Fabrication of Al2O3 Nano-Structure Functional Film on a Cellulose Insulation Polymer Surface and Its Space Charge Suppression Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
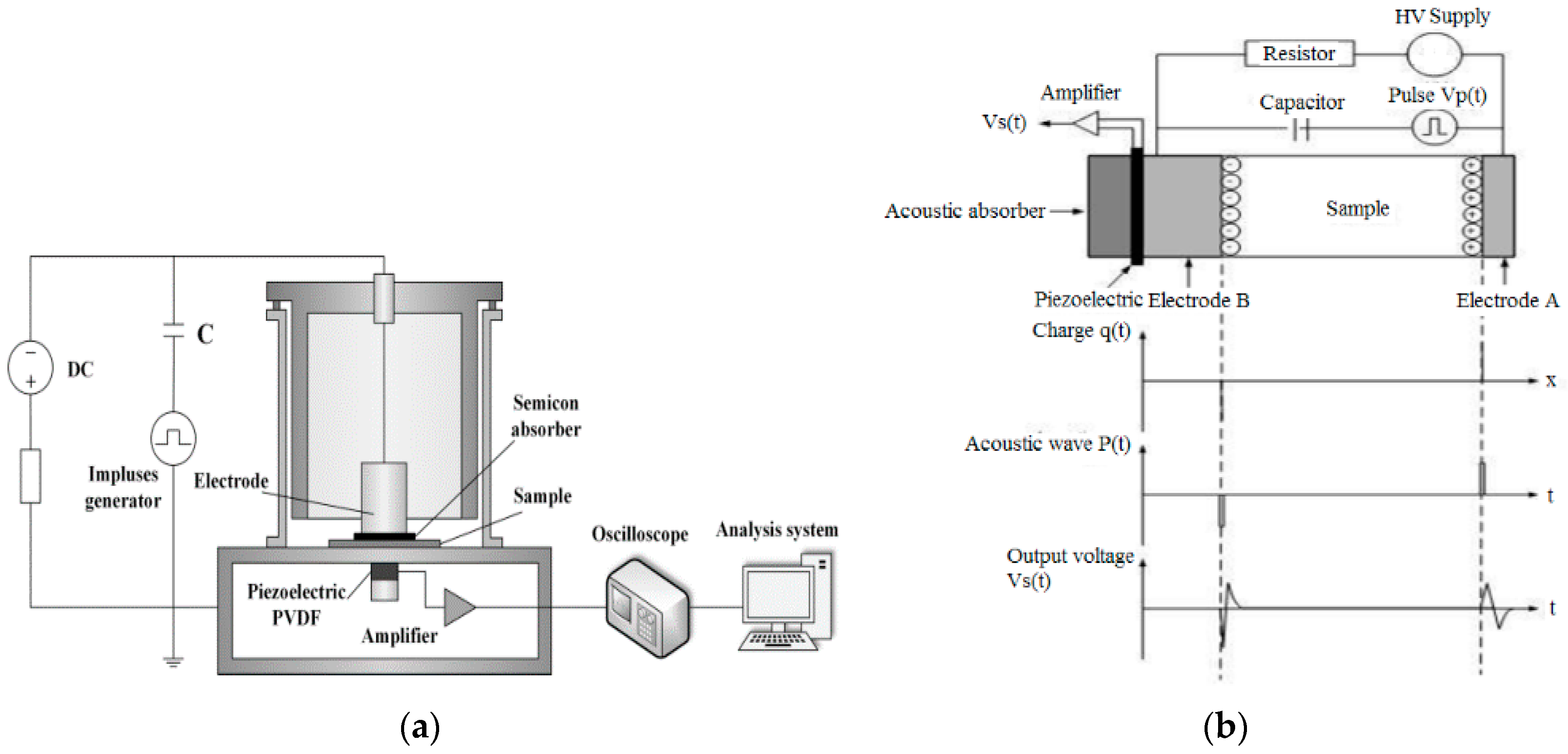
