Vesicles in Multiple Shapes: Fine-Tuning Polymersomes’ Shape and Stability by Setting Membrane Hydrophobicity
Abstract
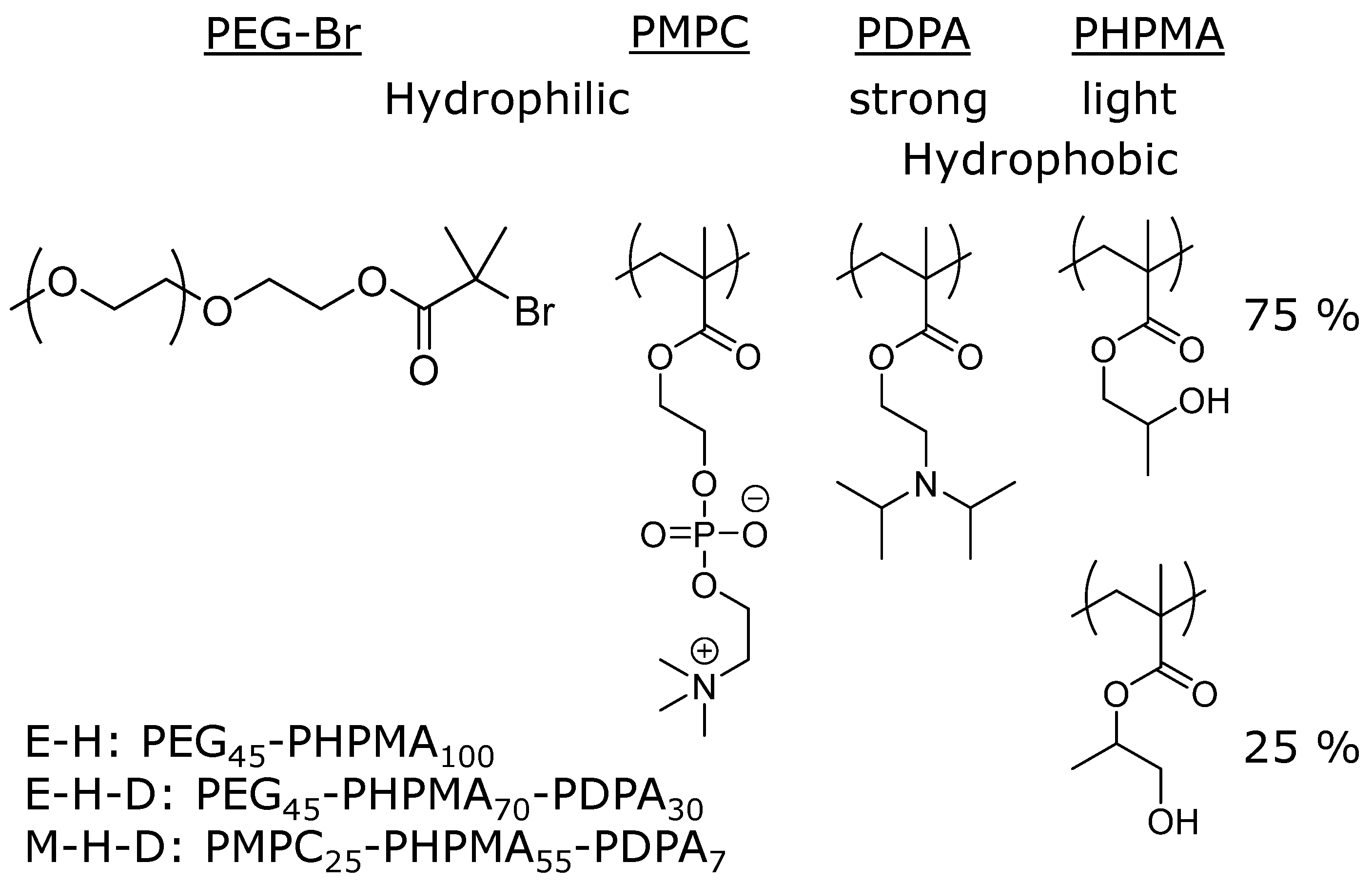
:1. Introduction
2. Experimental Section
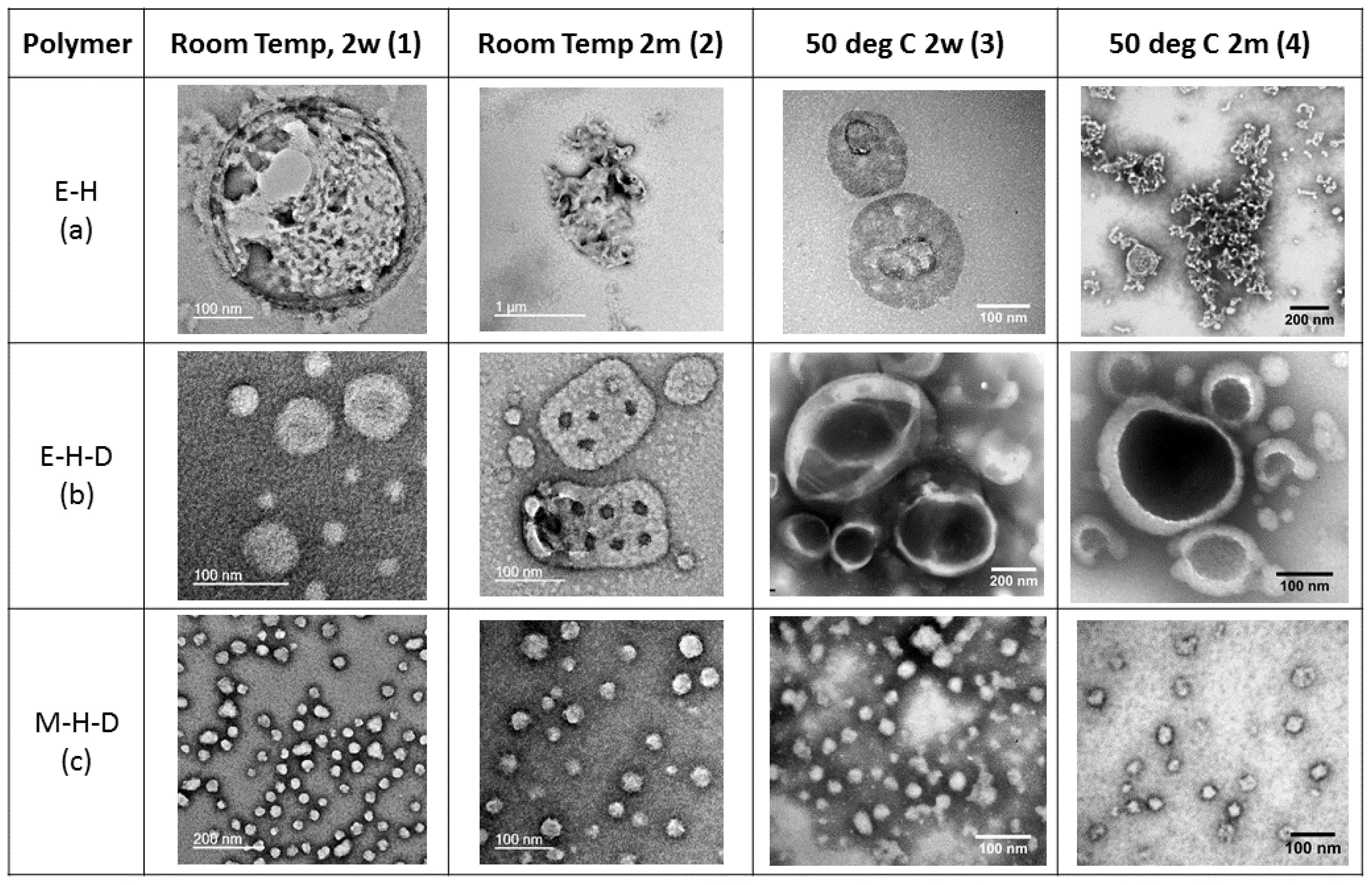
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Dongen, S.F.M.; de Hoog, H.P.M.; Peters, R.J.R.W.; Nallani, M.; Nolte, R.J.M.; van Hest, J.C.M. Biohybrid polymer capsules. Chem. Rev. 2009, 109, 6212–6274. [Google Scholar] [CrossRef] [PubMed]
- Brinkhuis, R.P.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymeric vesicles in biomedical applications. Polym. Chem. 2011, 2, 1449–1462. [Google Scholar] [CrossRef]
- Renggli, K.; Baumann, P.; Langowska, K.; Onaca, O.; Bruns, N.; Meier, W. Selective and responsive nanoreactors. Adv. Funct. Mater. 2011, 21, 1241–1259. [Google Scholar] [CrossRef]
- Pawar, P.V.; Gohil, S.V.; Jain, J.P.; Kumar, N. Functionalized polymersomes for biomedical applications. Polym. Chem. 2013, 4, 3160–3176. [Google Scholar] [CrossRef]
- Messager, L.; Gaitzsch, J.; Chierico, L.; Battaglia, G. Novel aspects of encapsulation and delivery using polymersomes. Curr. Opin. Pharmacol. 2014, 18, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gaitzsch, J.; Huang, X.; Voit, B. Engineering functional polymer capsules toward smart nanoreactors. Chem. Rev. 2016, 116, 1053–1093. [Google Scholar] [CrossRef] [PubMed]
- Gaitzsch, J.; Appelhans, D.; Wang, L.G.; Battaglia, G.; Voit, B. Synthetic Bio-nanoreactor: Mechanical and Chemical Control of Polymersome Membrane Permeability. Angew. Chem. Int. Ed. 2012, 51, 4448–4451. [Google Scholar] [CrossRef] [PubMed]
- Iyisan, B.; Janke, A.; Reichenbach, P.; Eng, L.M.; Appelhans, D.; Voit, B. Immobilized Multifunctional Polymersomes on Solid Surfaces: Infrared Light-Induced Selective Photochemical Reactions, pH Responsive Behavior, and Probing Mechanical Properties under Liquid Phase. ACS Appl. Mater. Interfaces 2016, 8, 15788–15801. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, C.; Massignani, M.; Fernyhough, C.; Blanazs, A.; Ryan, A.J.; Madsen, J.; Warren, N.J.; Armes, S.P.; Lewis, A.L.; Chirasatitsin, S.; et al. Controlling Polymersome Surface Topology at the Nanoscale by Membrane Confined Polymer/Polymer Phase Separation. ACS Nano 2011, 5, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Gaitzsch, J.; Appelhans, D.; Janke, A.; Strempel, M.; Schwille, P.; Voit, B. Cross-linked and pH sensitive supported polymer bilayers from polymersomes–studies concerning thickness, rigidity and fluidity. Soft Matter. 