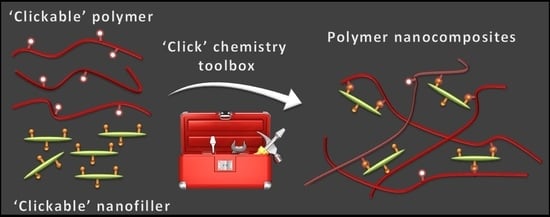
Polymer Nanocomposites via Click Chemistry Reactions
Abstract
:1. Introduction
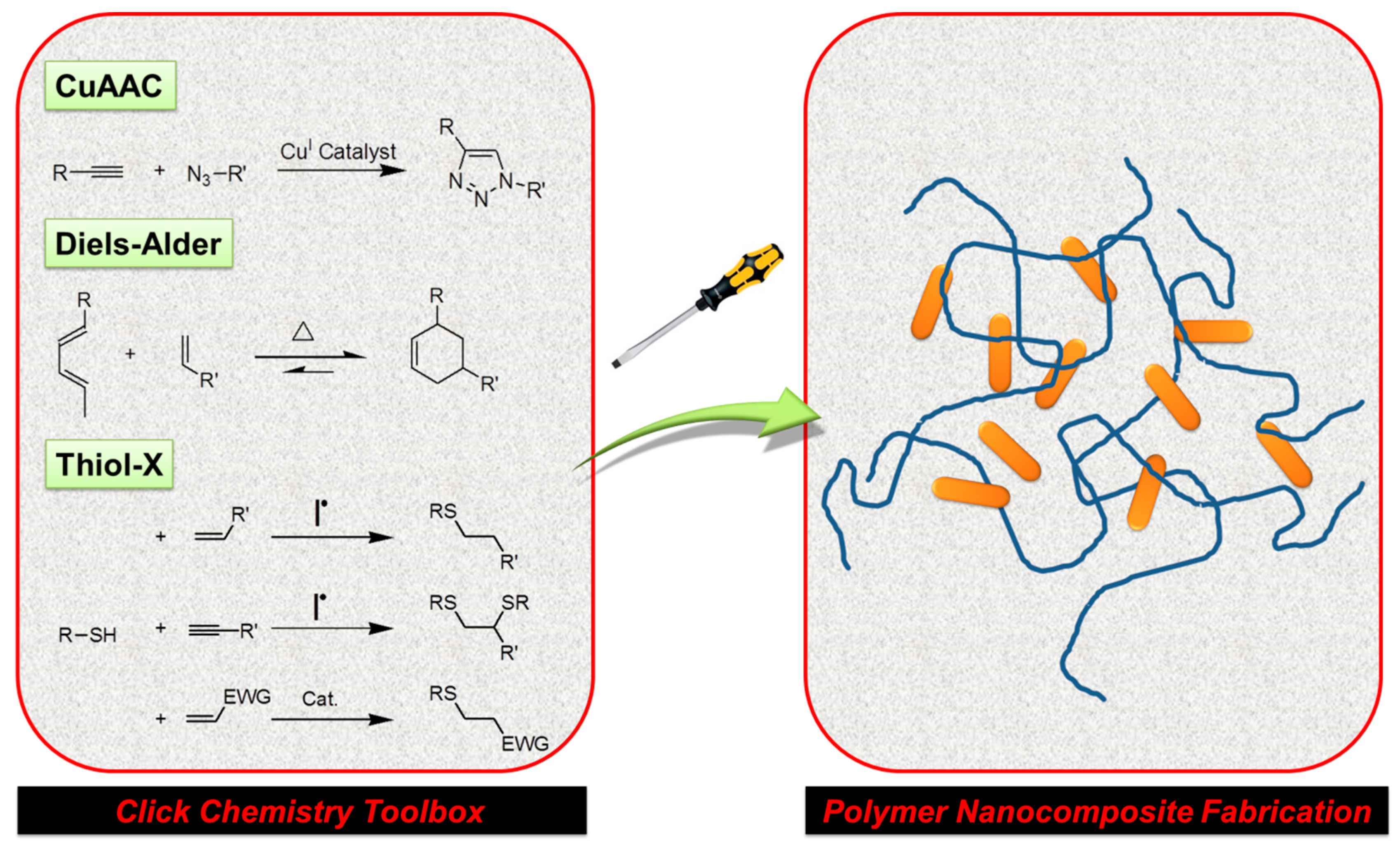
2. Click Chemistry-Based Methodologies in Polymer Nanocomposite Fabrication
2.1. Polymer Nanocomposites via CuAAC and Metal-Free Click Reactions
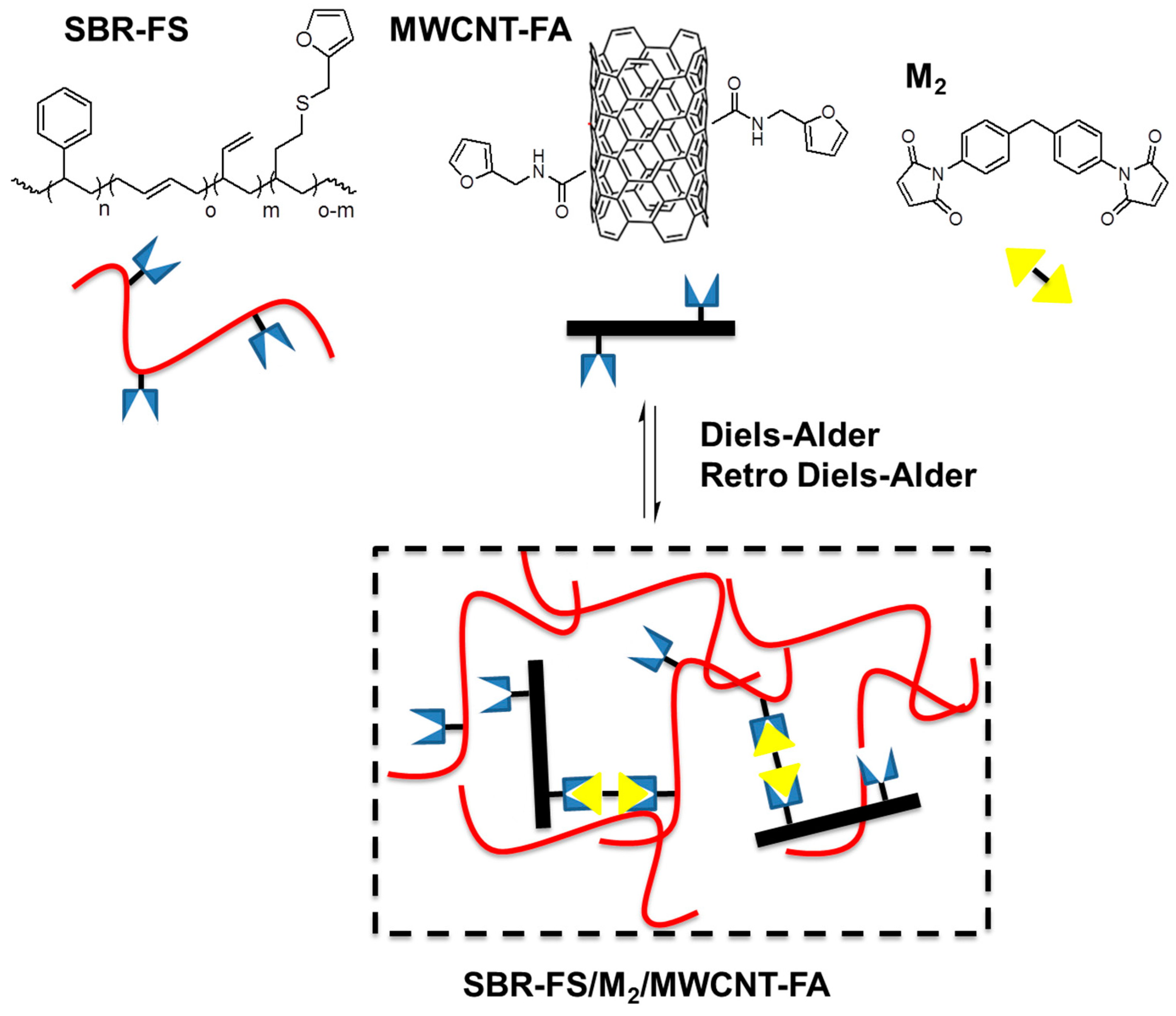
2.2. Polymer Nanocomposites via Diels–Alder Reactions
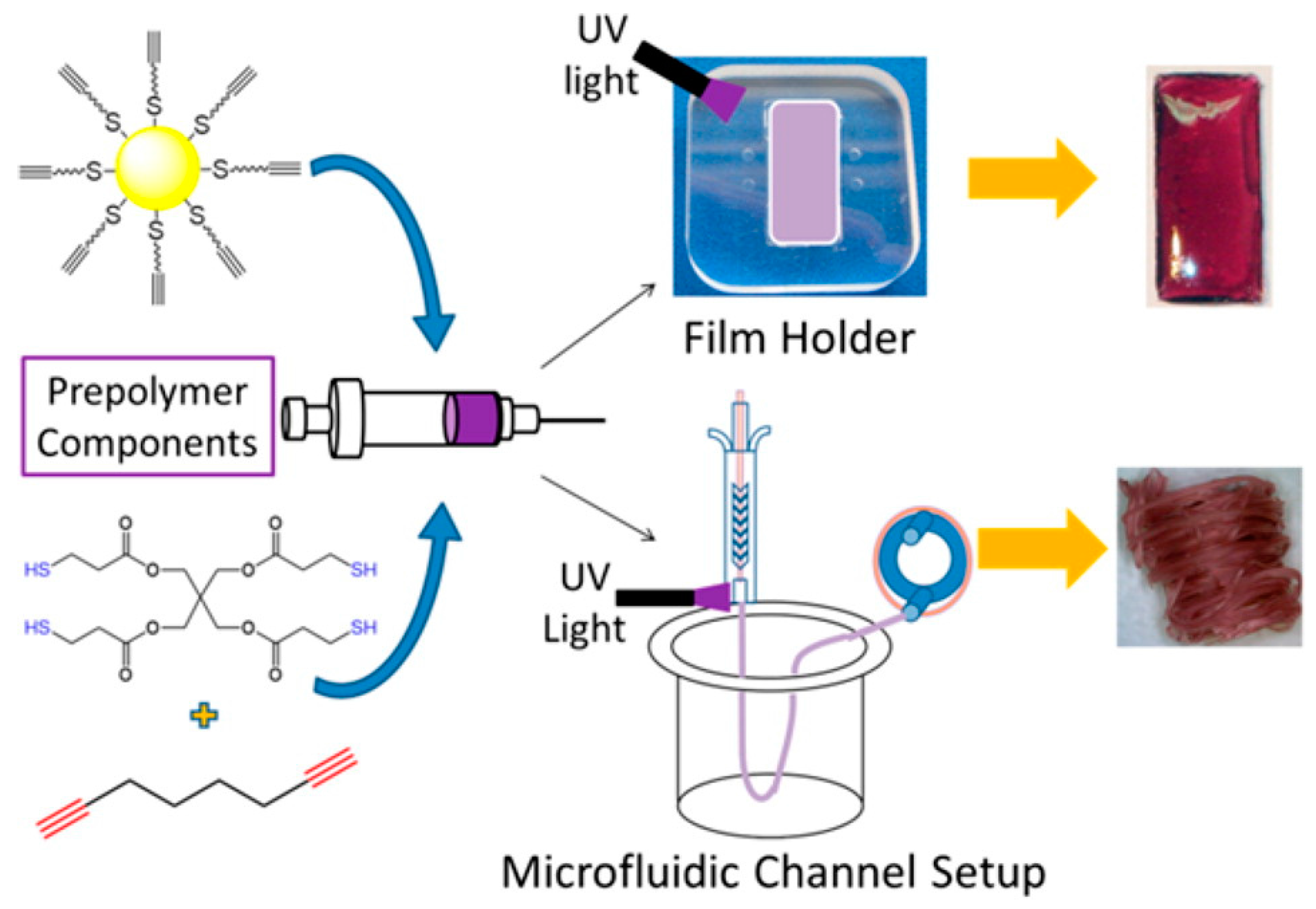
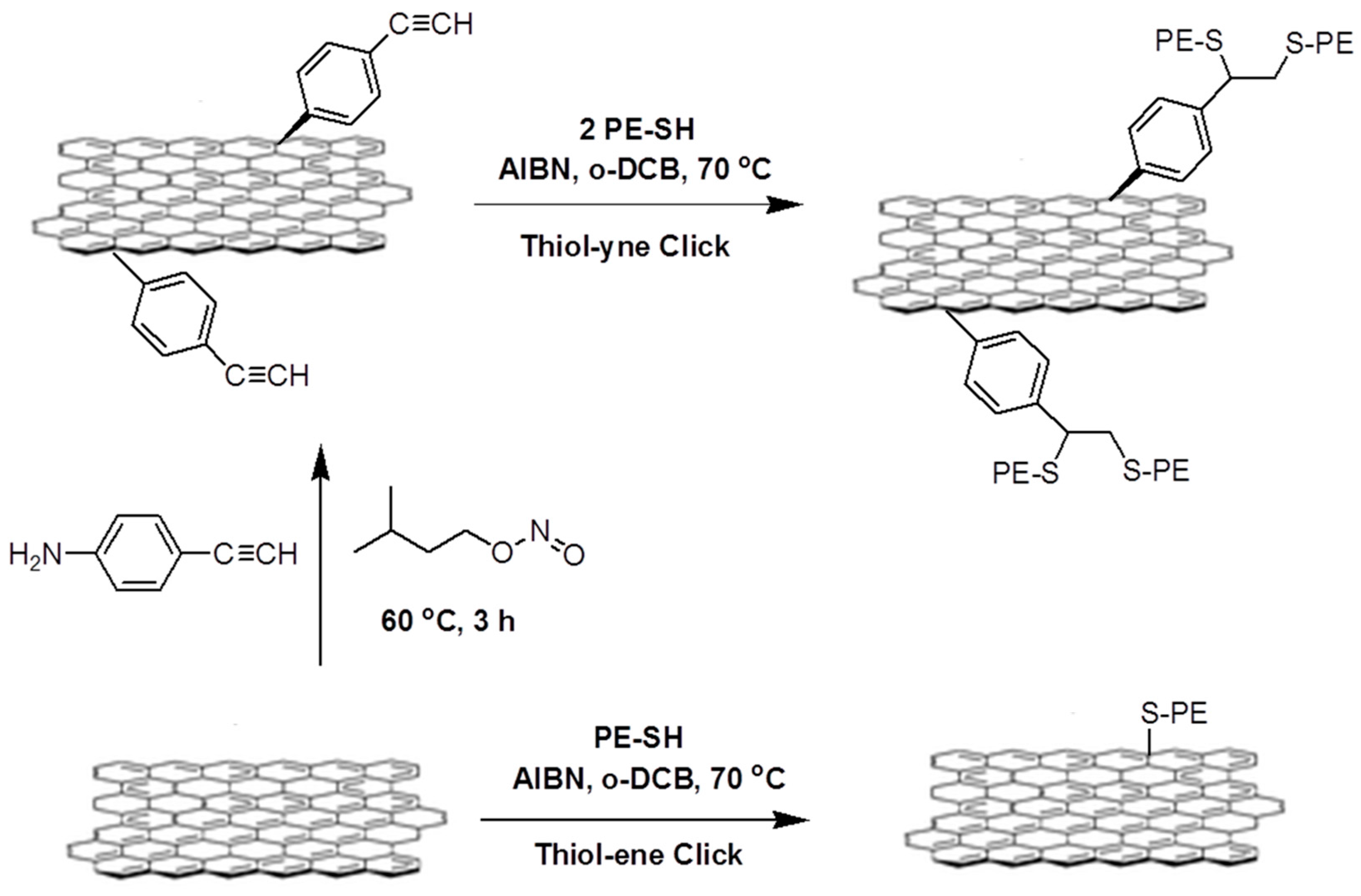
2.3. Polymer Nanocomposites via Thiol-X Reactions
3. General Overview and Future Perspectives
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Liu, T.; Burger, C.; Chu, B. Nanofabrication in polymer matrices. Prog. Polym. Sci. 2003, 28, 5–26. [Google Scholar] [CrossRef]
- Anandhan, S.; Bandyopadhyay, S. Polymer nanocomposites: From synthesis to applications. In Nanocomposites and Polymers with Analytical Methods; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Kolb, H.C.; Finn, M.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ chemistry in polymer and material science: An update. Macromol. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Vogt, A.P. Macromolecular engineering through click chemistry and other efficient transformations. Macromolecules 2010, 43, 1–13. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-catalyzed azide–alkyne cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Tasdelen, M.A.; Kiskan, B.; Yagci, Y. Externally stimulated click reactions for macromolecular syntheses. Prog. Polym. Sci. 2016, 52, 19–78. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites–processing, properties and applications: A review. Compos. Part B 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Alcock, J.R. Epoxy-based fibre reinforced nanocomposites. Adv. Eng. Mater. 2007, 9, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Vo, T.; Inam, F. Epoxy/graphene nanocomposites–processing and properties: A review. RSC Adv. 2015, 5, 73510–73524. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase:[1,2,3]-triazoles by regiospecific copper (I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic In Vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, N.; Huang, J.; Dufresne, A. Highly alkynyl-functionalization of cellulose nanocrystals and advanced nanocomposites thereof via click chemistry. Polym. Chem. 2015, 6, 4385–4395. [Google Scholar] [CrossRef]
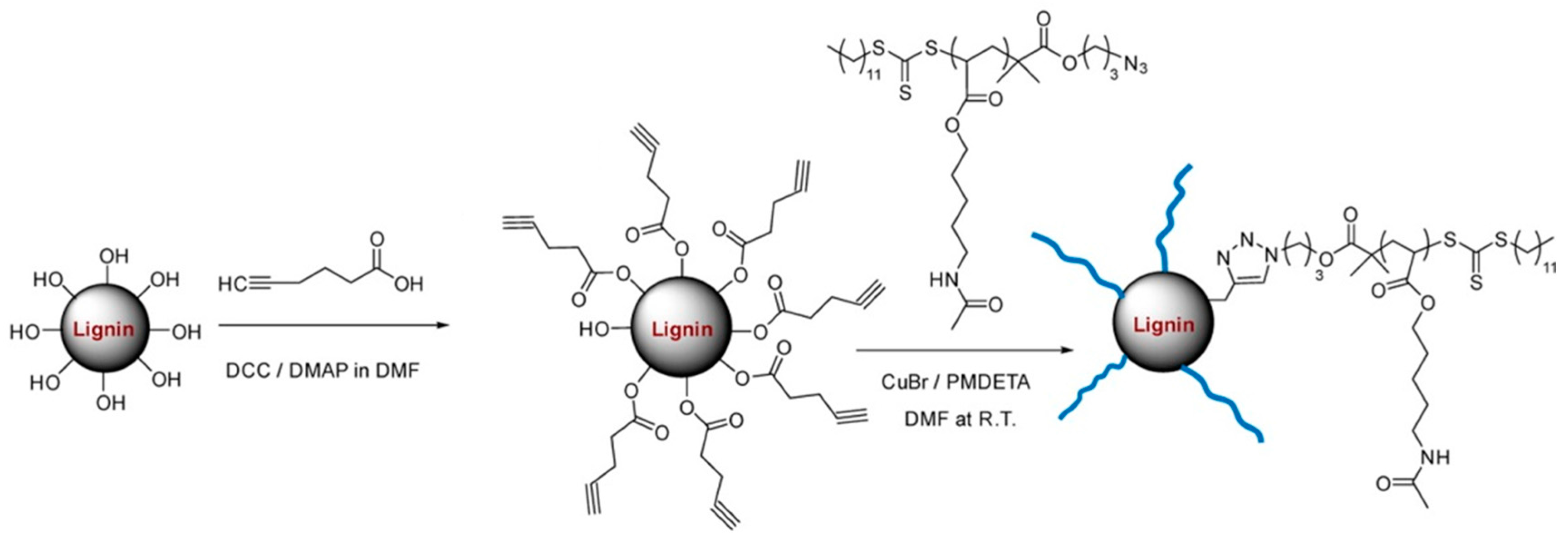
- Liu, H.; Chung, H. Self-healing properties of lignin-containing nanocomposite: Synthesis of lignin-graft-poly (5-acetylaminopentyl acrylate) via raft and click chemistry. Macromolecules 2016, 49, 7246–7256. [Google Scholar] [CrossRef]
- Viswanathan, V.; Laha, T.; Balani, K.; Agarwal, A.; Seal, S. Challenges and advances in nanocomposite processing techniques. Mater. Sci. Eng. 2006, 54, 121–285. [Google Scholar] [CrossRef]
- Kuchibhatla, S.; Karakoti, A.S.; Bera, D.; Seal, S. One dimensional nanostructured materials. Prog. Mater. Sci. 2007, 52, 699–913. [Google Scholar] [CrossRef]
- Tao, P.; Viswanath, A.; Schadler, L.S.; Benicewicz, B.C.; Siegel, R.W. Preparation and optical properties of indium tin oxide/epoxy nanocomposites with polyglycidyl methacrylate grafted nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
- Tchoul, M.N.; Fillery, S.P.; Koerner, H.; Drummy, L.F.; Oyerokun, F.T.; Mirau, P.A.; Durstock, M.F.; Vaia, R.A. Assemblies of titanium dioxide-polystyrene hybrid nanoparticles for dielectric applications. Chem. Mater. 2010, 22, 1749–1759. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, J.S.; Kwon, Y. Synthesis and thermal analysis of nano-aluminum/fluorinated polyurethane elastomeric composites for structural energetics. J. Nanosci. Nanotechnol. 2017, 17, 2488–2492. [Google Scholar] [CrossRef]
- Lakouraj, M.M.; Hasanzadeh, F.; Zare, E.N. Nanogel and super-paramagnetic nanocomposite of thiacalix[4]arene functionalized chitosan: Synthesis, characterization and heavy metal sorption. Iran. Polym. J. 2014, 23, 933–945. [Google Scholar] [CrossRef]
- Tian, Y.; Shi, W.; Luo, J.; Ma, F.; Mi, H.; Lei, Y. Carbazole-based conjugated polymer covalently coated fe3o4 nanoparticle as efficient and reversible hg2+ optical probe. J. Polym. Sci. Part A 2013, 51, 3636–3645. [Google Scholar] [CrossRef]
- Kantheti, S.; Narayan, R.; Raju, K.V. Pyrene-anchored ZnO nanoparticles through click reaction for the development of antimicrobial and fluorescent polyurethane nanocomposite. Polym. Int. 2015, 64, 267–274. [Google Scholar] [CrossRef]