2.2. Characterisation Methods
3. Results
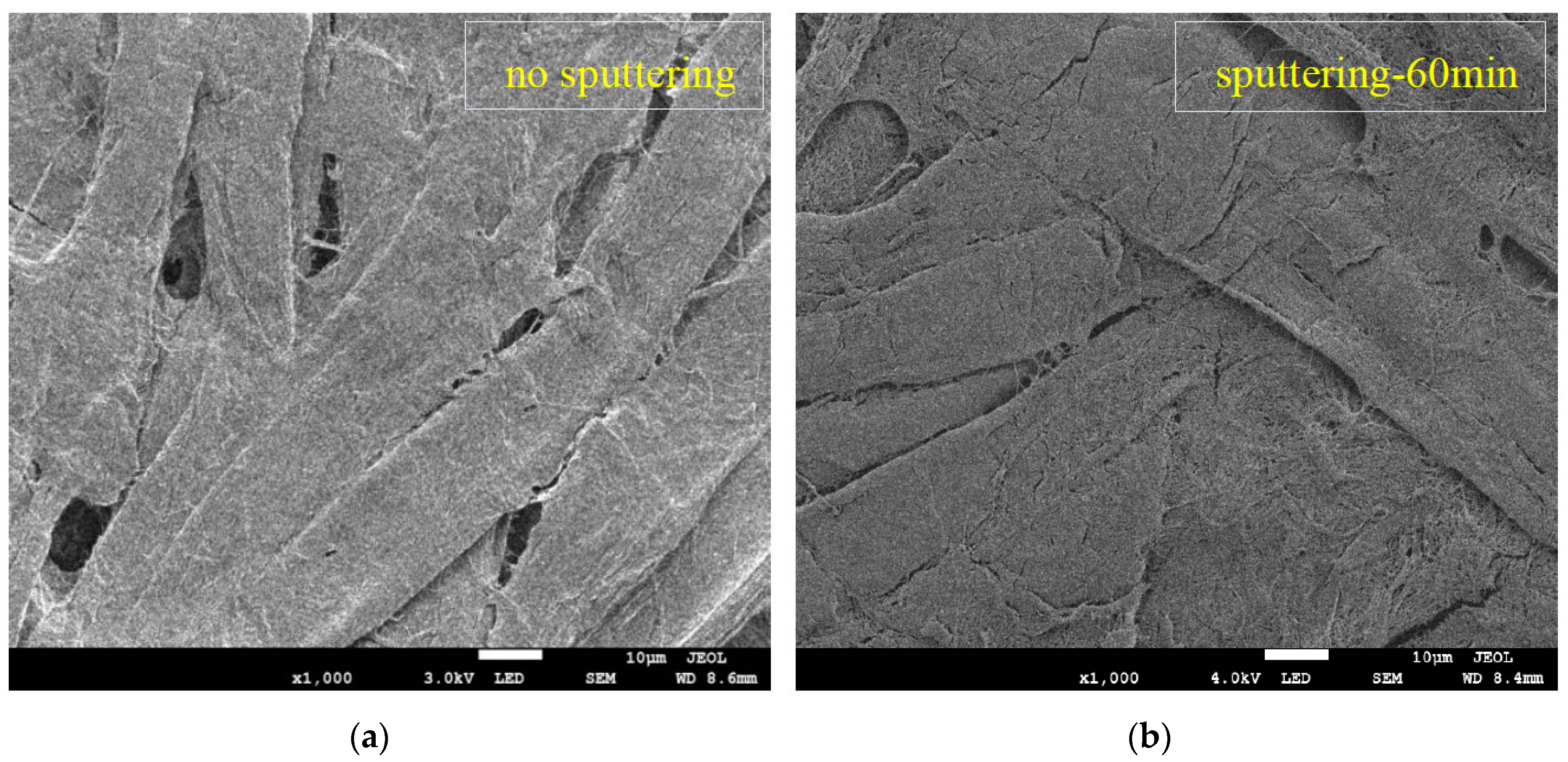
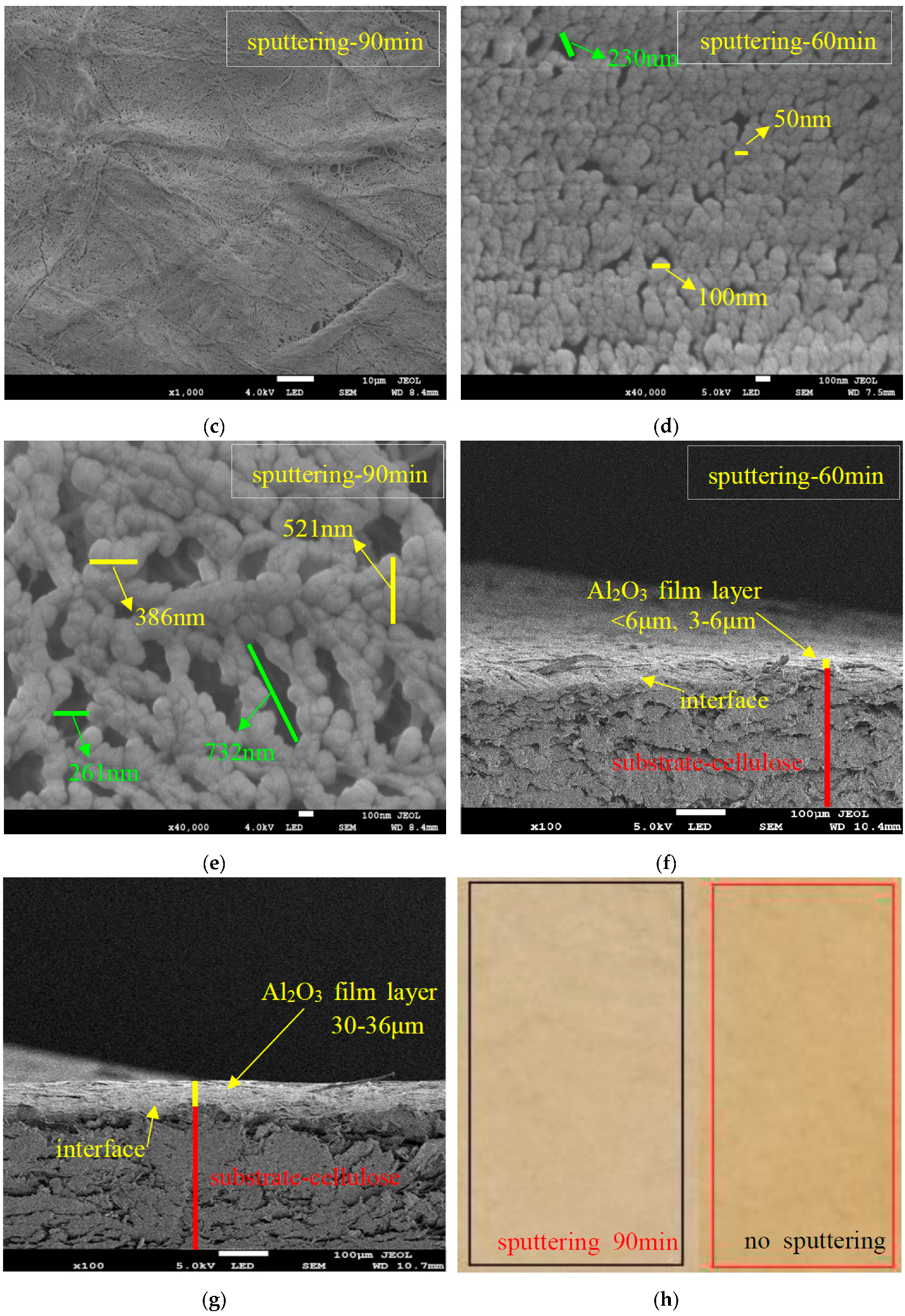