2014, 10, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, K.; Makowski, M.; Kappl, M.; Landfester, K.; Kroeger, A. Mechanical properties of poly (dimethylsiloxane)-block-poly (2-methyloxazoline) polymersomes probed by atomic force microscopy. Langmuir 2012, 28, 12629–12636. [Google Scholar] [CrossRef] [PubMed]
- Najer, A.; Wu, D.L.; Vasquez, D.; Palivan, C.G.; Meier, W. Polymer nanocompartments in broad-spectrum medical applications. Nanomedicine 2013, 8, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Le Meins, J.F.; Sandre, O.; Lecommandoux, S. Recent trends in the tuning of polymersomes' membrane properties. Eur. Phys. J. E Soft Matter 2011, 34, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Ryan, A.J.; Tomas, S. Polymeric vesicle permeability: A facile chemical assay. Langmuir 2006, 22, 4910–4913. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Ryan, A.J. Pathways of polymeric vesicle formation. J. Phys. Chem. B 2006, 110, 10272–10279. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Ryan, A.J.; Armes, S.P. Predictive phase diagrams for RAFT aqueous dispersion polymerization: effect of block copolymer composition, molecular weight, and copolymer concentration. Macromolecules 2012, 45, 5099–5107. [Google Scholar] [CrossRef]
- Warren, N.J.; Mykhaylyk, O.O.; Mahmood, D.; Ryan, A.J.; Armes, S.P. RAFT aqueous dispersion polymerization yields poly (ethylene glycol)-based diblock copolymer nano-objects with predictable single phase morphologies. J. Am. Chem. Soc. 2014, 136, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.R.; Mykhaylyk, O.O.; Semsarilar, M.; Boerakker, M.; Wyman, P.; Armes, S.P. How do spherical diblock copolymer nanoparticles grow during RAFT alcoholic dispersion polymerization? Macromolecules 2016, 49, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A critical appraisal of RAFT-mediated polymerization-induced self-assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Madsen, J.; Battaglia, G.; Ryan, A.J.; Armes, S.P. Mechanistic insights for block copolymer morphologies: how do worms form vesicles? J. Am. Chem. Soc. 2011, 133, 16581–16587. [Google Scholar] [CrossRef] [PubMed]