- Oz, E.; Uyar, T.; Esen, H.; Tasdelen, M.A. Simultaneous photoinduced electron transfer and photoinduced cuaac processes for antibacterial thermosets. Prog. Org. Coat. 2017, 105, 252–257. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Messing, M.E.; Ye, L. Temperature and ph dual-responsive core-brush nanocomposite for enrichment of glycoproteins. ACS Appl. Mater. Interfaces 2017, 9, 8985–8995. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.; Krentz, T.M.; Nelson, J.K.; Schadler, L.S.; Bell, M.; Benicewicz, B.; Hillborg, H.; Zhao, S. Dielectric breakdown strength of epoxy bimodal-polymer-brush-grafted core functionalized silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 563–570. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. Poss related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, K.; Zheng, S. Hepta (3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(N-isopropylacrylamide) telechelics: Synthesis and behavior of physical hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Kuo, S.-W. Synthesis and characterization of polyhedral oligomeric silsesquioxane (poss) with multifunctional benzoxazine groups through click chemistry. Polymer 2010, 51, 3948–3955. [Google Scholar] [CrossRef]
- Arslan, I.; Tasdelen, M.A. POSS-based hybrid thermosets via photoinduced copper-catalyzed azide-alkyne cycloaddition click chemistry. Des. Monomers Polym. 2016, 19, 155–160. [Google Scholar] [CrossRef]
- Tinmaz, H.B.; Arslan, I.; Tasdelen, M.A. Star polymers by photoinduced copper-catalyzed azide-alkyne cycloaddition click chemistry. J. Polym. Sci. Part A 2015, 53, 1687–1695. [Google Scholar] [CrossRef]
- Doganci, E.; Tasdelen, M.A.; Yilmaz, F. Synthesis of miktoarm star-shaped polymers with poss core via a combination of cuaac click chemistry, atrp, and rop techniques. Macromol. Chem. Phys. 2015, 216, 1823–1830. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kuo, S.W. Self-assembly and secondary structures of linear polypeptides tethered to polyhedral oligomeric silsesquioxane nanoparticles through click chemistry. J. Polym. Sci. Part A 2011, 49, 2127–2137. [Google Scholar] [CrossRef]
- Islam, M.; Bach, L.G.; Park, J.M.; Hong, S.S.; Lim, K.T. Synthesis and characterization of poly (hema-co-mma)-g-poss nanocomposites by combination of reversible addition fragmentation chain transfer polymerization and click chemistry. J. Appl. Polym. Sci. 2013, 127, 1569–1577. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Baird, D.G. Preparation of polymer–clay nanocomposites and their properties. Adv. Polym. Technol. 2006, 25, 270–285. [Google Scholar] [CrossRef]
- Solomon, D.H.; Loft, B.C. Reactions catalyzed by minerals. Part III. The mechanism of spontaneous interlamellar polymerizations in aluminosilicates. J. Appl. Polym. Sci. 1968, 12, 1253–1262. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Kreutzer, J.; Yagci, Y. In Situ synthesis of polymer/clay nanocomposites by living and controlled/living polymerization. Macromol. Chem. Phys. 2010, 211, 279–285. [Google Scholar] [CrossRef]
- Altinkok, C.; Uyar, T.; Tasdelen, M.A.; Yagci, Y. In Situ synthesis of polymer/clay nanocomposites by type ii photoinitiated free radical polymerization. J. Polym. Sci. Part A 2011, 49, 3658–3663. [Google Scholar] [CrossRef]
- Ozkose, U.U.; Altinkok, C.; Yilmaz, O.; Alpturk, O.; Tasdelen, M.A. In-Situ preparation of poly (2-ethyl-2-oxazoline)/clay nanocomposites via living cationic ring-opening polymerization. Eur. Polym. J. 2017, 88, 586–593. [Google Scholar] [CrossRef]
- Oral, A.; Tasdelen, M.A.; Demirel, A.L.; Yagci, Y. Poly(cyclohexene oxide)/clay nanocomposites by photoinitiated cationic polymerization via activated monomer mechanism. J. Polym. Sci. Part A 2009, 47, 5328–5335. [Google Scholar] [CrossRef]
- Yenice, Z.; Tasdelen, M.A.; Oral, A.; Guler, C.; Yagci, Y. Poly(styrene-b-tetrahydrofuran)/clay nanocomposites by mechanistic transformation. J. Polym. Sci. Part A 2009, 47, 2190–2197. [Google Scholar] [CrossRef]
- Dizman, C.; Ates, S.; Uyar, T.; Tasdelen, M.A.; Torun, L.; Yagci, Y. Polysulfone/clay nanocomposites by In Situ photoinduced crosslinking polymerization. Macromol. Mater. Eng. 2011, 296, 1101–1106. [Google Scholar] [CrossRef]
- Karamane, M.; Raihane, M.; Tasdelen, M.A.; Uyar, T.; Lahcini, M.; Ilsouk, M.; Yagci, Y. Preparation of fluorinated methacrylate/clay nanocomposite via in-situ polymerization: Characterization, structure, and properties. J. Polym. Sci. Part A 2017, 55, 411–418. [Google Scholar] [CrossRef]
- Demir, K.D.; Tasdelen, M.A.; Uyar, T.; Kawaguchi, A.W.; Sudo, A.; Endo, T.; Yagci, Y. Synthesis of polybenzoxazine/clay nanocomposites by in situ thermal ring-opening polymerization using intercalated monomer. J. Polym. Sci. Part A 2011, 49, 4213–4220. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Pavlidou, S.; Papaspyrides, C. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Aydin, M.; Tasdelen, M.A.; Uyar, T.; Jockusch, S.; Turro, N.J.; Yagci, Y. Polystyrene/clay nanocomposites by atom transfer radical nitroxide coupling chemistry. J. Polym. Sci. Part A 2013, 51, 1024–1028. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Van Camp, W.; Goethals, E.; Dubois, P.; Du Prez, F.; Yagci, Y. Polytetrahydrofuran/clay nanocomposites by in situ polymerization and “click” chemistry processes. Macromolecules 2008, 41, 6035–6040. [Google Scholar] [CrossRef]