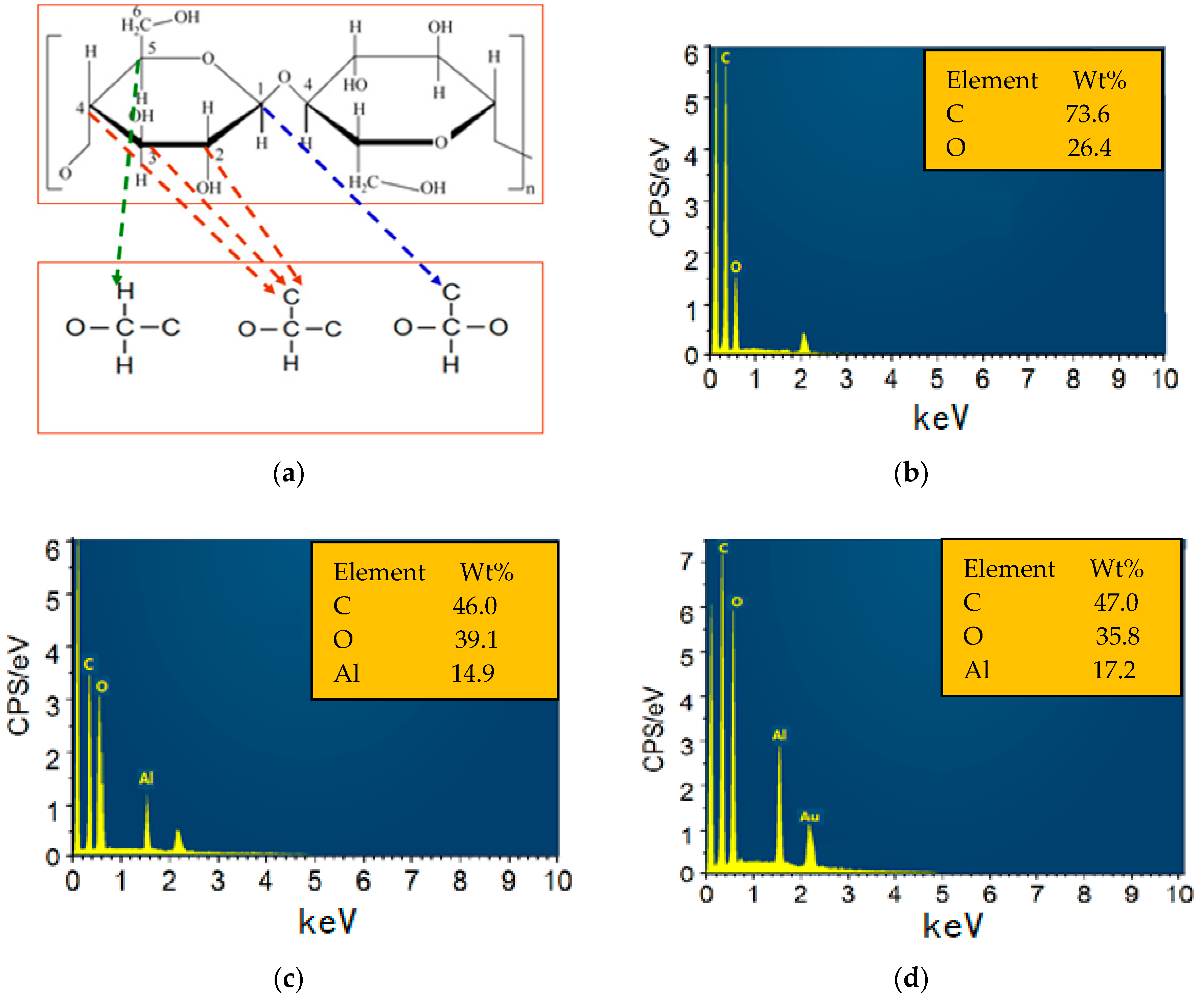
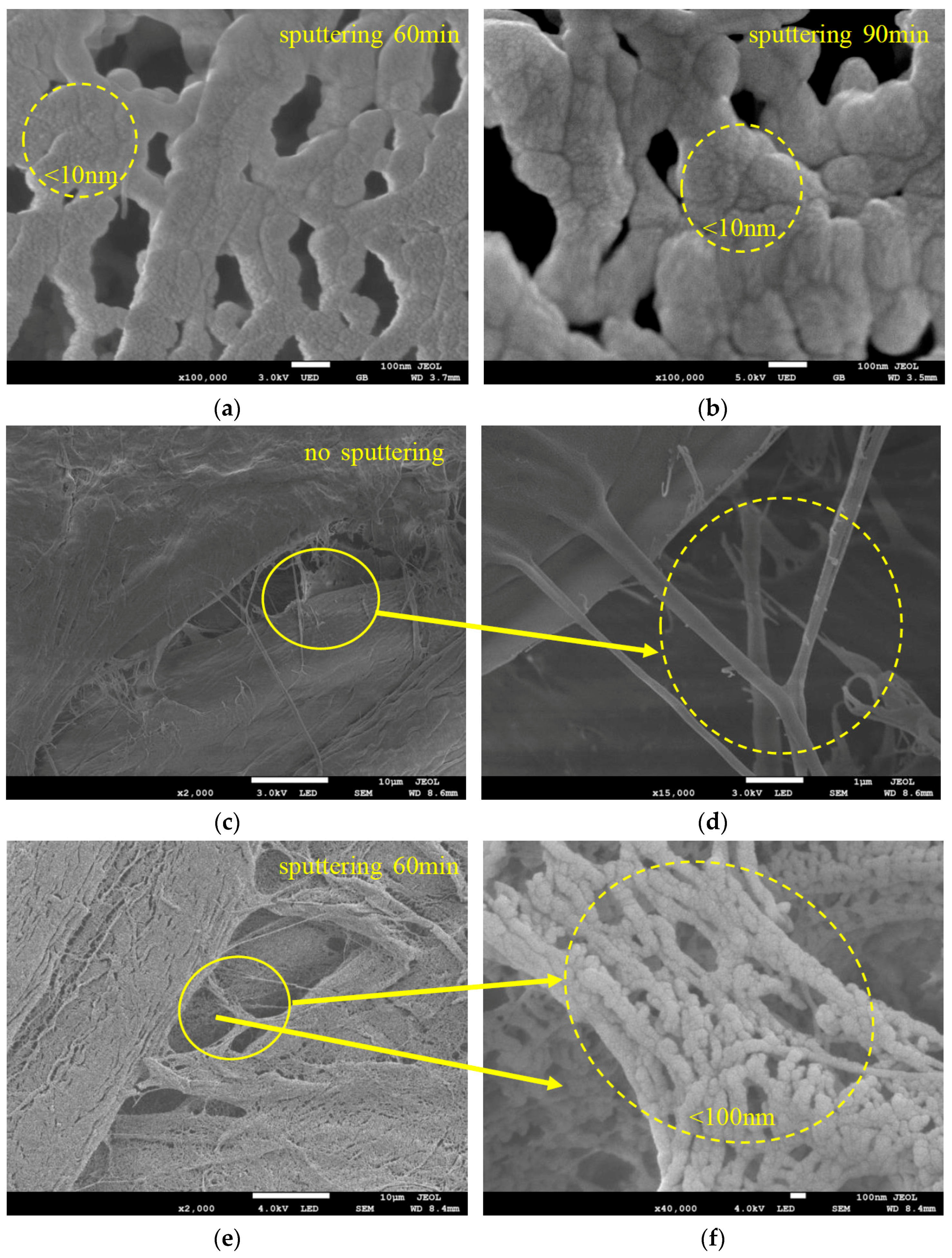
