Ferrocene-Modified Block Copolymers for the Preparation of Smart Porous Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials
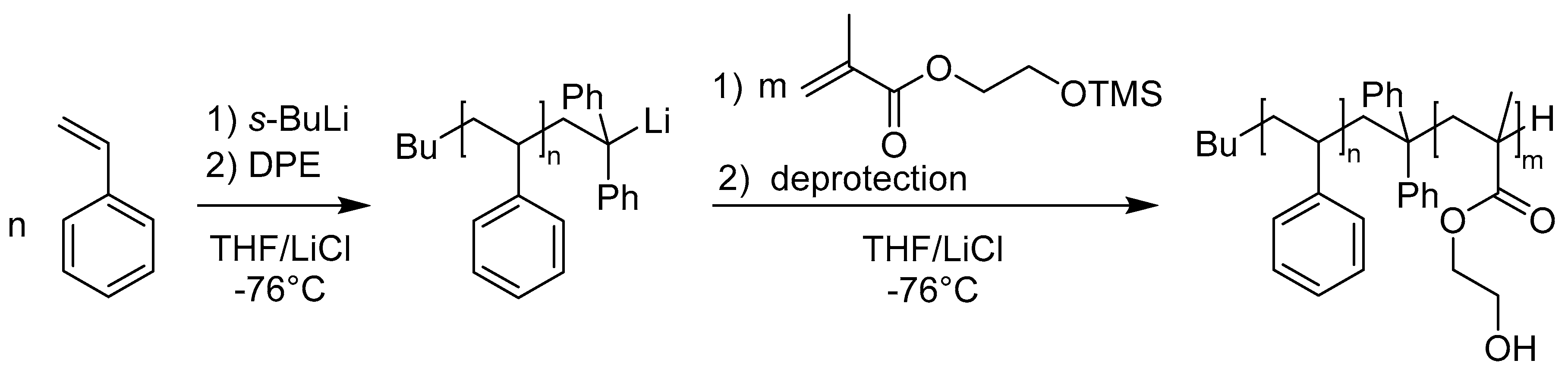
2.3. Block Copolymer Synthesis
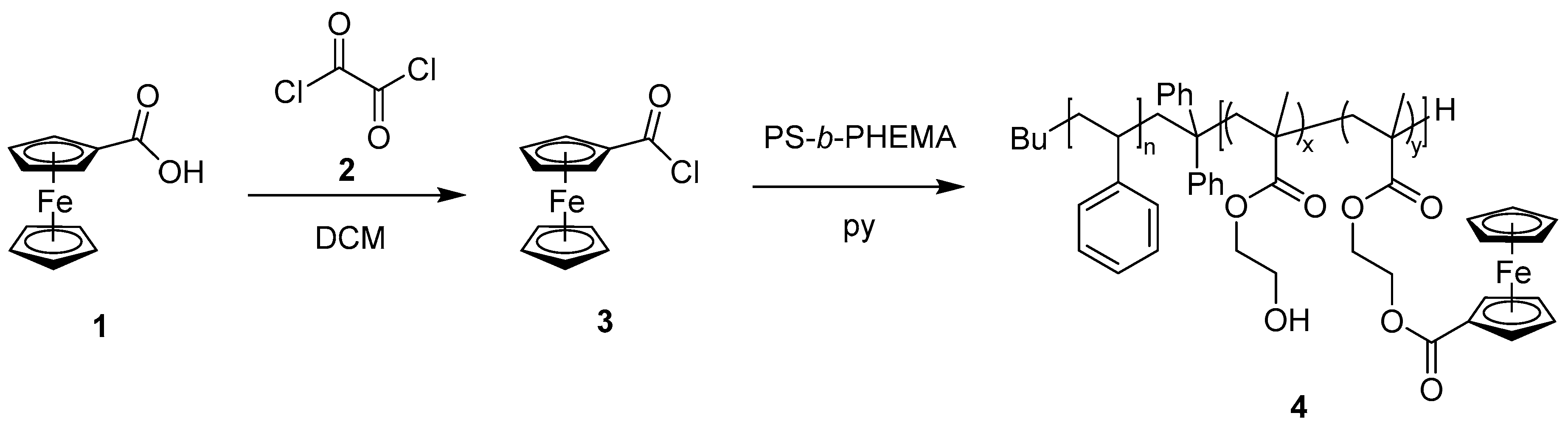
2.4. Block Copolymer Modification with Ferrocene Acid
2.5. Membrane Formation with Ferrocene-Functionalized Block Copolymers
3. Results
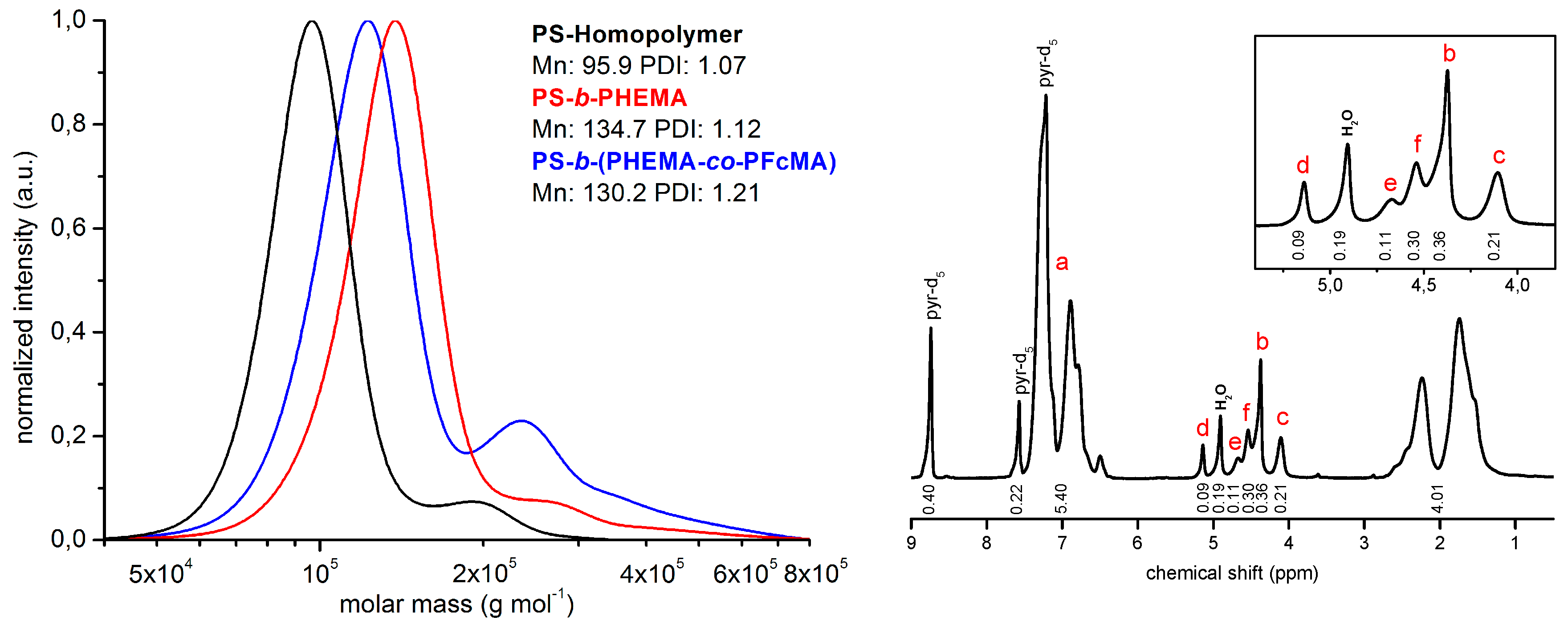
3.1. Polymer Synthesis and Characterization
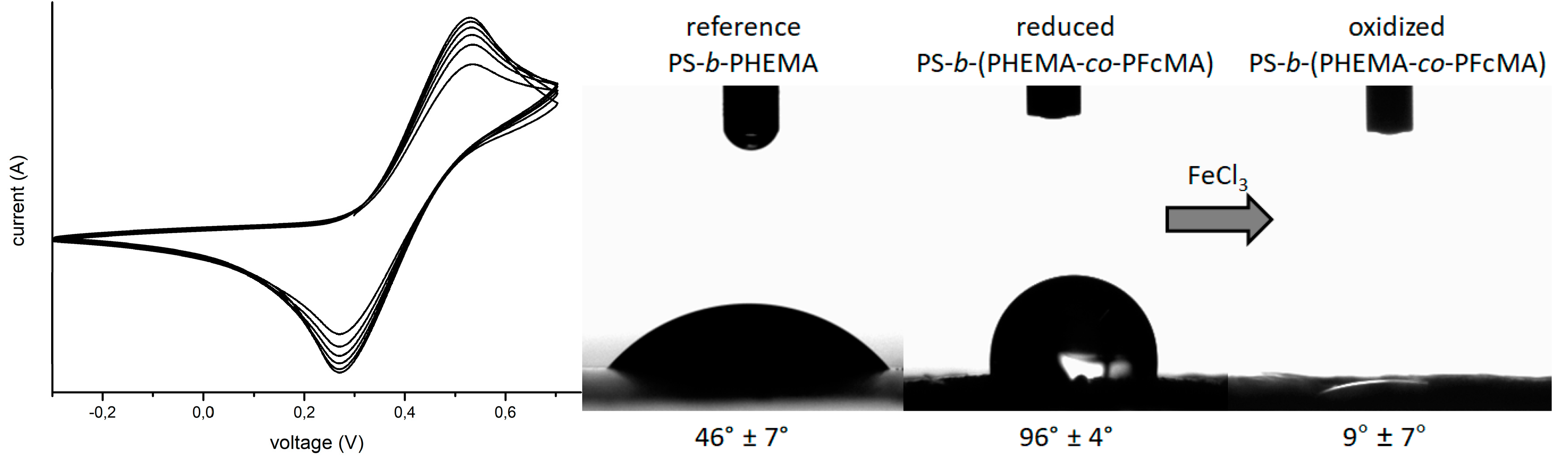
3.2. Functionalization of PHEMA-Containing Block Copolymers with Redox-Responsive Moieties
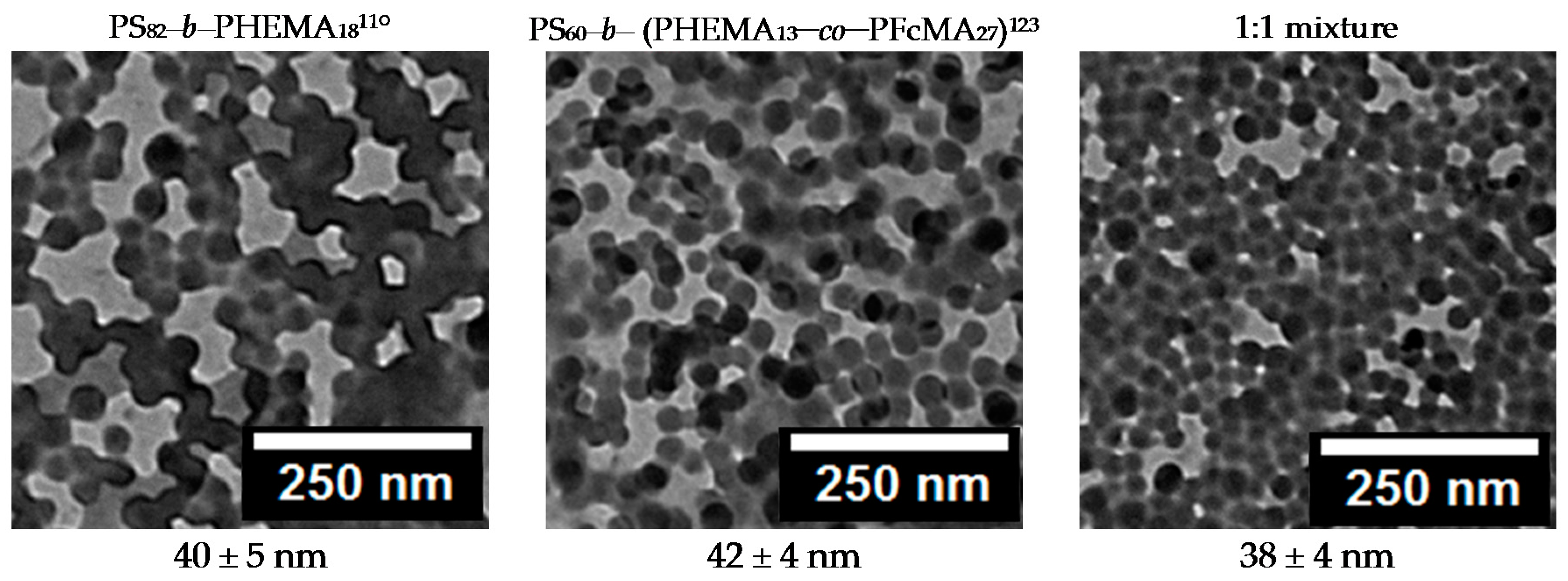
3.3. Membrane Preparation by Self-Assembly and Non-Solvent-Induced Phase Separation (SNIPS) Process
3.4. Stimuli-Responsiveness of the Membranes and Water Flux Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Zhao, C.; Nie, S.; Tang, M.; Sun, S. Polymeric pH-sensitive membranes—A review. Prog. Polym. Sci. 2011, 36, 1499–1520. [Google Scholar] [CrossRef]
- Kaner, P.; Bengani-Lutz, P.; Sadeghi, I.; Asatekin, A. Responsive filtration membranes by polymer self-assembly. Technology 2016, 4, 217–228. [Google Scholar] [CrossRef]
- Chu, L.; Xie, R.; Ju, X. Stimuli-responsive Membranes: Smart Tools for Controllable Mass-transfer and Separation Processes. Chin. J. Chem. Eng. 2011, 19, 891–903. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nature 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Urban, M.W. Recent advances and challenges in designing stimuli-responsive polymers. Prog. Polym. Sci. 2010, 35, 3–23. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Meng, H.; Li, G.; Ibekwe, S.I. A review of stimuli-responsive polymers for smart textile applications. Smart Mater. Struct. 2012, 21, 053001. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, X.; Ito, K.; Hong, L.; Ishizone, T.; Yokoyama, H.; Ulbricht, M. Tunable magneto-responsive mesoporous block copolymer membranes. J. Membr. Sci. 2017, 544, 406–415. [Google Scholar] [CrossRef]