- Oral, A.; Tasdelen, M.A.; Demirel, A.L.; Yagci, Y. Poly(methyl methacrylate)/clay nanocomposites by photoinitiated free radical polymerization using intercalated monomer. Polymer 2009, 50, 3905–3910. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Poly(epsilon-caprolactone)/clay nanocomposites via “click” chemistry. Eur. Polym. J. 2011, 47, 937–941. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Ye, Y.-S.; Syu, Y.-J.; Hwang, B.-J.; Chang, F.-C. Synthesis and characterization of sulfonated polytriazole-clay proton exchange membrane by in situ polymerization and click reaction for direct methanol fuel cells. J. Power Sources 2012, 208, 144–152. [Google Scholar] [CrossRef]
- Arcudi, F.; Cavallaro, G.; Lazzara, G.; Massaro, M.; Milioto, S.; Noto, R.; Riela, S. Selective functionalization of halloysite cavity by click reaction: Structured filler for enhancing mechanical properties of bionanocomposite films. J. Phys. Chem. C 2014, 118, 15095–15101. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Bartneck, M.; Pan, Y.; Tacke, F. One-step fabrication of biocompatible multifaceted nanocomposite gels and nanolayers. Biomacromolecules 2017, 18, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Segura, J.L.; Salavagione, H.J. Graphene in copper catalyzed azide-alkyne cycloaddition reactions: Evolution from [60]fullerene and carbon nanotubes strategies. Curr. Org. Chem. 2013, 17, 1680–1693. [Google Scholar] [CrossRef]
- Jin, Z.; McNicholas, T.P.; Shih, C.-J.; Wang, Q.H.; Paulus, G.L.; Hilmer, A.J.; Shimizu, S.; Strano, M.S. Click chemistry on solution-dispersed graphene and monolayer cvd graphene. Chem. Mater. 2011, 23, 3362–3370. [Google Scholar] [CrossRef]
- Castelaín, M.; Martínez, G.; Merino, P.; Martín-Gago, J.Á.; Segura, J.L.; Ellis, G.; Salavagione, H.J. Graphene functionalisation with a conjugated poly (fluorene) by click coupling: Striking electronic properties in solution. Chem. Eur. J. 2012, 18, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lai, Z.; Feng, J.; Wu, P. Graphene oxide sheets covalently functionalized with block copolymers via click chemistry as reinforcing fillers. J. Mater. Chem. 2011, 21, 9271–9278. [Google Scholar] [CrossRef]
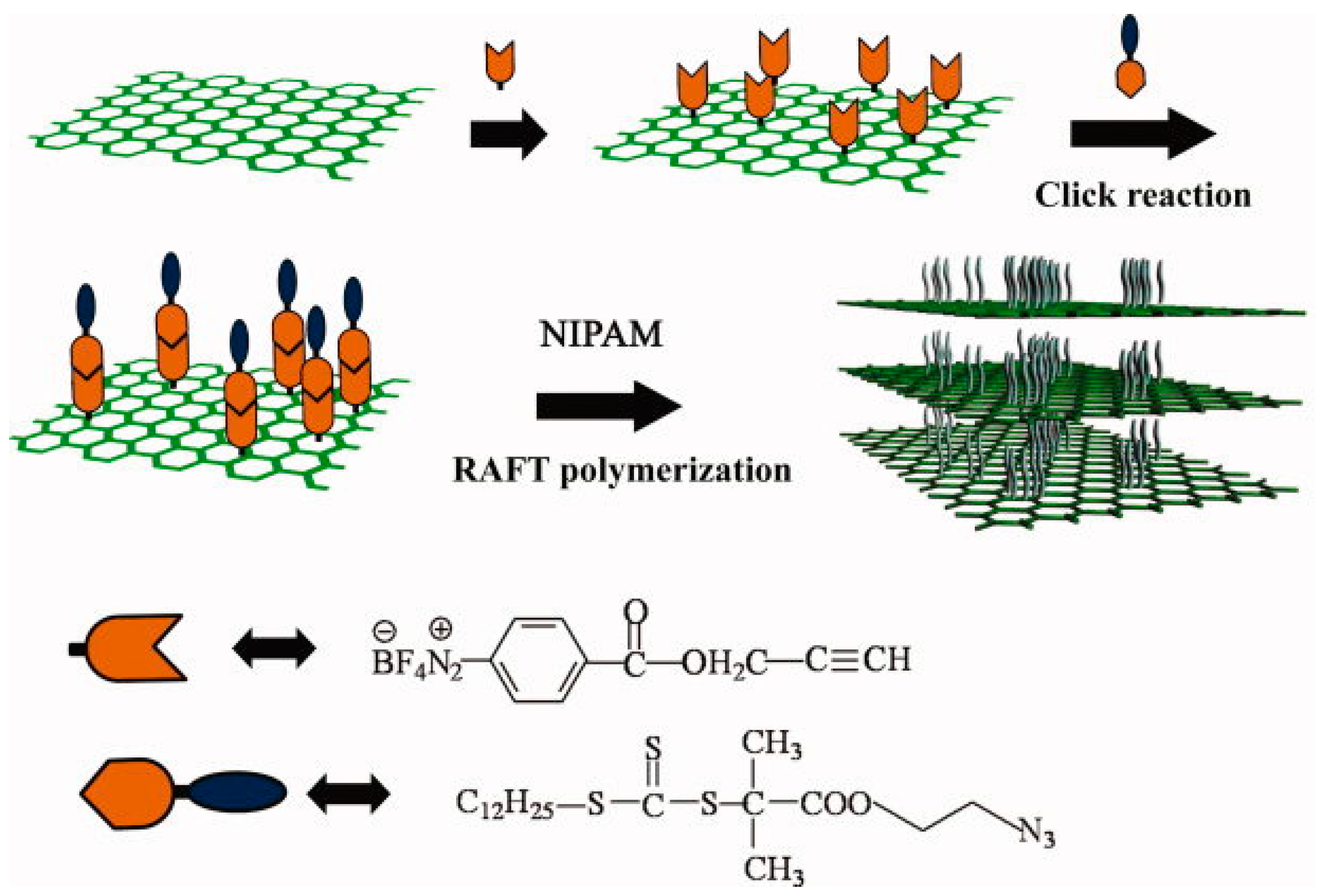
- Yang, Y.; Song, X.; Yuan, L.; Li, M.; Liu, J.; Ji, R.; Zhao, H. Synthesis of pnipam polymer brushes on reduced graphene oxide based on click chemistry and raft polymerization. J. Polym. Sci. Part A 2012, 50, 329–337. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.; Li, L. Water-soluble poly(N-isopropylacrylamide)–graphene sheets synthesized via click chemistry for drug delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, G.; Li, Y.; Li, Y.; Huang, X. Click synthesis of graphene/poly (N-(2-hydroxypropyl) methacrylamide) nanocomposite via “grafting-onto” strategy at ambient temperature. RSC Adv. 2014, 4, 60920–60928. [Google Scholar] [CrossRef]
- Yadav, S.K.; Yoo, H.J.; Cho, J.W. Click coupled graphene for fabrication of high-performance polymer nanocomposites. J. Polym. Sci. Part B 2013, 51, 39–47. [Google Scholar] [CrossRef]
- Han, N.R.; Cho, J.W. Click coupled stitched graphene sheets and their polymer nanocomposites with enhanced photothermal and mechanical properties. Compos. Part A 2016, 87, 78–85. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Yadav, S.K.; Cho, J.W. Synthesis of click-coupled graphene sheets with hyperbranched polyurethane: Effective exfoliation and enhancement of nanocomposite properties. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Nia, A.S.; Rana, S.; Döhler, D.; Osim, W.; Binder, W.H. Nanocomposites via a direct graphene-promoted “click”-reaction. Polymer 2015, 79, 21–28. [Google Scholar]
- Yang, X.; Zhang, X.; Ma, Y.; Huang, Y.; Wang, Y.; Chen, Y. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 2710–2714. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Q.; Sun, Y.; Bai, H.; Shi, G. Three-dimensional self-assembly of graphene oxide and DNA into multifunctional hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Guo, Z.; Liu, Z.; Zhang, W.; Wan, M.; Yang, B. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B 2013, 120, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.; Mei, K.-C.; Klippstein, R.; Costa, P.M.; Hodgins, N.; Wang, J.T.-W.; Festy, F.; Abbate, V.; Hider, R.C.; Chan, K.L.A. Solvent-free click-mechanochemistry for the preparation of cancer cell targeting graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 18920–18923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.-M.; Liu, T.; Tian, R.; Liu, S.-X.; Han, Y.-N.; Wang, Q.-Q.; Sheng, W.-J. Synthesis of novel temperature responsive PEG-b-[PCL-g-P (MEO 2 MA-co-OEGMA)]-b-PEG (tBG) triblock-graft copolymers and preparation of tBG/graphene oxide composite hydrogels via click chemistry. React. Funct. Polym. 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Ryu, H.J.; Mahapatra, S.S.; Yadav, S.K.; Cho, J.W. Synthesis of click-coupled graphene sheet with chitosan: Effective exfoliation and enhanced properties of their nanocomposites. Eur. Polym. J. 2013, 49, 2627–2634. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Pierre, C.; Dikin, D.A.; Ruoff, R.S.; Ramanathan, T.; Brinson, L.C.; Torkelson, J.M. Polymer- graphite nanocomposites: Effective dispersion and major property enhancement via solid-state shear pulverization. Macromolecules 2008, 41, 1905–1908. [Google Scholar] [CrossRef]
