Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Material
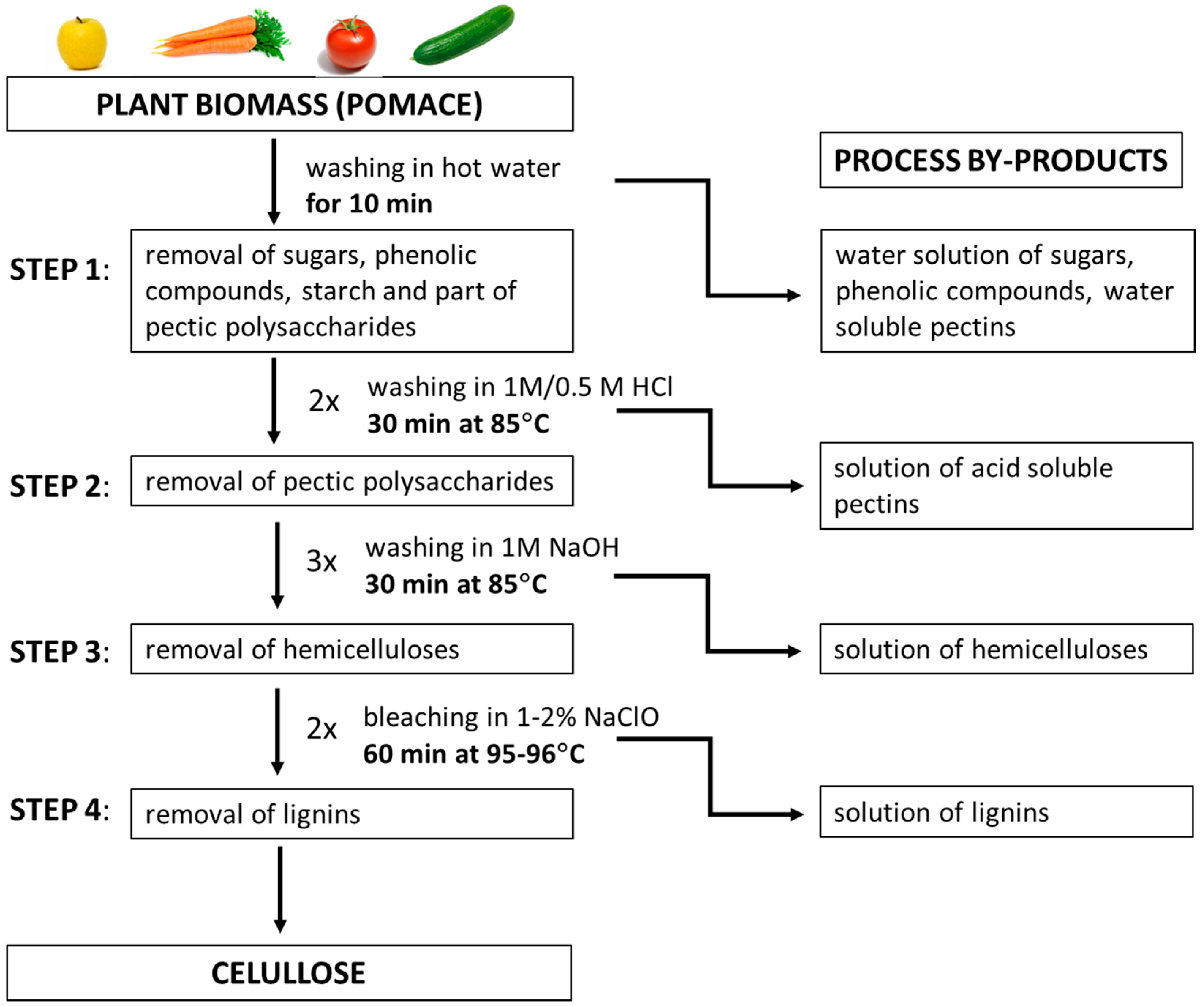
2.2. Fractioning of Plant Biomass
2.3. Characterization of Plant Material
2.4. The Hemicelluloses Yield Was Estimated
2.5. Spectroscopic Investigation of Cellulose
2.6. Scanning Electron Microscopy (SEM)
2.7. AFM Images and Microfibrils Diameter Measurement
2.8. X-ray Diffractometry (XRD)
3. Results
3.1. The Composition of Plant Pomaces
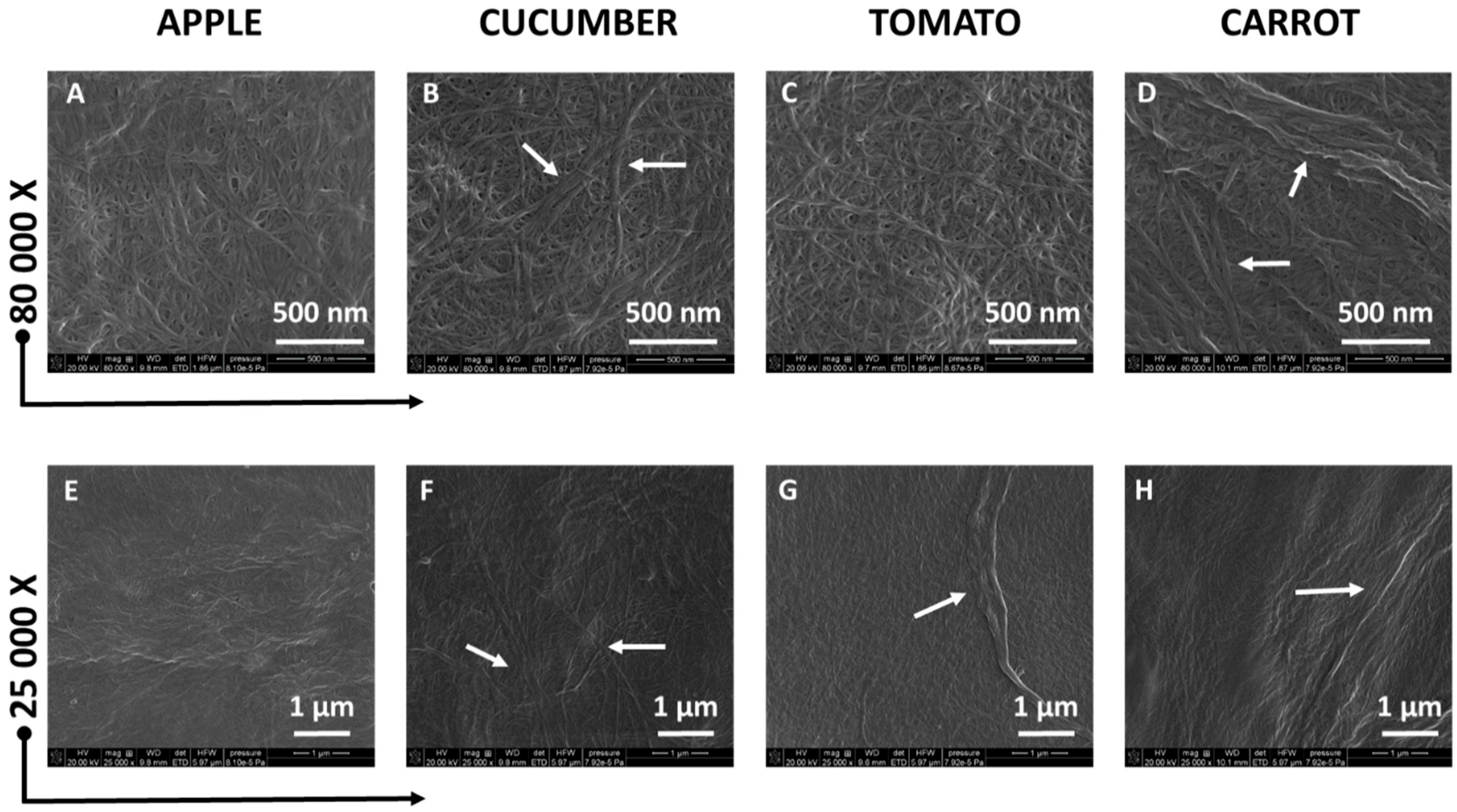
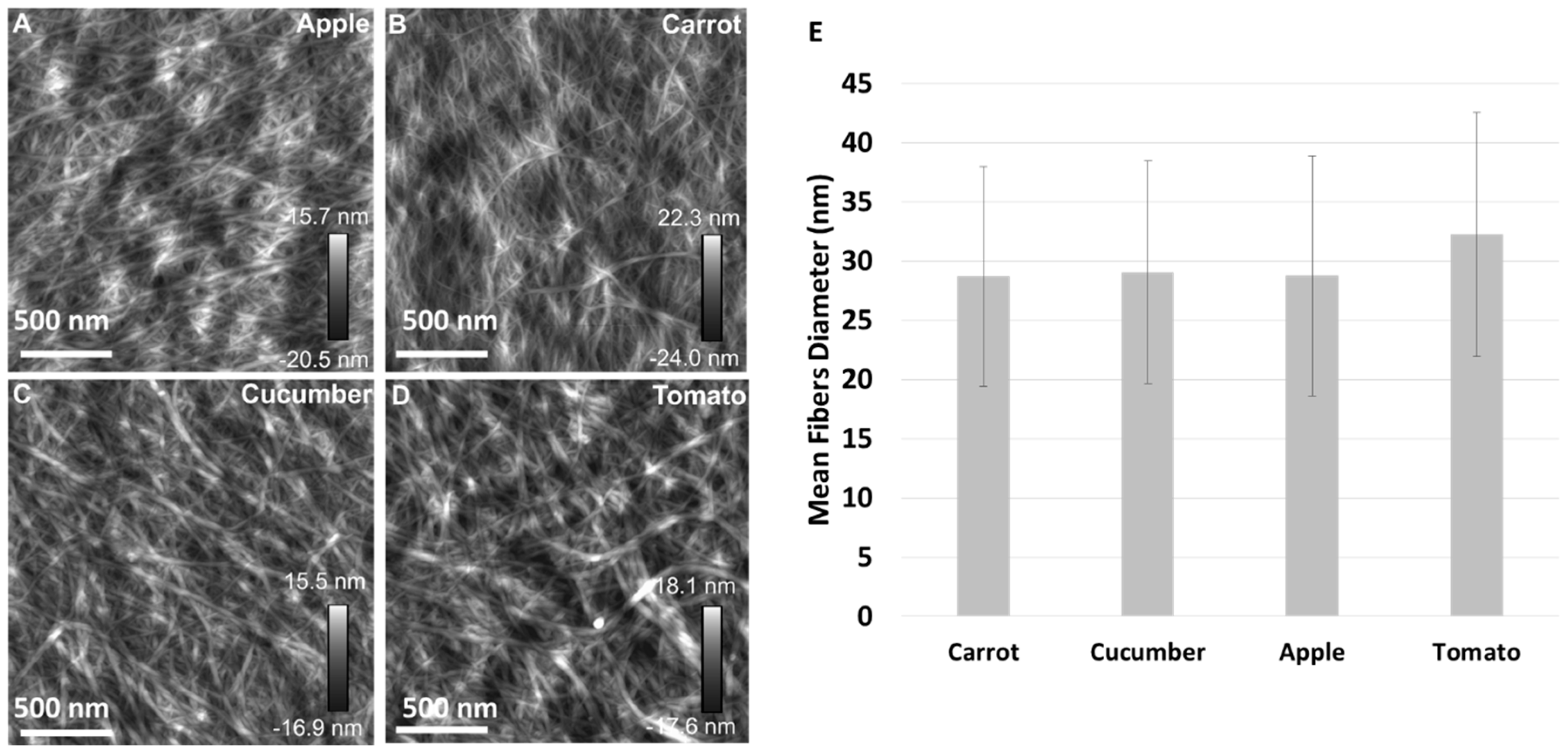
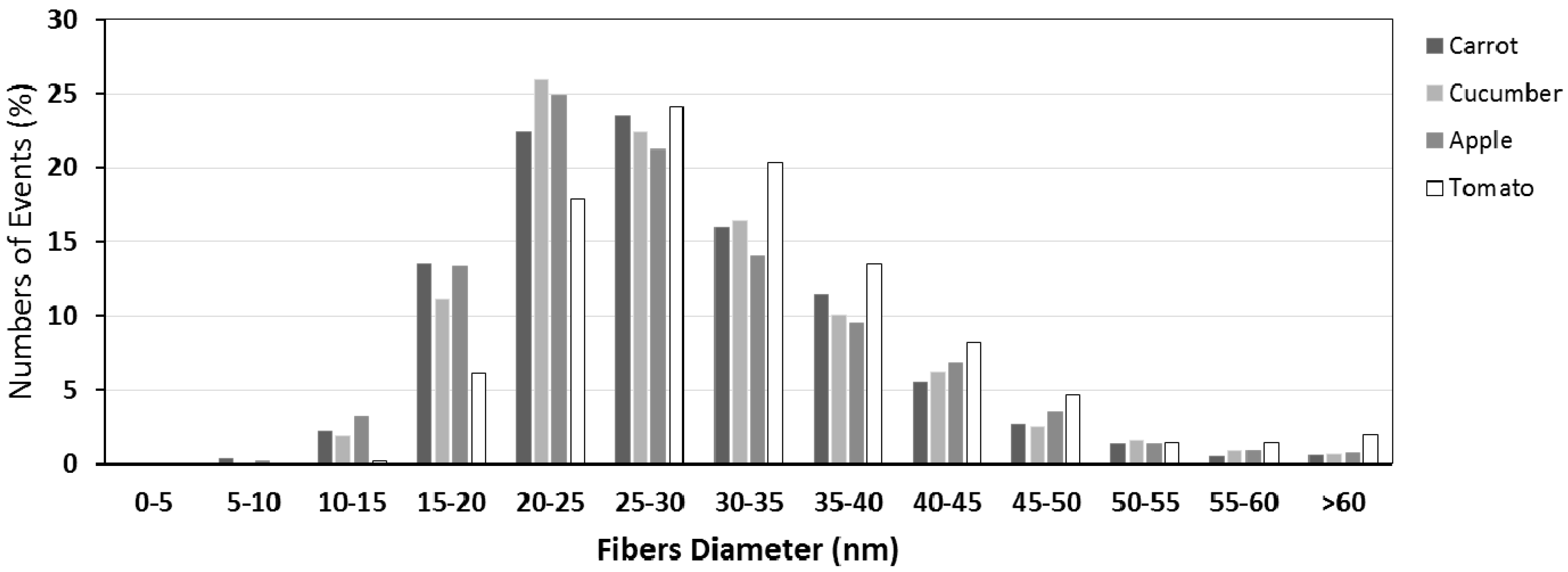