3.1. SEM/EDS of the Sputtered Film
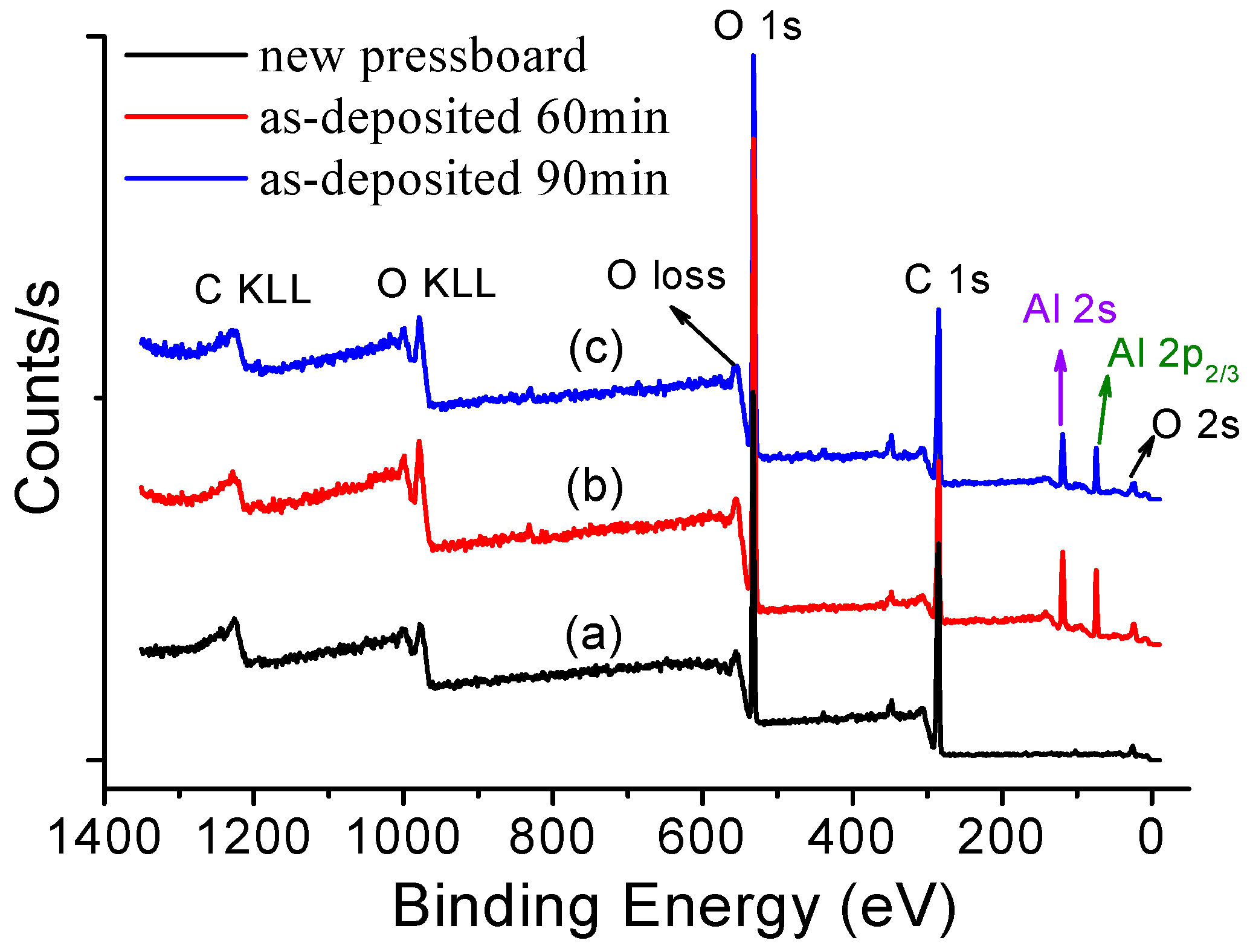
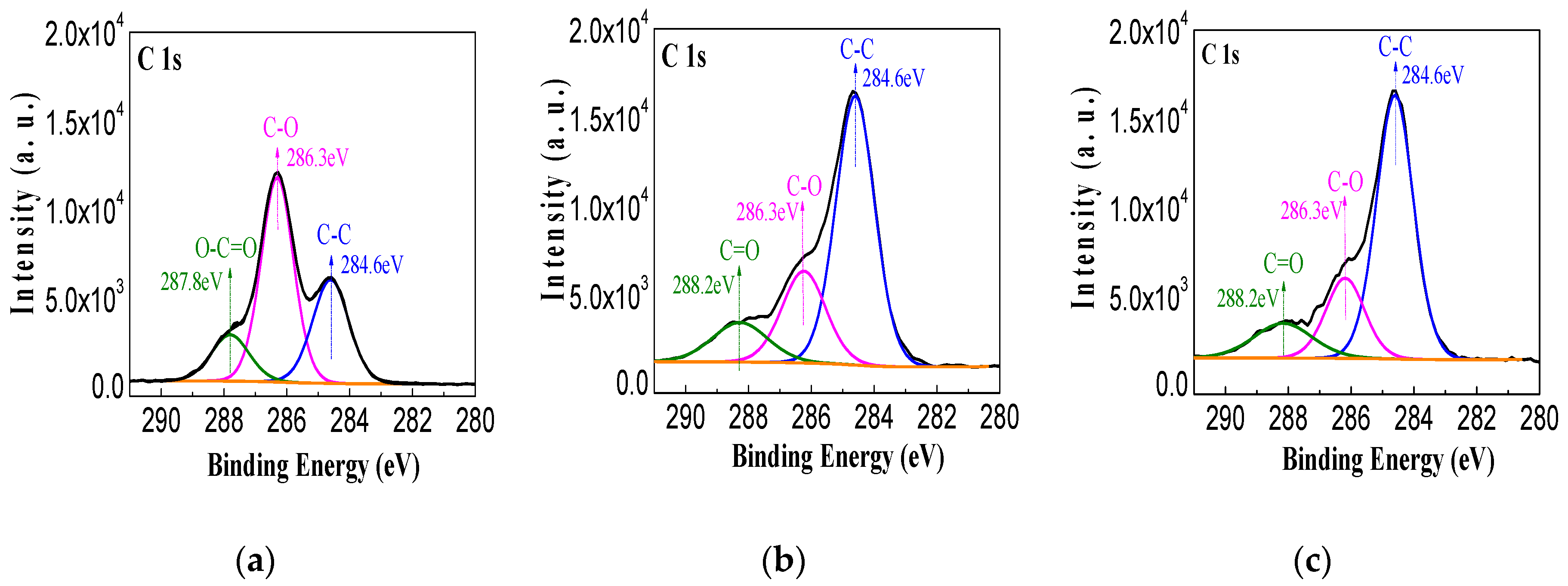