- Mazumdar, P.; Chockalingam, S.; Rattan, S. Strategy to synthesise nano-engineered polymer nanocomposite with a mechanically strong interface: A highly flexible ammonia gas sensor. RSC Adv. 2016, 6, 73269–73281. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Teresi, R.; Gambarotti, C. Silanol-POSS as dispersing agents for carbon nanotubes in polyamide. Polym. Eng. Sci. 2017, 57, 588–594. [Google Scholar] [CrossRef]
- Bensghaïer, A.; Salmi, Z.; Le Droumaguet, B.; Mekki, A.; Mohamed, A.A.; Beji, M.; Chehimi, M.M. Diazonium interface chemistry and click polymerization: A novel route for carbon nanotube-polytriazole nanocomposites. Surf. Interface Anal. 2016, 48, 509–513. [Google Scholar] [CrossRef]
- Inglis, A.J.; Barner-Kowollik, C. Ultra rapid approaches to mild macromolecular conjugation. Macromol. Rapid Commun. 2010, 31, 1247–1266. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The diels–alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Sanyal, A. Diels–alder cycloaddition-cycloreversion: A powerful combo in materials design. Macromol. Chem. Phys. 2010, 211, 1417–1425. [Google Scholar] [CrossRef]
- Tasdelen, M.A. Diels–alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Hu, X.; Meng, J. Effect of organoclay on the curing reactions in bismaleimide/diallyl bisphenol a resin. J. Polym. Sci. Part A 2005, 43, 994–1006. [Google Scholar] [CrossRef]
- Hayden, H.; Gun’ko, Y.K.; Perova, T.S. Chemical modification of multi-walled carbon nanotubes using a tetrazine derivative. Chem. Phys. Lett. 2007, 435, 84–89. [Google Scholar] [CrossRef]
- Sakellariou, G.; Ji, H.; Mays, J.W.; Hadjichristidis, N.; Baskaran, D. Controlled covalent functionalization of multiwalled carbon nanotubes using [4 + 2] cycloaddition of benzocyclobutenes. Chem. Mater. 2007, 19, 6370–6372. [Google Scholar] [CrossRef]
- Sakellariou, G.; Ji, H.; Mays, J.W.; Baskaran, D. Enhanced polymer grafting from multiwalled carbon nanotubes through living anionic surface-initiated polymerization. Chem. Mater. 2008, 20, 6217–6230. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Hadjichristidis, N.; Penott, E.K.; Lorenzo, A.T.; Müller, A.J. Surface modification of multiwalled carbon nanotubes with biocompatible polymers via ring opening and living anionic surface initiated polymerization. Kinetics and crystallization behavior. J. Polym. Sci. Part A 2009, 47, 4379–4390. [Google Scholar] [CrossRef]
- Priftis, D.; Petzetakis, N.; Sakellariou, G.; Pitsikalis, M.; Baskaran, D.; Mays, J.W.; Hadjichristidis, N. Surface-initiated titanium-mediated coordination polymerization from catalyst-functionalized single and multiwalled carbon nanotubes. Macromolecules 2009, 42, 3340–3346. [Google Scholar] [CrossRef]
- Pérez, R.A.; López, J.V.; Hoskins, J.N.; Zhang, B.; Grayson, S.M.; Casas, M.T.; Puiggalí, J.; Müller, A.J. Nucleation and antinucleation effects of functionalized carbon nanotubes on cyclic and linear poly(ε-caprolactones). Macromolecules 2014, 47, 3553–3566. [Google Scholar] [CrossRef]
- Chang, C.-M.; Liu, Y.-L. Functionalization of multi-walled carbon nanotubes with furan and maleimide compounds through diels–alder cycloaddition. Carbon 2009, 47, 3041–3049. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chang, C.-M.; Hsu, K.-Y.; Liu, Y.-L. Poly(lactide)-functionalized and Fe3O4 nanoparticle-decorated multiwalled carbon nanotubes for preparation of electrically-conductive and magnetic poly (lactide) films and electrospun nanofibers. J. Mater. Chem. 2012, 22, 4855–4860. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Chang, C.-M.; Liu, Y.-L. Benzoxazine-functionalized multi-walled carbon nanotubes for preparation of electrically-conductive polybenzoxazines. Polymer 2012, 53, 106–112. [Google Scholar] [CrossRef]
- Willocq, B.; Bose, R.; Khelifa, F.; Garcia, S.; Dubois, P.; Raquez, J.-M. Healing by the joule effect of electrically conductive poly(ester-urethane)/carbon nanotube nanocomposites. J. Mater. Chem. A 2016, 4, 4089–4097. [Google Scholar] [CrossRef]
- García-García, J.M.; Bernal, M.; Verdejo, R.; López-Manchado, M.A.; Doncel-Pérez, E.; Garrido, L.; Quijada-Garrido, I. Semiconductive bionanocomposites of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and mwcnts for neural growth applications. J. Polym. Sci. Part B 2014, 52, 349–360. [Google Scholar] [CrossRef]
- Chen, Z.; Dai, X.J.; Lamb, P.R.; de Celis Leal, D.R.; Fox, B.L.; Chen, Y.; du Plessis, J.; Field, M.; Wang, X. Practical amine functionalization of multi-walled carbon nanotubes for effective interfacial bonding. Plasma Process. Polym. 2012, 9, 733–741. [Google Scholar] [CrossRef]
