 |
| Entry | FC3 | PS (%) | Mn (kDa); PDI | Tensile Test | Cyclic Tensile Test | ||
|---|---|---|---|---|---|---|---|
| Tensile Strength (N/mm2) | Elongation at Break (%) | Elastic Recovery at 1st Cycle (%) | Elastic Recovery at 10th Cycle (%) | ||||
| 1 | 0.22 | 37 | 121; 1.73 | 5.09 | 490 | 65 | 55 |
| 2 | 0.22 | 33 | 120; 1.84 | 2.68 | 300 | 90 | 85 |
| 3 | 0.24 | 42 | 111; 1.71 | 6.10 | 470 | 61 | 54 |
| 4 | 0.27 | 38 | 125; 1.72 | 4.25 | 490 | 78 | 66 |
| 5 | 0.25 | 32 | 109; 1.69 | 5.22 | 770 | 77 | 64 |
| 6 | 0.30 | 39 | 134 1.67 | 2.55 | 290 | 88 | 84 |
| 7 | 0.29 | 34 | 106; 1.76 | 2.90 | 480 | 91 | 81 |
| 8 | 0.28 | 32 | 110; 1.75 | 2.56 | 520 | 85 | 74 |
| 9 | 0.30 | 34 | 94; 1.74 | 1.82 | 440 | 81 | 75 |
| 10 | 0.28 | 36 | 105; 1.79 | 2.69 | 550 | 85 | 76 |
| 11 | 0.29 | 33 | 114; 1.63 | 1.64 | 220 | 81 | broken |
| 12 | SEBS | - | - | 19.2 | 720 | 89 | 86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.S.; Park, S.S.; Kim, S.D.; Kwon, S.J.; Baek, J.W.; Lee, B.Y. Polystyrene Chain Growth from Di-End-Functional Polyolefins for Polystyrene-Polyolefin-Polystyrene Block Copolymers. Polymers 2017, 9, 481. https://doi.org/10.3390/polym9100481
Kim CS, Park SS, Kim SD, Kwon SJ, Baek JW, Lee BY. Polystyrene Chain Growth from Di-End-Functional Polyolefins for Polystyrene-Polyolefin-Polystyrene Block Copolymers. Polymers. 2017; 9(10):481. https://doi.org/10.3390/polym9100481
Chicago/Turabian StyleKim, Chung Sol, Seung Soo Park, Sung Dong Kim, Su Jin Kwon, Jun Won Baek, and Bun Yeoul Lee. 2017. "Polystyrene Chain Growth from Di-End-Functional Polyolefins for Polystyrene-Polyolefin-Polystyrene Block Copolymers" Polymers 9, no. 10: 481. https://doi.org/10.3390/polym9100481