- Cetintas, M.; de Grooth, J.; Hofman, A.; van der Kooij, H.M.; Loos, K.; de Vos, W.M.; Kamperman, M. Free-standing thermo-responsive nanoporous membranes from high molecular weight PS-PNIPAM block copolymers synthesized via RAFT polymerization. Polym. Chem. 2017, 17, 2235–2243. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, X.; Wu, G.; Wen, P.; Wang, L.; Huang, Y.; Huang, X. Programmable Modulation of Membrane Permeability of Proteinosome upon Multiple Stimuli Responses. ACS Macro Lett. 2016, 5, 961–966. [Google Scholar] [CrossRef]
- Lee, B.Y.; Hyun, S.; Jeon, G.; Kim, E.Y.; Kim, J.; Kim, W.J.; Kim, J.K. Bioinspired Dual Stimuli-Responsive Membranous System with Multiple On-Off Gates. ACS Appl. Mater. Interfaces 2016, 8, 11758–11764. [Google Scholar] [CrossRef] [PubMed]
- Lokuge, I.; Wang, X.; Bohn, P.W. Temperature-Controlled Flow Switching in Nanocapillary Array Membranes Mediated by Poly(N-isopropylacrylamide) Polymer Brushes Grafted by Atom Transfer Radical Polymerization. Langmuir 2007, 23, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ito, K.; Hong, L.; Ishizone, T.; Yokoyama, H. Tunable Thermoresponsive Mesoporous Block Copolymer Membranes. Macromolecules 2016, 49, 7886–7896. [Google Scholar] [CrossRef]
- Rüttiger, C.; Mehlhase, S.; Vowinkel, S.; Cherkashinin, G.; Liu, N.; Dietz, C.; Stark, R.W.; Biesalski, M.; Gallei, M. Redox-mediated flux control in functional paper. Polymer 2016, 98, 429–436. [Google Scholar] [CrossRef]
- Scheid, D.; von der Luhe, M.; Gallei, M. Synthesis of Breathing Metallopolymer Hollow Spheres for Redox-Controlled Release. Macromol. Rapid Commun. 2016, 37, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sui, X.; Hempenius, M.A.; Vancso, G.J. Electrografting of Stimuli-Responsive, Redox Active Organometallic Polymers to Gold from Ionic Liquids. J. Am. Chem. Soc. 2014, 136, 7865–7868. [Google Scholar] [CrossRef] [PubMed]