- Hu, L.; Hecht, D.S.; Gruner, G. Carbon nanotube thin films: Fabrication, properties, and applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, I.; Kumar, J.; Bhatnagar, C.; Dixit, S.K.; Bhatnagar, P.K.; Mathur, P.C.; Covas, J.A.; da Conceicao Paiva, M. Enhancement in the performance of multi-walled carbon nanotube: Poly(methylmethacrylate) composite thin film ethanol sensors through appropriate nanotube functionalization. Mater. Sci. Semiconduct. Process. 2015, 31, 166–174. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Wang, D. Enhancement of mechanical and self-healing performance in multiwall carbon nanotube/rubber composites via diels–alder bonding. Macromol. Mater. Eng. 2016, 301, 535–541. [Google Scholar] [CrossRef]
- Zhu, J.; Waengler, C.; Lennox, R.B.; Schirrmacher, R. Preparation of water-soluble maleimide-functionalized 3 nm gold nanoparticles: A new bioconjugation template. Langmuir 2012, 28, 5508–5512. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Astrain, C.; Ahmed, I.; Kendziora, D.; Guaresti, O.; Eceiza, A.; Fruk, L.; Corcuera, M.; Gabilondo, N. Effect of maleimide-functionalized gold nanoparticles on hybrid biohydrogels properties. RSC Adv. 2015, 5, 50268–50277. [Google Scholar] [CrossRef]
- García-Astrain, C.; Hernández, R.; Guaresti, O.; Fruk, L.; Mijangos, C.; Eceiza, A.; Gabilondo, N. Click crosslinked chitosan/gold nanocomposite hydrogels. Macromol. Mater. Eng. 2016, 301, 1295–1300. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, M.; Chen, S.; Yuan, M.; Guo, Y.; Song, Y.; Liu, H.; Li, Y. Organic–inorganic nanohybrids via directly grafting gold nanoparticles onto conjugated copolymers through the diels–alder reaction. Langmuir 2008, 24, 11967–11974. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Kao, T.-H.; Li, H.-Y.; Liu, Y.-L. Preparation of polybenzoxazine-functionalized Fe3O4 nanoparticles through In Situ diels–alder polymerization for high performance magnetic polybenzoxazine/Fe3O4 nanocomposites. Compos. Sci. Technol. 2012, 72, 1562–1567. [Google Scholar] [CrossRef]
- Engel, T.; Kickelbick, G. Thermoreversible reactions on inorganic nanoparticle surfaces: Diels–alder reactions on sterically crowded surfaces. Chem. Mater. 2013, 25, 149–157. [Google Scholar] [CrossRef]
- Engel, T.; Kickelbick, G. Self-healing nanocomposites from silica–polymer core–shell nanoparticles. Polym. Int. 2014, 63, 915–923. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Self-healing polymer nanocomposites based on diels-alder-reactions with silica nanoparticles: The role of the polymer matrix. Polymer 2015, 69, 357–368. [Google Scholar] [CrossRef]
- Chuo, T.-W.; Liu, Y.-L. Preparation of self-healing organic–inorganic nanocomposites with the reactions between methacrylated polyhedral oligomeric silsesquioxanes and furfurylamine. Compos. Sci. Technol. 2015, 118, 236–243. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Wang, X.; Lin, T. A thermally healable polyhedral oligomeric silsesquioxane (POSS) nanocomposite based on diels–alder chemistry. Chem. Commun. 2013, 49, 6755–6757. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Miljevic, M.; Ahmed, I.; Martin, L.; Eceiza, A.; Fruk, L.; Corcuera, M.; Gabilondo, N. Designing hydrogel nanocomposites using TiO2 as clickable cross-linkers. J. Mater. Sci. 2016, 51, 5073–5081. [Google Scholar] [CrossRef]
- Arslan, M.; Gevrek, T.N.; Lyskawa, J.; Szunerits, S.; Boukherroub, R.; Sanyal, R.; Woisel, P.; Sanyal, A. Bioinspired anchorable thiol-reactive polymers: Synthesis and applications toward surface functionalization of magnetic nanoparticles. Macromolecules 2014, 47, 5124–5134. [Google Scholar] [CrossRef]
- Watson, M.A.; Lyskawa, J.L.; Zobrist, C.D.; Fournier, D.; Jimenez, M.; Traisnel, M.; Gengembre, L.O.; Woisel, P. A “clickable” titanium surface platform. Langmuir 2010, 26, 15920–15924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, B.; Yin, D.; Wei, J.; Wang, Z.; Xiong, R.; Shi, J.; Liu, Z.; Lei, Q. Flexible self-healing nanocomposites for recoverable motion sensor. Nano Energy 2015, 17, 1–9. [Google Scholar] [CrossRef]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Louarn, G.; Aubert, P.-H.; Alain-Rizzo, V.; Galmiche, L.; Audebert, P.; Miomandre, F. Polypyrrole-modified graphene sheet nanocomposites as new efficient materials for supercapacitors. Carbon 2016, 105, 510–520. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Deng, L.; Zhao, S.; Gao, Y.; Jiang, K.; Sun, R.; Wong, C. In Situ polymerization of mechanically reinforced, thermally healable graphene oxide/polyurethane composites based on diels–alder chemistry. J. Mater. Chem. A 2014, 2, 20642–20649. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Sun, R.; Wong, C.-P. A covalently cross-linked reduced functionalized graphene oxide/polyurethane composite based on diels–alder chemistry and its potential application in healable flexible electronics. J. Mater. Chem. C 2017, 5, 220–228. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis: A first update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Ten Brummelhuis, N.; Diehl, C.; Schlaad, H. Thiol–ene modification of 1,2-polybutadiene using UV light or sunlight. Macromolecules 2008, 41, 9946–9947. [Google Scholar] [CrossRef]