- Sigg, S.J.; Postupalenko, V.; Duskey, J.T.; Palivan, C.G.; Meier, W. Stimuli-responsive codelivery of oligonucleotides and drugs by self-assembled peptide nanoparticles. Biomacromolecules 2016, 17, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Warren, N.J.; Mykhaylyk, O.O.; Ryan, A.J.; Williams, M.; Doussineau, T.; Dugourd, P.; Antoine, R.; Portale, G.; Armes, S.P. Testing the vesicular morphology to destruction: Birth and death of diblock copolymer vesicles prepared via polymerization-induced self-assembly. J. Am. Chem. Soc. 2015, 137, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.T.; Warren, N.J.; Lewis, A.L.; Armes, S.P.; Battaglia, G. Effect of pH and Temperature on PMPC–PDPA Copolymer Self-Assembly. Macromolecules 2013, 46, 1400–1407. [Google Scholar] [CrossRef]
- Chambon, P.; Blanazs, A.; Battaglia, G.; Armes, S.P. Facile synthesis of methacrylic ABC triblock copolymer vesicles by RAFT aqueous dispersion polymerization. Macromolecules 2012, 45, 5081–5090. [Google Scholar] [CrossRef]
- Ruiz-Perez, L.; Madsen, J.; Themistou, E.; Gaitzsch, J.; Messager, L.; Armes, S.P.; Battaglia, G. Nanoscale detection of metal-labeled copolymers in patchy polymersomes. Polym. Chem. 2015, 6, 2065–2068. [Google Scholar] [CrossRef]
- Gaitzsch, J.; Chudasama, V.; Morecroft, E.; Messager, L.; Battaglia, G. Synthesis of an Amphiphilic Miktoarm Star Terpolymer for Self-Assembly into Patchy Polymersomes. ACS Macro Lett. 2016, 5, 351–354. [Google Scholar] [CrossRef]
- Carcouet, C.C.M.C.; Esteves, A.C.C.; Hendrix, M.M.R.M.; van Benthem, R.A.T.M.; de With, G. Fine-Tuning of Superhydrophobicity Based on Monolayers of Well-defined Raspberry Nanoparticles with Variable Dual-roughness Size and Ratio. Adv. Funct. Mater. 2014, 24, 5745–5752. [Google Scholar] [CrossRef]
- Gaitzsch, J.; Appelhans, D.; Grafe, D.; Schwille, P.; Voit, B. Photo-crosslinked and pH sensitive polymersomes for triggering the loading and release of cargo. Chem. Commun. 2011, 47, 3466–3468. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Le Men, L.; Borsali, R.; Lai-Kee-Him, J.; Brisson, A.; Armes, S.P.; Lewis, A.L. Phosphorylcholine-based pH-responsive diblock copolymer micelles as drug delivery vehicles: light scattering, electron microscopy, and fluorescence experiments. Biomacromolecules 2006, 7, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Save, M.; Weaver, J.V.M.; Armes, S.P.; McKenna, P. Atom transfer radical polymerization of hydroxy-functional methacrylates at ambient temperature: comparison of glycerol monomethacrylate with 2-hydroxypropyl. Macromolecules 2002, 35, 1152–1159. [Google Scholar] [CrossRef]