3.2. Cellulose Morphology Analysis: SEM and AFM
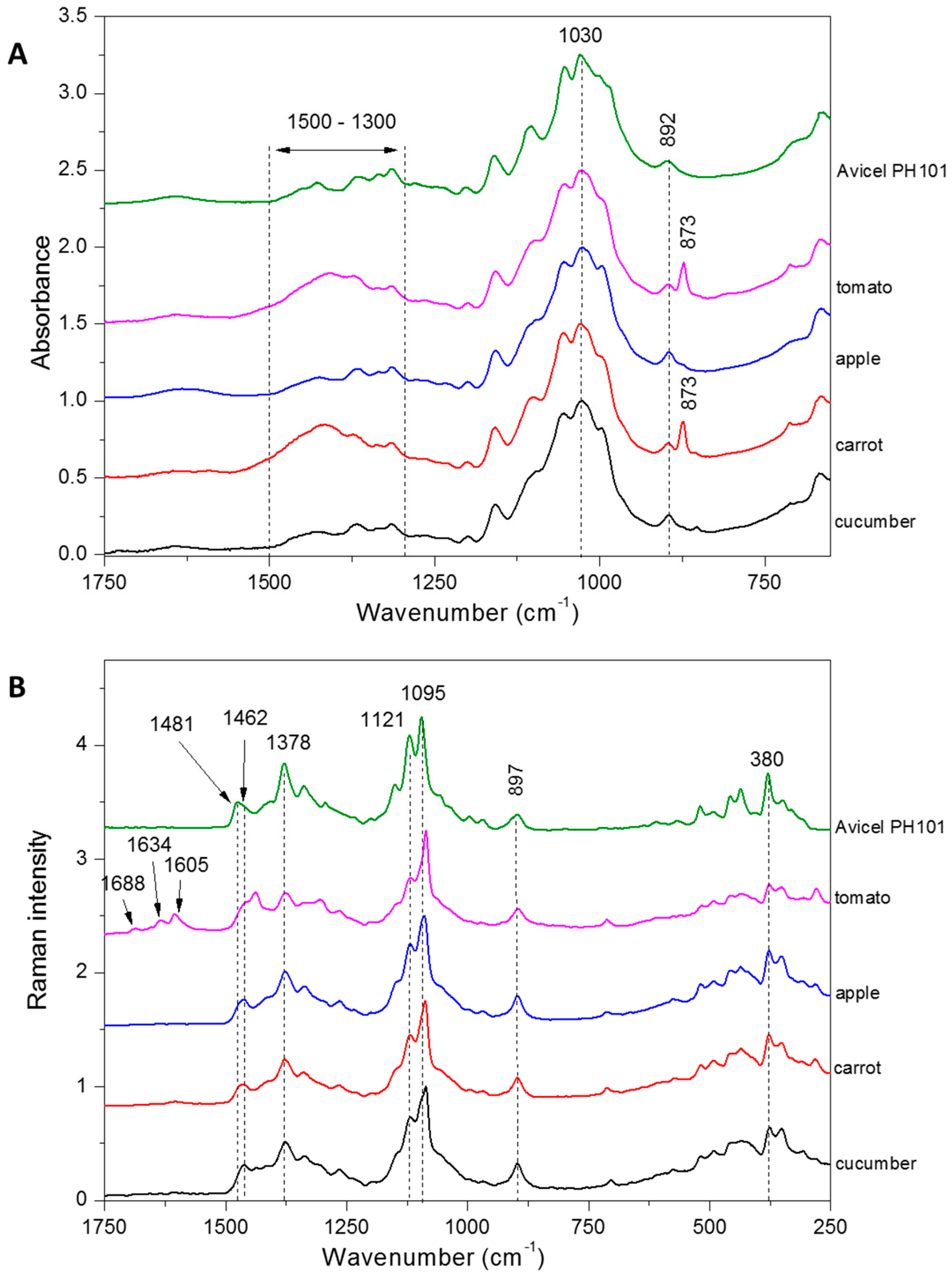
3.3. Cellulose Structure
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and vegetable processing by-/co-products: Can they be used as functional feed ingredients in animal nutrition to produce novel value-added products? In Proceedings of the 3rd International ISEKI Food Conference ISEKI_Food 2014, Athens, Greece, 21–23 May 2014. [Google Scholar]
- Chylińska, M.; Szymańska-Chargot, M.; Kruk, B.; Zdunek, A. Study on dietary fiber by Fourier