- Scheid, D.; Lederle, C.; Vowinkel, S.; Schäfer, C.G.; Stühn, B.; Gallei, M. Redox- and mechano-chromic response of metallopolymer-based elastomeric colloidal crystal films. J. Mater. Chem. C 2014, 2, 2583–2590. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Elbert, J.; Barner-Kowollik, C.; Gallei, M. Individually Addressable Thermo- and Redox-Responsive Block Copolymers by Combining Anionic Polymerization and RAFT Protocols. Macromol. Rapid Commun. 2014, 35, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jańczewski, D.; Ma, Y.; van Ingen, L.; Ee Sim, C.; Goh, Q.; Xu, J.; Vancso, G.J. Electrochemically controlled release of molecular guests from redox responsive polymeric multilayers and devices. Eur. Polym. J. 2013, 49, 2477–2484. [Google Scholar] [CrossRef]
- Elbert, J.; Gallei, M.; Rüttiger, C.; Brunsen, A.; Didzoleit, H.; Stühn, B.; Rehahn, M. Ferrocene Polymers for Switchable Surface Wettability. Organometallics 2013, 32, 5873–5878. [Google Scholar] [CrossRef]
- Staff, R.H.; Gallei, M.; Mazurowski, M.; Rehahn, M.; Berger, R.; Landfester, K.; Crespy, D. Patchy nanocapsules of poly(vinylferrocene)-based block copolymers for redox-responsive release. ACS Nano 2012, 6, 9042–9049. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Martin, C.R. Redox Modulation of Electroosmotic Flow in a Carbon Nanotube Membrane. J. Am. Chem. Soc. 2004, 126, 6226–6227. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mitta, G.; Marmisolle, W.A.; Trautmann, C.; Toimil-Molares, M.E.; Azzaroni, O. Nanofluidic Diodes with Dynamic Rectification Properties Stemming from Reversible Electrochemical Conversions in Conducting Polymers. J. Am. Chem. Soc. 2015, 137, 15382–15385. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Tahir, M.N.; Siwy, Z.; Neumann, R.; Tremel, W.; Ensinger, W. Hydrogen peroxide sensing with horseradish peroxidase-modified polymer single conical nanochannels. Anal. Chem. 2011, 83, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ahmed, I.; Ramirez, P.; Nasir, S.; Mafe, S.; Niemeyer, C.M.; Ensinger, W. A redox-sensitive nanofluidic diode based on nicotinamide-modified asymmetric nanopores. Sens. Actuators B Chem. 2017, 240, 895–902. [Google Scholar] [CrossRef]
- Elbert, J.; Krohm, F.; Rüttiger, C.; Kienle, S.; Didzoleit, H.; Balzer, B.N.; Hugel, T.; Stühn, B.; Gallei, M.; Brunsen, A. Polymer-Modified Mesoporous Silica Thin Films for Redox-Mediated Selective Membrane Gating. Adv. Funct. Mater. 2014, 24, 1591–1601. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, W.-F.; Hempenius, M.A.; Möhwald, H.; Vancso, G.J. Redox-controlled molecular permeability of composite-wall microcapsules. Nat. Mater. 2006, 5, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, X.; Sui, X.; Hempenius, M.A.; Vancso, G.J. Breathing Pores on Command: Redox-Responsive Spongy Membranes from Poly(ferrocenylsilane)s. Angew. Chem. Int. Ed. 2014, 53, 13789–13793. [Google Scholar] [CrossRef] [PubMed]
- Folkertsma, L.; Zhang, K.; Czakkel, O.; de Boer, H.L.; Hempenius, M.A.; van den Berg, A.; Odijk, M.; Vancso, G.J. Synchrotron SAXS and Impedance Spectroscopy Unveil Nanostructure Variations in Redox-Responsive Porous Membranes from Poly(ferrocenylsilane) Poly(ionic liquid)s. Macromolecules 2017, 50, 296–302. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Membrane Technology in the Chemical Industry, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Nunes, S.P. Block Copolymer Membranes for Aqueous Solution Applications. Macromolecules 2016, 49, 2905–2916. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Nehache, S.; Deratani, A.; Quemener, D. Filtration membranes from self-assembled block copolymers—A review on recent progress. Eur. Phys. J. Spec. Top. 2015, 224, 1883–1897. [Google Scholar] [CrossRef]