- Rosilo, H.; Kontturi, E.; Seitsonen, J.; Kolehmainen, E.; Ikkala, O. Transition to reinforced state by percolating domains of intercalated brush-modified cellulose nanocrystals and poly (butadiene) in cross-linked composites based on thiol–ene click chemistry. Biomacromolecules 2013, 14, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Uygun, M.; Tasdelen, M.A.; Yagci, Y. Influence of type of initiation on thiol–ene “click” chemistry. Macromol. Chem. Phys. 2010, 211, 103–110. [Google Scholar] [CrossRef]
- Parambath Kanoth, B.; Claudino, M.; Johansson, M.; Berglund, L.A.; Zhou, Q. Biocomposites from natural rubber: Synergistic effects of functionalized cellulose nanocrystals as both reinforcing and cross-linking agents via free-radical thiol–ene chemistry. ACS Appl. Mater. Interfaces 2015, 7, 16303–16310. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.D.; Capadona, J.R.; Marasco, P.D.; Rowan, S.J. Bioinspired water-enhanced mechanical gradient nanocomposite films that mimic the architecture and properties of the squid beak. J. Am. Chem. Soc. 2013, 135, 5167–5174. [Google Scholar] [CrossRef] [PubMed]
- Schyrr, B.; Pasche, S.P.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors based on porous cellulose nanocrystal–poly(vinyl alcohol) scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef] [PubMed]
- Garber, L.; Chen, C.; Kilchrist, K.V.; Bounds, C.; Pojman, J.A.; Hayes, D. Thiol-acrylate nanocomposite foams for critical size bone defect repair: A novel biomaterial. J. Biomed. Mater. Res. Part A 2013, 101, 3531–3541. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.C.; Elupula, R.; Rehm, C.; Adireddy, S.; Grayson, S.M.; Chrisey, D.B. Click-in ferroelectric nanoparticles for dielectric energy storage. ACS Appl. Mater. Interfaces 2015, 7, 17819–17825. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Huang, X.; Zhu, M.; Xie, L.; Tanaka, T.; Jiang, P. Combining raft polymerization and thiol–ene click reaction for core–shell structured polymer@ batio3 nanodielectrics with high dielectric constant, low dielectric loss, and high energy storage capability. ACS Appl. Mater. Interfaces 2014, 6, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Cramer, N.B.; Scott, J.P.; Bowman, C.N. Photopolymerizations of thiol–ene polymers without photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Y.; Zhao, B.; Li, S.; Zhang, Y.; Gao, C. Sequentially hetero-functional, topological polymers by step-growth thiol-yne approach. Sci. Rep. 2014, 4, 4387. [Google Scholar] [CrossRef] [PubMed]
- Bae, J. Thiol-ene/clay nanocomposite thin film as novel transparent barrier. Polym. Int. 2012, 61, 895–900. [Google Scholar] [CrossRef]
- Owusu-Adom, K.; Schall, J.; Guymon, C.A. Photopolymerization behavior of thiol–acrylate monomers in clay nanocomposites. Macromolecules 2009, 42, 3275–3284. [Google Scholar] [CrossRef]
- Kim, S.K.; Guymon, C.A. Photopolymerization behavior in nanocomposites formed with thiol–acrylate and polymerizable organoclays. J. Polym. Sci. Part A 2011, 49, 465–475. [Google Scholar] [CrossRef]
- Boyd, D.A.; Naciri, J.; Fontana, J.; Pacardo, D.B.; Shields, A.R.; Verbarg, J.; Spillmann, C.M.; Ligler, F.S. Facile fabrication of color tunable film and fiber nanocomposites via thiol click chemistry. Macromolecules 2014, 47, 695–704. [Google Scholar] [CrossRef]
- Phillips, J.P.; Mackey, N.M.; Confait, B.S.; Heaps, D.T.; Deng, X.; Todd, M.L.; Stevenson, S.; Zhou, H.; Hoyle, C.E. Dispersion of gold nanoparticles in UV-cured, thiol–ene films by precomplexation of gold–thiol. Chem. Mater. 2008, 20, 5240–5245. [Google Scholar] [CrossRef]
- Boyd, D.A.; Bezares, F.J.; Pacardo, D.B.; Ukaegbu, M.; Hosten, C.; Ligler, F.S. Small-molecule detection in thiol–yne nanocomposites via surface-enhanced raman spectroscopy. Anal. Chem. 2014, 86, 12315–12320. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Functional PEG-hydrogels convey gold nanoparticles from silicon and aid cell adhesion onto the nanocomposites. Chem. Mater. 2017, 29, 2008–2015. [Google Scholar] [CrossRef]
- Mardare, L.; Benea, L. Development of Anticorrosive Polymer Nanocomposite Coating for Corrosion Protection in Marine Environment; Materials Science and Engineering Conference Series; IOP: London, UK, 2017; p. 012056. [Google Scholar]
- Nazari, M.H.; Shi, X. Polymer-based nanocomposite coatings for anticorrosion applications. In Industrial Applications for Intelligent Polymers and Coatings; Springer: Berlin, Germany, 2016; pp. 373–398. [Google Scholar]