transform-infrared spectroscopy and chemometric methods. Food Chem. 2016, 196, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Nawirska, A.; Uklańska, C. Waste products from fruit and vegetable processing as potential sources for food enrichment in dietary fibre. Acta Sci. Pol. Technol. Aliment. 2008, 7, 35–42. [Google Scholar]
- Nawrocka, A.; Miś, A.; Szymańska-Chargot, M. Characteristics of relationships between structure of gluten proteins and dough rheology—Influence of dietary fibres studied by FT-Raman spectroscopy. Food Biophys. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Bakshi, M.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy. Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Cybulska, J.; Zdunek, A. Sensing the structural differences of cellulose from apple and bacterial cell wall materials by Raman and FT-IR spectroscopy. Sensors 2011, 11, 5543–5560. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Barakat, A.; de Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; Lopes, A.M.D.C.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xiao, W.; Ji, G.; Zhang, Y.; Cao, Y.; Han, L. Regularity and mechanism of wheat straw properties change in ball milling process at cellular scale. Bioresour Technol. 2017, 241, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. 2016. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Agricultural_products (accessed on 18 September 2017).
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations (FAOSTAT). Food and Agricultural Commodities Production for, Production of Tomato by Countries. 2014. Available online: http://faostat3.fao.org/home/E 2014 (accessed on 18 September 2017).