- Gu, Y.; Wiesner, U. Tailoring Pore Size of Graded Mesoporous Block Copolymer Membranes: Moving from Ultrafiltration toward Nanofiltration. Macromolecules 2015, 48, 6153–6159. [Google Scholar] [CrossRef]
- Zhang, Y.; Sargent, J.L.; Boudouris, B.W.; Phillip, W.A. Nanoporous membranes generated from self-assembled block polymer precursors: Quo Vadis? J. Appl. Polym. Sci. 2015, 132, 41683. [Google Scholar]
- She, M.-S.; Lo, T.-Y.; Hsueh, H.-Y.; Ho, R.-M. Nanostructured thin films of degradable block copolymers and their applications. NPG Asia Mater. 2013, 5, e42. [Google Scholar] [CrossRef]
- Jackson, E.A.; Hillmyer, M.A. Nanoporous Membranes Derived from Block Copolymers: From Drug Delivery to Water Filtration. ACS Nano 2010, 4, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.; Ulbricht, M.; Müller, A.H.E. Self-Supporting, Double Stimuli-Responsive Porous Membranes From Polystyrene-block-poly(N,N-dimethylaminoethyl methacrylate) Diblock Copolymers. Adv. Funct. Mater. 2009, 19, 1040–1045. [Google Scholar] [CrossRef]
- Hörenz, C.; Pietsch, C.; Goldmann, A.S.; Barner-Kowollik, C.; Schacher, F.H. Phase Inversion Membranes from Amphiphilic Diblock Terpolymers. Adv. Mater. Interfaces 2015, 2, 1500042. [Google Scholar] [CrossRef]
- Clodt, J.I.; Filiz, V.; Rangou, S.; Buhr, K.; Abetz, C.; Höche, D.; Hahn, J.; Jung, A.; Abetz, V. Double Stimuli-Responsive Isoporous Membranes via Post-Modification of pH-Sensitive Self-Assembled Diblock Copolymer Membranes. Adv. Funct. Mater. 2013, 23, 731–738. [Google Scholar] [CrossRef]