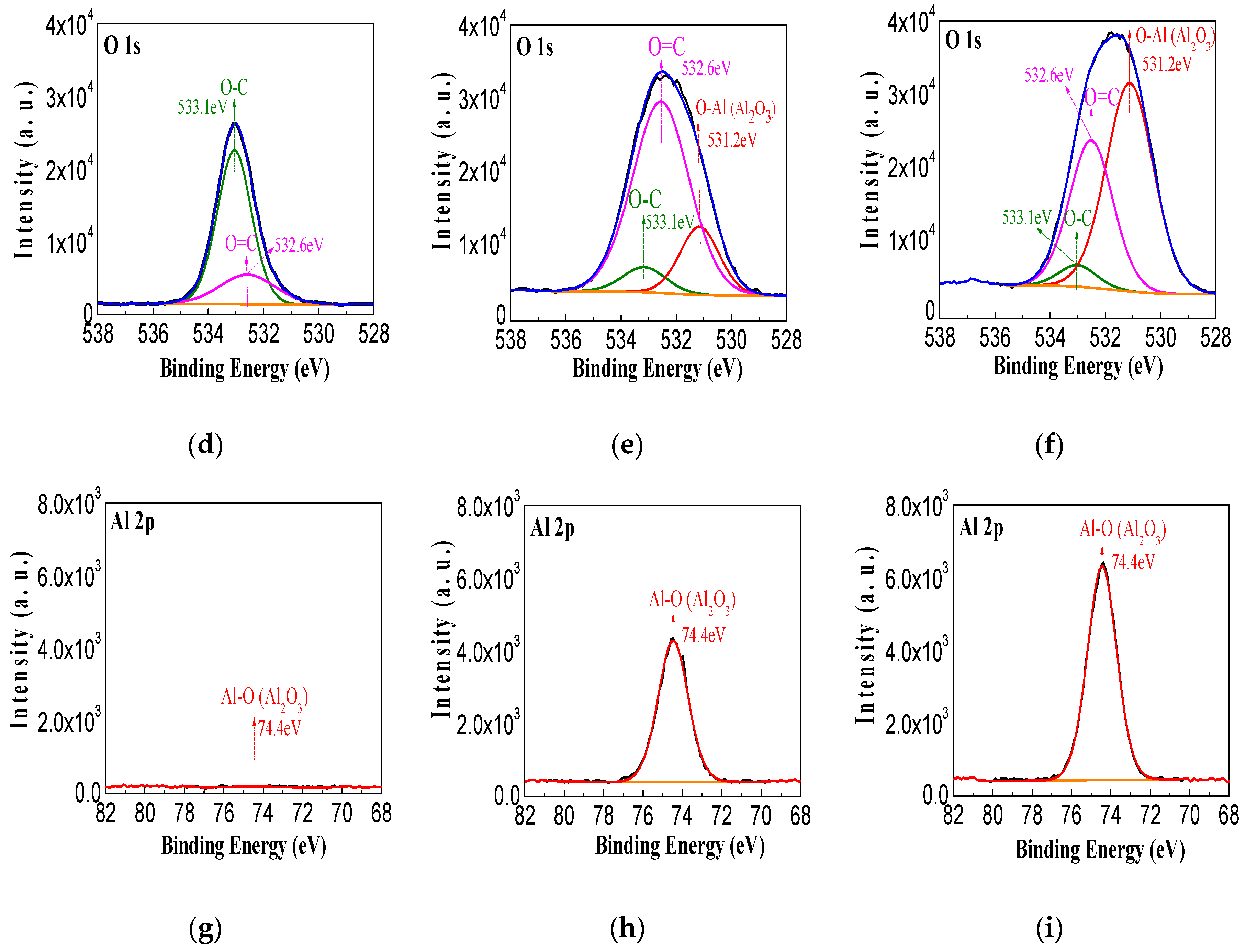
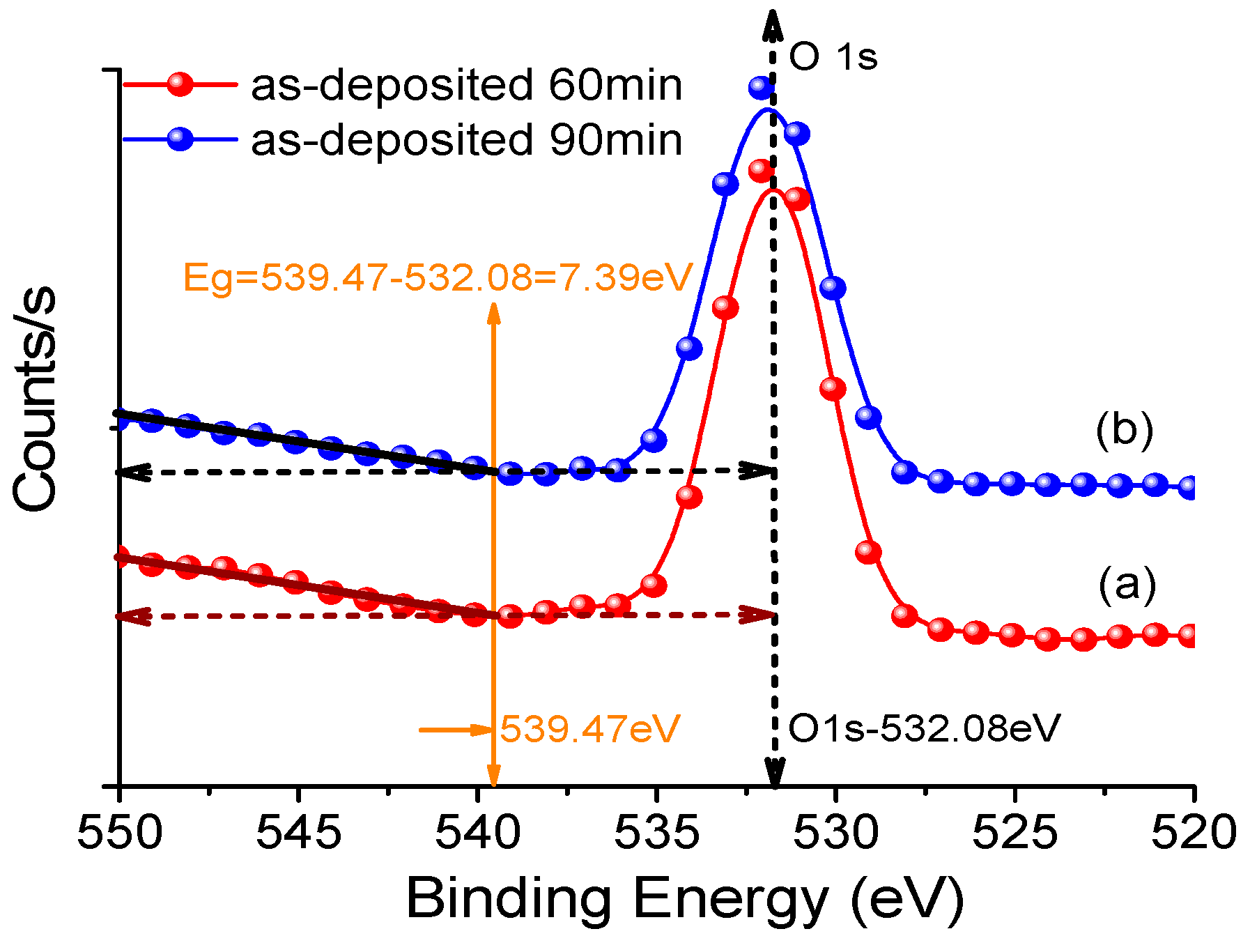
3.2. XPS of the Sputtered Film