- Majumdar, D.; Blanton, T.N.; Schwark, D.W. Clay–polymer nanocomposite coatings for imaging application. Appl. Clay Sci. 2003, 23, 265–273. [Google Scholar] [CrossRef]
- Bae, J.; Lee, J.; Park, C.S.; Kwon, O.S.; Lee, C.-S. Fabrication of photo-crosslinkable polymer/silica sol–gel hybrid thin films as versatile barrier films. J. Ind. Eng. Chem. 2016, 38, 61–66. [Google Scholar] [CrossRef]
- Mülazim, Y.; Çakmakçi, E.; Kahraman, M.V. Properties of thiol–ene photocurable highly hydrophobic and oleophobic nanocomposite coatings on abs and hips substrates. Adv. Polym. Technol. 2013, 32. [Google Scholar] [CrossRef]
- Chollet, B.; Li, M.; Martwong, E.; Bresson, B.; Fretigny, C.; Tabeling, P.; Tran, Y. Multiscale surface-attached hydrogel thin films with tailored architecture. ACS Appl. Mater. Interfaces 2016, 8, 11729–11738. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, S.; Yu, B.; Tang, G.; Xing, W.; Hu, Y. Poss-functionalized polyphosphazene nanotube: Preparation and effective reinforcement on uv-curable epoxy acrylate nanocomposite coatings. RSC Adv. 2016, 6, 3025–3031. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Song, L.; Xing, W.; Tang, G.; Hu, W.; Hu, Y. Preparation and thermal stability of UV-cured epoxy-based coatings modified with octamercaptopropyl poss. Thermochim. Acta 2013, 568, 130–139. [Google Scholar] [CrossRef]
- Acar, S.B.; Ozcelik, M.; Uyar, T.; Tasdelen, M.A. Polyhedral oligomeric silsesquioxane-based hybrid networks obtained via thiol-epoxy click chemistry. Iran. Polym. J. 2017, 26, 405–411. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, Y. Preparation and properties of polyhedral oligomeric silsesquioxane and carbon nanotube grafted carbon fiber hierarchical reinforcing structure. J. Mater. Chem. 2011, 21, 2867–2870. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, Y.; Liu, L.; Bai, Y.; Xu, L. Formation of a carbon fiber/polyhedral oligomeric silsesquioxane/carbon nanotube hybrid reinforcement and its effect on the interfacial properties of carbon fiber/epoxy composites. Carbon 2011, 49, 2624–2632. [Google Scholar] [CrossRef]
- Tzur-Balter, A.; Shatsberg, Z.; Beckerman, M.; Segal, E.; Artzi, N. Mechanism of erosion of nanostructured porous silicon drug carriers in neoplastic tissues. Nat. Commun. 2015, 6, 6208. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.; Ranjan, S.; Correia, A.M.; Mäkilä, E.M.; Kinnunen, S.M.; Zhang, H.; Shahbazi, M.-A.; Almeida, P.V.; Salonen, J.J.; Ruskoaho, H.J. In Vitro and In Vivo assessment of heart-homing porous silicon nanoparticles. Biomaterials 2016, 94, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Almeida, P.V.; Correia, A.; Herranz-Blanco, B.; Shrestha, N.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; Santos, H.A. Intracellular responsive dual delivery by endosomolytic polyplexes carrying DNA anchored porous silicon nanoparticles. J. Control. Release 2017, 249, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Feld, A.; Koll, R.; Fruhner, L.S.; Krutyeva, M.; Pyckhout-Hintzen, W.; Weiß, C.; Heller, H.; Weimer, A.; Schmidtke, C.; Appavou, M.-S. Nanocomposites of highly monodisperse encapsulated superparamagnetic iron oxide nanocrystals homogeneously dispersed in a poly(ethylene oxide) melt. ACS Nano 2017, 11, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, S.V.; Vallo, C.I. Facile preparation of silver-based nanocomposites via thiol-methacrylate ‘click’photopolymerization. Eur. Polym. J. 2016, 79, 163–175. [Google Scholar] [CrossRef]
- Jin, F.; Zheng, M.-L.; Zhang, M.-L.; Zhao, Z.-S.; Duan, X.-M. A facile layer-by-layer assembly method for the fabrication of fluorescent polymer/quantum dot nanocomposite thin films. RSC Adv. 2014, 4, 33206–33214. [Google Scholar] [CrossRef]
- Salavagione, H.J. Promising alternative routes for graphene production and functionalization. J. Mater. Chem. A 2014, 2, 7138–7146. [Google Scholar] [CrossRef]
- Luong, N.D.; Sinh, L.H.; Johansson, L.S.; Campell, J.; Seppälä, J. Functional graphene by thiol-ene click chemistry. Chem. Eur. J. 2015, 21, 3183–3186. [Google Scholar] [CrossRef] [PubMed]
- Castelaín, M.; Martínez, G.; Marco, C.; Ellis, G.; Salavagione, H.J. Effect of click-chemistry approaches for graphene modification on the electrical, thermal, and mechanical properties of polyethylene/graphene nanocomposites. Macromolecules 2013, 46, 8980–8987. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Xing, W.; Yang, H.; Wang, X.; Song, L.; Hu, Y.; Lo, S. Enhanced thermal and mechanical properties of functionalized graphene/thiol-ene systems by photopolymerization technology. Chem. Eng. J. 2013, 228, 318–326. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Liu, L.; Yang, H.; Tai, Q.; Lo, S.; Song, L.; Hu, Y. Click-chemistry approach for graphene modification: Effective reinforcement of UV-curable functionalized graphene/polyurethane acrylate nanocomposites. RSC Adv. 2015, 5, 13502–13506. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, S.W.; Cho, K.Y. Improved thermal conductivity of graphene encapsulated poly(methyl methacrylate) nanocomposite adhesives with low loading amount of graphene. Compos. Sci. Technol. 2014, 94, 147–154. [Google Scholar] [CrossRef]