- Qiu, X.; Yu, H.; Karunakaran, M.; Pradeep, N.; Nunes, S.P.; Peinemann, K.-V. Selective Separation of Similarly Sized Proteins with Tunable Nanoporous Block Copolymer Membranes. ACS Nano 2013, 7, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Gallei, M.; Rangou, S.; Filiz, V.; Buhr, K.; Bolmer, S.; Abetz, C.; Abetz, V. The Influence of Magnesium Acetate on the Structure Formation of Polystyrene-block-poly(4-vinylpyridine)-Based Integral-Asymmetric Membranes. Macromol. Chem. Phys. 2013, 214, 1037–1046. [Google Scholar] [CrossRef]
- Nunes, S.P.; Behzad, A.R.; Hooghan, B.; Sougrat, R.; Karunakaran, M.; Pradeep, N.; Vainio, U.; Peinemann, K.V. Switchable pH-Responsive Polymeric Membranes Prepared via Block Copolymer Micelle Assembly. ACS Nano 2011, 5, 3516–3522. [Google Scholar] [CrossRef] [PubMed]
- Peinemann, K.V.; Abetz, V.; Simon, P.F.W. Asymmetric superstructure formed in a block copolymer via phase separation. Nat. Mater. 2007, 6, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Schöttner, S.; Schaffrath, H.-J.; Gallei, M. Poly(2-hydroxyethyl methacrylate)-Based Amphiphilic Block Copolymers for High Water Flux Membranes and Ceramic Templates. Macromolecules 2016, 49, 7286–7295. [Google Scholar] [CrossRef]
- Breit, B.; Breuninger, D. Practical Synthesis of Enantiomerically Pure 2-(Diphenylphosphanyl)ferrocene Carboxylic Acid. Synthesis 2005, 16, 2782–2786. [Google Scholar] [CrossRef]
- Sanders, R.; Mueller-Westerhoff, U.T. The lithiation of ferrocene and ruthenocene: A retraction and an improvement. J. Organomet. Chem. 1996, 512, 219–224. [Google Scholar] [CrossRef]
- Rieger, J. The Glass Transition Temperature of Polystyrene. J. Therm. Anal. 1996, 46, 965–972. [Google Scholar] [CrossRef]
- Zhu, P.; Song, F.; Ma, P.; Wang, Y.; Chen, C.; Feng, J. Morphology-controlled self-assembly of a ferrocene–porphyrin based NO2gas sensor: Tuning the semiconducting nature via solvent–solute interaction. J. Mater. Chem. C 2016, 4, 10471–10478. [Google Scholar] [CrossRef]
- Gallei, M.; Klein, R.; Rehahn, M. Silacyclobutane-Mediated Re-Activation of “Sleeping” Polyvinylferrocene Macro-Anions: A Powerful Access to Novel Metalloblock Copolymers. Macromolecules 2010, 43, 1844–1854. [Google Scholar] [CrossRef]