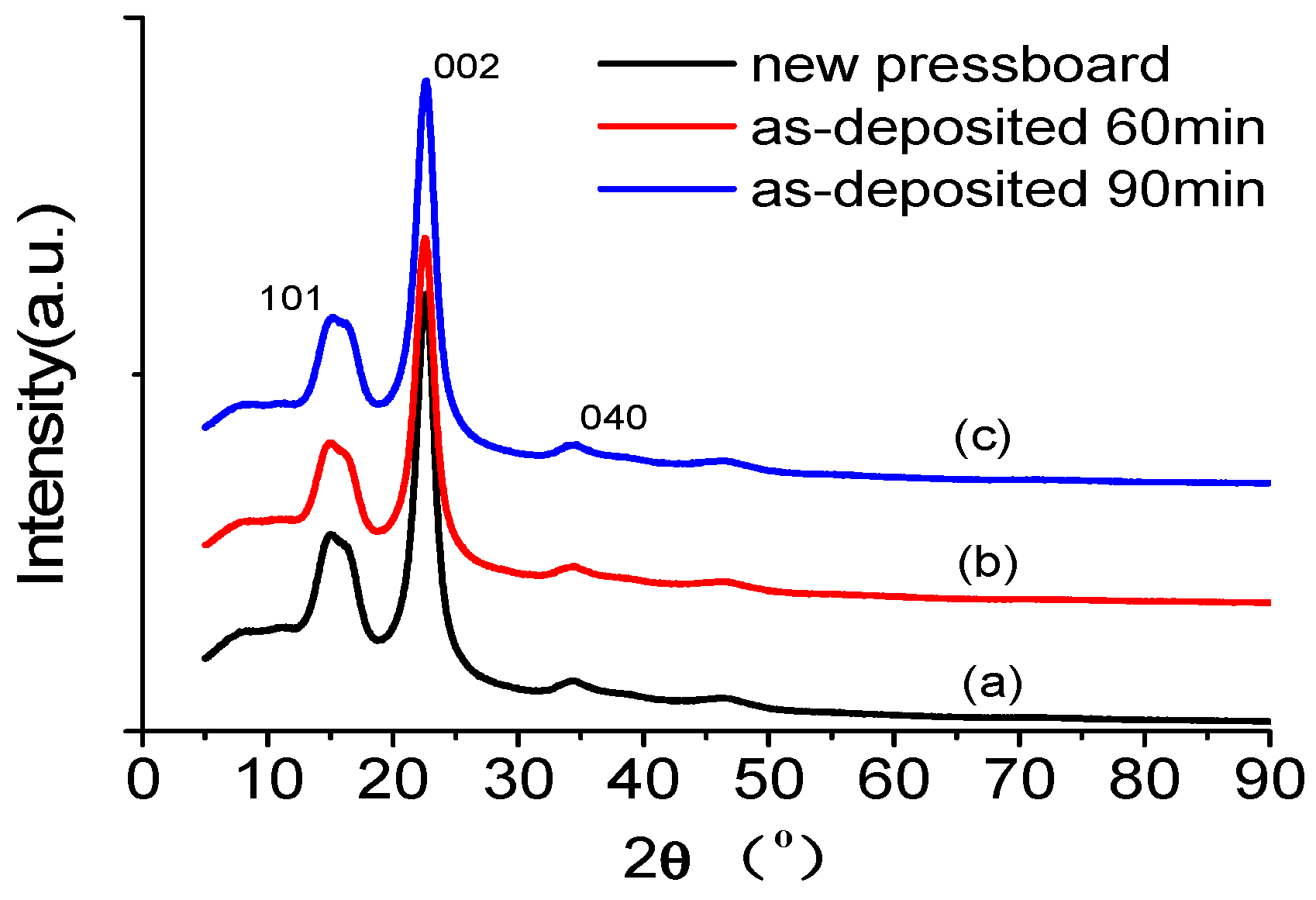
3.3. XRD of the Sputtered Pressboard with Nano-Structured Al2O3 Film