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Kaur, D.; Wani, A.A.; Oberoi, D.P.S.; Sogi, D. Effect of extraction conditions on lycopene extractions from tomato processing waste skin using response surface methodology. Food Chem. 2008, 108, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Cha, M.; Shin, S.R.; Kim, K.S. Enzymatic production of a soluble-fibre hydrolysate from carrot pomace and its sugar composition. Food Chem. 2005, 92, 151–157. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K.; Chau, K.V. Development of perforation-mediated modified atmosphere packaging for fresh-cut vegetables. In Processing Foods: Quality Optimization and Process Assessment; Oliveira, F.A.R., Oliveira, J.C., Eds.; CRC Press: Boca Raton, FL, USA, 1999; p. 389. [Google Scholar]
- Tarazona-Díaz, M.P.; Aguayo, E. Assessment of by-products from fresh-cut products for reuse as bioactive compounds. Food Sci. Technol. Int. 2013, 19, 439–446. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.E.; Rodrigues Marcelino, H.; Salgado Gomes, M.C.; Oliveira, E.; Nagashima, T., Jr.; Tabosa Egito, E. Xylan, a promising hemicellulose for pharmaceutical use, products and applications of biopolymers. In Products and Application of Biopolymers; Verbeek, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 61–84. [Google Scholar]
- Persin, Z.; Stana-Kleinschek, K.; Foster, T.J.; Van Dam, J.E.G.; Boeriu, C.G.; Navard, P. Challenges and opportunities in polysaccharides research and technology: The EPNOE views for the next decade in the areas of materials, food and health care. Carbohydr. Polym. 2011, 84, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, P.B.S.; Coelho, L.C.B.B.; Teixeira, J.A.; Carneiro-da-Cunha, M.G. Approaches in biotechnological applications of natural polymers. AIMS Mol. Sci. 2016, 3, 386–425. [Google Scholar] [CrossRef] [Green Version]
- Kollarigowda, R.H. Novel polysaccharide nanowires; synthesized from pectin-modified methacrylate. RSC Adv. 2015, 5, 102143–102146. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Zdunek, A. Rheological and chemical properties of pectin enriched fractions from different sources extracted with citric acid. Carbohydr. Polym. 2017, 156, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Voragen, F.; Beldman, G.; Schols, H. Chemistry and Enzymology of Pectins. In Advanced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 379–398. [Google Scholar]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New insights into an old polymer are starting to gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, J.-X.; Xu, F.; Sun, R.-C.; Baird, M.S. Influence of steam pressure on the physicochemical properties of degraded hemicelluloses obtained from steam-exploded Lespedeza stalks. BioResources 2010, 5, 1717–1732. [Google Scholar]
- Menon, V.; Prakash, G.; Rao, M. Value added products from hemicelluloses: Biotechnological perspective. Glob. J. Biochem. 2010, 1, 36–67. [Google Scholar]
- Hallac, B.B.; Ragauska, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefin. 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Shokri, J.; Adibkia, K. Application of Cellulose and Cellulose Derivatives in Pharmaceutical Industries. In Cellulose—Medical, Pharmaceutical and Electronic Applications; van de Ven, T., Godbout, L., Eds.; InTech: Rijeka, Croatia, 2013; pp. 47–66. ISBN 978-953-51-1191-7. [Google Scholar]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.H.L.; Morais, A.R.C.; Da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current Pretreatment Technologies for the Development of Cellulosic Ethanol and Biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Lemeunier, C.; Thibault, J.F. Alkaline extraction of xyloglucan from depectinised apple pomace: optimization and characterisation. Carbohydr. Polym. 1995, 28, 209–216. [Google Scholar] [CrossRef]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Anzelmo, G.; Fiorentino, G.; Nicolaus, B.; Tommonaro, G.P. Di Donato, Polysaccharides from Wastes of Vegetable Industrial Processing: New Opportunities for Their Eco-Friendly Re-Use. In Biotechnology of Biopolymers; Elnashar, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 33–56. [Google Scholar]
- Szymańska-Chargot, M.; Chylińska, M.; Farooq, M. Method of Nanocellulose Preparation form Fruit Pomace, Nanocellulose Films and Method of Nanocellulose Films Preparation. Patent Application n. P.420890, 17 March 2017. (In Polish). [Google Scholar]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Szymańska-Chargot, M.; Zdunek, A. Use of FT-IR spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Chylińska, M.; Kruk, B.; Zdunek, A. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr. Polym. 2015, 115, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J.; Zdunek, A.; Psonka-Antonczyk, K.M.; Stokke, B.T. The relation of apple texture with cell wall nanostructure studied using an atomic force microscopy. Carbohydr. Polym. 2013, 92, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M.; Szaja, A.; Szymańska-Chargot, M. Hydrodynamic cavitation of brewery spent grain diluted by wastewater. Chem. Eng. J. 2017, 313, 946–956. [Google Scholar] [CrossRef]