- Mazurowski, M.; Sondergeld, K.; Elbert, J.; Kim, C.J.; Li, J.; Frielinghaus, H.; Gallei, M.; Stühn, B.; Rehahn, M. Polystyrene Brushes on Fully Deuterated Organic Nanoparticles by Surface-Initiated Nitroxide-Mediated Radical Polymerization. Macromol. Chem. Phys. 2013, 214, 1094–1106. [Google Scholar] [CrossRef]
- Rüttiger, C.; Appold, M.; Didzoleit, H.; Eils, A.; Dietz, C.; Stark, R.W.; Stühn, B.; Gallei, M. Structure Formation of Metallopolymer-Grafted Block Copolymers. Macromolecules 2016, 49, 3415–3426. [Google Scholar] [CrossRef]
- Scheid, D.; Cherkashinin, G.; Ionescu, E.; Gallei, M. Single-source magnetic nanorattles by using convenient emulsion polymerization protocols. Langmuir 2014, 30, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Mazurowski, M.; Gallei, M.; Li, J.; Didzoleit, H.; Stühn, B.; Rehahn, M. Redox-Responsive Polymer Brushes Grafted from Polystyrene Nanoparticles by Means of Surface Initiated Atom Transfer Radical Polymerization. Macromolecules 2012, 45, 8970–8981. [Google Scholar] [CrossRef]
- Nunes, S.P.; Karunakaran, M.; Pradeep, N.; Behzad, A.R.; Hooghan, B.; Sougrat, R.; He, H.; Peinemann, K.V. From micelle supramolecular assemblies in selective solvents to isoporous membranes. Langmuir 2011, 27, 10184–10190. [Google Scholar] [CrossRef] [PubMed]
- Radjabian, M.; Abetz, C.; Fischer, B.; Meyer, A.; Abetz, V. Influence of Solvent on the Structure of an Amphiphilic Block Copolymer in Solution and in Formation of an Integral Asymmetric Membrane. ACS Appl. Mater. Interfaces 2017, 9, 31224–31234. [Google Scholar] [CrossRef] [PubMed]