- Ratcliffe, L.P.D.; Blanazs, A.; Williams, C.N.; Brown, S.L.; Armes, S.P. RAFT polymerization of hydroxy-functional methacrylic monomers under heterogeneous conditions: effect of varying the core-forming block. Polym. Chem. 2014, 5, 3643–3655. [Google Scholar] [CrossRef]
- Lomas, H.; Massignani, M.; Abdullah, K.A.; Canton, I.; Lo Presti, C.; MacNeil, S.; Du, J.Z.; Blanazs, A.; Madsen, J.; Armes, S.P.; et al. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss. 2008, 139, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Lomas, H.; Du, J.Z.; Canton, I.; Madsen, J.; Warren, N.; Armes, S.P.; Lewis, A.L.; Battaglia, G. Efficient Encapsulation of Plasmid DNA in pH-Sensitive PMPC–PDPA Polymersomes: Study of the Effect of PDPA Block Length on Copolymer–DNA Binding Affinity. Macromol. Biosci. 2010, 10, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; Massignani, M.; Patikarnmonthon, N.; Chierico, L.; Robertson, J.; Renshaw, S.A.; Warren, N.J.; Madsen, J.P.; Armes, S.P.; Lewis, A.L.; et al. Fully synthetic polymer vesicles for intracellular delivery of antibodies in live cells. FASEB J. 2013, 27, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; Ryan, A.J. The evolution of vesicles from bulk lamellar gels. Nat. Mater. 2005, 4, 869–876. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitzsch, J.; Messager, L.; Morecroft, E.; Meier, W. Vesicles in Multiple Shapes: Fine-Tuning Polymersomes’ Shape and Stability by Setting Membrane Hydrophobicity. Polymers 2017, 9, 483. https://doi.org/10.3390/polym9100483
Gaitzsch J, Messager L, Morecroft E, Meier W. Vesicles in Multiple Shapes: Fine-Tuning Polymersomes’ Shape and Stability by Setting Membrane Hydrophobicity. Polymers. 2017; 9(10):483. https://doi.org/10.3390/polym9100483
Chicago/Turabian StyleGaitzsch, Jens, Lea Messager, Eloise Morecroft, and Wolfgang Meier. 2017. "Vesicles in Multiple Shapes: Fine-Tuning Polymersomes’ Shape and Stability by Setting Membrane Hydrophobicity" Polymers 9, no. 10: 483. https://doi.org/10.3390/polym9100483