- Renard, C.M.G.C. Variability in cell wall preparations: Quantification and comparison of common methods. Carbohydr. Polym. 2005, 60, 512–522. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Gehin-Delval, C. Physicochemical properties of cell wall materials from apple, kiwifruit and tomato. Eur. Food Res. Technol. 2008, 227, 607–618. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, B. Macromolecular Physic; Academic Press: New York, NY, USA, 1973; Volume 1. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1962, 29, 786–794. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M.; Heitor, L.; Zattera, A.J. Native cellulose: structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-J.; Kamdem, D.P. Chemical composition, crystallinity and crystallite cellulose size in Populus hybrids and aspen. Cellul. Chem. Technol. 2009, 43, 229–234. [Google Scholar]
- Zdunek, A.; Kozioł, A.; Pieczywek, P.M.; Cybulska, J. Evaluation of the Nanostructure of Pectin, Hemicellulose and Cellulose in the Cell Walls of Pears of Different Texture and Firmness. Food Bioprocess Technol. 2014, 7, 3525–3535. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Shulz, H.; Baranska, M. Identification and quantification of valuable plant substances. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Rösch, P.; Schmitt, M.; Popp, J.; Zdunek, A. Raman imaging of changes in the polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta 2016, 243, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant Methods 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schenzel, K.; Fischer, S.; Brendler, E. New method for determining the degree of cellulose I crystallinity by means of FT Raman spectroscopy. Cellulose 2005, 12, 223–231. [Google Scholar] [CrossRef]
- Gierlinger, N.; Keplinger, T.; Harrington, M. Imaging of plan cell walls by confocal Raman microscopy. Nat. Protoc. 2012, 7, 1694–1708. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Pieczywek, P.M.; Chylińska, M.; Zdunek, A. Hyperspectral image analysis of Raman maps of plant cell walls for blind spectra characterization by nonnegative matrix factorization algorithm. Chemom. Intell. Lab. Syst. 2016, 151, 136–145. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Ardjmand, M.; Najafi, G. Lignocellulosic biomass to bioethanol, a comprehensive review with focus on pretreatment. Renew. Sustain. Energy. Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Gindl, W. Cellulose Fibril- and Whisker-Reinforced Polymer Nanocomposites. In Recent Advances in Polymer Nanocomposites; Thomas, S., Zaikov, G., Valsaraj, K.T., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 269–284. [Google Scholar]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Khor, W.C.; Rabaey, K.; Vervaeren, H. Low temperature calcium hydroxide treatment enhances anaerobic methane production from (extruded) biomass. Bioresour. Technol. 2015, 176, 181–188. [Google Scholar] [CrossRef] [PubMed]
| POMACE | Dry Matter Content | Cellulose | Hemicellulose | Lignin | NDF |
|---|---|---|---|---|---|
| % | g/100 g DRY POMACE | g/100 g DRY POMACE | g/100 g DRY POMACE | g/100 g DRY POMACE | |
| Carrot | 13.10 (±0.17) | 10.01 (±0.20) | 5.73 (±0.27) | 2.50 (±0.19) | 18.24 (±0.28) |
| Tomato | 6.03 (±0.22) | 8.60 (±0.34) | 5.33 (±0.92) | 5.85 (±0.32) | 19.77 (±1.52) |
| Cucumber | 5.57 (±0.05) | 16.13 (±0.90) | 4.33 (±1.32) | 4.51 (±0.54) | 24.98 (±0.52) |
| Apple | 17.84 (±0.50) | 8.81 (±0.51) | 5.44 (±0.49) | 2.98 (±0.67) | 17.22 (±1.21) |
| POMACE | Polysaccharide Fraction | ||||
|---|---|---|---|---|---|
| KOH | WSP | CSP | DASP | TOTAL | |
| mg Xyl/g CWM | mg GalA/g CWM | mg GalA/g CWM | mg GalA/g CWM | mg GalA/g CWM | |
| Carrot | 99.88 (±0.49) | 12.21 (±0.15) | 43.77 (±0.60) | 130.49 (±0.59) | 186.48 (±1.34) |
| Tomato | 89.64 (±1.60) | 60.57 (±0.63) | 67.02 (±0.44) | 8.29 (±0.08) | 135.87 (±1.15) |
| Cucumber | 56.24 (±1.94) | 16.25 (±0.91) | 71.84 (±0.73) | 71.68 (±1.75) | 159.76 (±3.39) |
| Apple | 70.93 (±1.34) | 22.40 (±1.26) | 51.29 (±0.54) | 78.41 (±0.74) | 152.10 (±2.55) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. https://doi.org/10.3390/polym9100495
Szymańska-Chargot M, Chylińska M, Gdula K, Kozioł A, Zdunek A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers. 2017; 9(10):495. https://doi.org/10.3390/polym9100495
Chicago/Turabian StyleSzymańska-Chargot, Monika, Monika Chylińska, Karolina Gdula, Arkadiusz Kozioł, and Artur Zdunek. 2017. "Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces" Polymers 9, no. 10: 495. https://doi.org/10.3390/polym9100495