- Liras, M.; García, O.; Quijada-Garrido, I.; Ellis, G.; Salavagione, H.J. Homogenous thin layer coated graphene via one pot reaction with multidentate thiolated pmmas. J. Mater. Chem. C 2014, 2, 1723–1729. [Google Scholar] [CrossRef]
- Castelaín, M.; Martínez, G.; Ellis, G.; Salavagione, H.J. A versatile chemical tool for the preparation of conductive graphene-based polymer nanocomposites. Chem. Commun. 2013, 49, 8967–8969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Wang, W.; Guo, A.; Li, J.; Li, H. Efficient and robust reactions for polyethylene covalently grafted carbon nanotubes. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Su, Z.; Liu, Y.; Xie, Q.; Chen, L.; Zhang, Y.; Meng, Y.; Li, Y.; Fu, Y.; Ma, M.; Yao, S. Preparation of thiolated polymeric nanocomposite for sensitive electroanalysis of dopamine. Biosens. Bioelectron. 2012, 36, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Zhang, Y.; Chen, L.; Gu, T.; Huang, S.; Liu, Y.; Sun, L.; Xie, Q.; Yao, S. Amperometric determination of ascorbic acid using multiwalled carbon nanotube-thiolated polyaniline composite modified glassy carbon electrode. J. Electroanal. Chem. 2013, 709, 19–25. [Google Scholar] [CrossRef]
- Su, Z.; Liu, Y.; Zhang, Y.; Xie, Q.; Chen, L.; Huang, Y.; Fu, Y.; Meng, Y.; Li, X.; Ma, M. Thiol–ene chemistry guided preparation of thiolated polymeric nanocomposite for anodic stripping voltammetric analysis of Cd2+ and Pb2+. Analyst 2013, 138, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, D.J.; Lee, N.; Kim, B.H.; Choi, S.H.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for ph controlled drug release and dual modal imaging. J. Mater. Chem. 2011, 21, 16869–16872. [Google Scholar] [CrossRef]
- De France, K.J.; Chan, K.J.; Cranston, E.D.; Hoare, T. Enhanced mechanical properties in cellulose nanocrystal-poly(oligoethylene glycol methacrylate) injectable nanocomposite hydrogels through control of physical and chemical cross-linking. Biomacromolecules 2016, 17, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Mäkilä, E.; Fan, J.; Herranz-Blanco, B.; Wang, C.-F.; Rosa, R.; Ribeiro, A.J.; Salonen, J.; Hirvonen, J. Microfluidic assisted one-step fabrication of porous silicon@ acetalated dextran nanocomposites for precisely controlled combination chemotherapy. Biomaterials 2015, 39, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Knall, A.-C.; Slugovc, C. Inverse electron demand diels–alder (iedda)-initiated conjugation: A (high) potential click chemistry scheme. Chem. Soc. Rev. 2013, 42, 5131–5142. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liang, G. A biocompatible, highly efficient click reaction and its applications. Org. Biomol. Chem. 2014, 12, 865–871. [Google Scholar] [CrossRef] [PubMed]



| Click Reaction | Advantages | Limitations | Comments |
|---|---|---|---|
| CuAAC click reaction | High selectivity, rapid and quantitative transformations, tolerance to diverse organic solvents and water, stability, orthogonality. | Need to use (toxic) metal catalyst. Removal of the catalyst. | Particularly suitable for effective coupling of nanofillers and matrices. Provides improvement in interfacial compatibility. |
| Metal-free click reaction | Selectivity, high reactivity, biocompatibility and stability. | Substrates such as strained cyclooctynes are not so common and expensive materials. | Suitable if toxic metal catalyst is an issue, especially in biomedical applications. |
| Diels–Alder reaction | Activation through heating (could be beneficial in certain cases). Most of the time no byproduct formation. Reversibility of the reaction. | Heating requirement (could be a problem in certain cases), relatively prolonged reactions times. | Nanofiller surface can act as a substrate which resolves the destructive surface chemical treatments. Reversible nature is useful in self-healing materials. |
| Thiol-ene and thiol-yne reactions | High efficiency, high conversions, UV or heat-triggered activation. | Thiols are prone to many side reactions and have low self-stability. Especially the volatile thiols have disagreeable odors. | Reaction mechanism may induce more homogeneous network formation and reduces the network shrinkage. UV-triggered nature might be useful in coating applications. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, M.; Tasdelen, M.A. Polymer Nanocomposites via Click Chemistry Reactions. Polymers 2017, 9, 499. https://doi.org/10.3390/polym9100499
Arslan M, Tasdelen MA. Polymer Nanocomposites via Click Chemistry Reactions. Polymers. 2017; 9(10):499. https://doi.org/10.3390/polym9100499
Chicago/Turabian StyleArslan, Mehmet, and Mehmet Atilla Tasdelen. 2017. "Polymer Nanocomposites via Click Chemistry Reactions" Polymers 9, no. 10: 499. https://doi.org/10.3390/polym9100499