- Phillip, W.A.; O’Neill, B.; Rodwogin, M.; Hillmyer, M.A.; Cussler, E.L. Self-assembled block copolymer thin films as water filtration membranes. ACS Appl. Mater. Interfaces 2010, 2, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.P.; Sougrat, R.; Hooghan, B.; Anjum, D.H.; Behzad, A.R.; Zhao, L.; Pradeep, N.; Pinnau, I.; Vainio, U.; Peinemann, K.V. Ultraporous Films with Uniform Nanochannels by Block Copolymer Micelles Assembly. Macromolecules 2010, 43, 8079–8085. [Google Scholar] [CrossRef]
- Su, X.; Tan, K.-J.; Elbert, J.; Rüttiger, C.; Gallei, M.; Jamison, T.F.; Hatton, T.A. Asymmetric Faradaic systems for selective electrochemical separations. Energy Environ. Sci. 2017, 10, 1272–1283. [Google Scholar] [CrossRef]


| Block Copolymer | Mn a | Đ b | xHEMA c |
|---|---|---|---|
| PS75-b-PHEMA2580 | 80 | 1.14 | 21.3 |
| PS84-b-PHEMA16100 | 100 | 1.07 | 13.0 |
| PS82-b-PHEMA18106 | 106 | 1.12 | 14.9 |
| PS82-b-PHEMA18110 | 110 | 1.18 | 14.5 |
| PS75-b-PHEMA25112 | 112 | 1.11 | 20.6 |
| Block Copolymer | Mn a | Đ b | DF c |
|---|---|---|---|
| PS72-b- (PHEMA17–co–PFcMA11)112 | 112 | 1.41 | 19 |
| PS61-b- (PHEMA10–co–PFcMA29)113 | 113 | 1.77 | 53 |
| PS77-b- (PHEMA13–co–PFcMA11)115 | 115 | 1.59 | 30 |
| PS72-b- (PHEMA5–co–PFcMA23)117 | 117 | 1.76 | 66 |
| PS78-b- (PHEMA14–co–PFcMA17)121 | 121 | 1.37 | 20 |
| PS60-b- (PHEMA13–co–PFcMA27)123 | 123 | 1.21 | 43 |
| PS64-b- (PHEMA12–co–PFcMA24)132 | 132 | 1.66 | 44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöttner, S.; Hossain, R.; Rüttiger, C.; Gallei, M. Ferrocene-Modified Block Copolymers for the Preparation of Smart Porous Membranes. Polymers 2017, 9, 491. https://doi.org/10.3390/polym9100491
Schöttner S, Hossain R, Rüttiger C, Gallei M. Ferrocene-Modified Block Copolymers for the Preparation of Smart Porous Membranes. Polymers. 2017; 9(10):491. https://doi.org/10.3390/polym9100491
Chicago/Turabian StyleSchöttner, Sebastian, Rimjhim Hossain, Christian Rüttiger, and Markus Gallei. 2017. "Ferrocene-Modified Block Copolymers for the Preparation of Smart Porous Membranes" Polymers 9, no. 10: 491. https://doi.org/10.3390/polym9100491