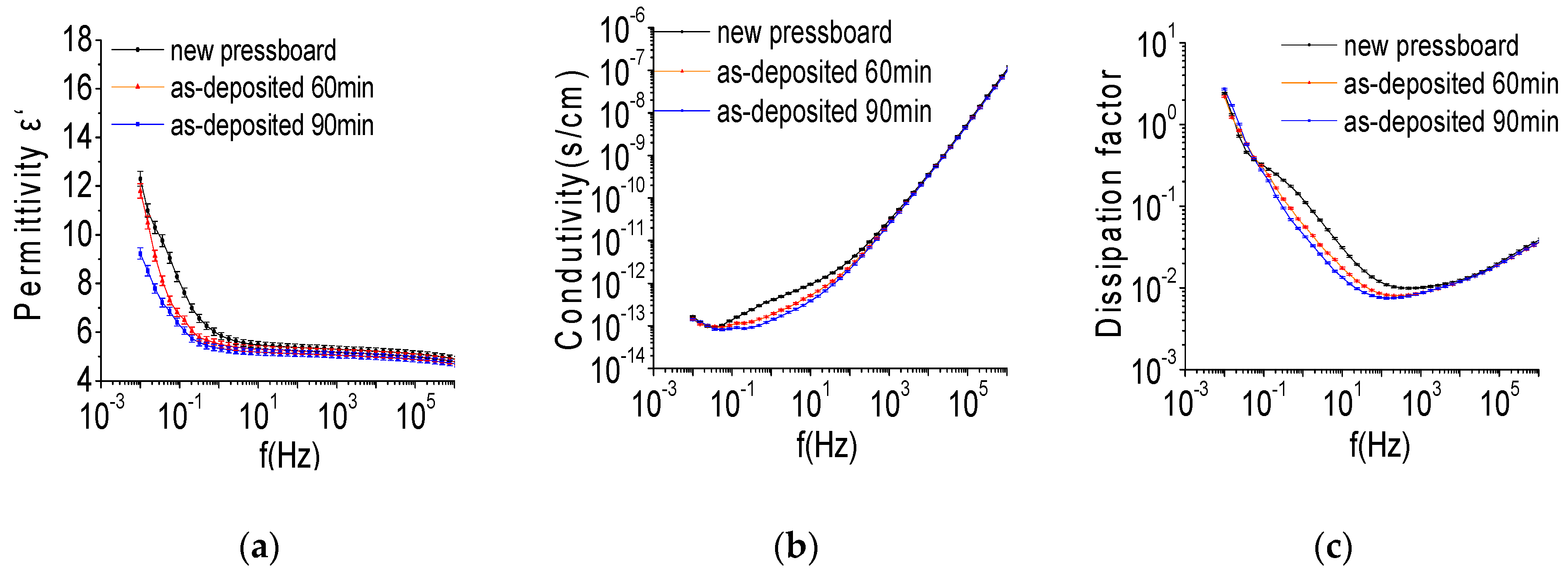
3.4. Frequency Dielectric Property of the Sputtered Pressboard
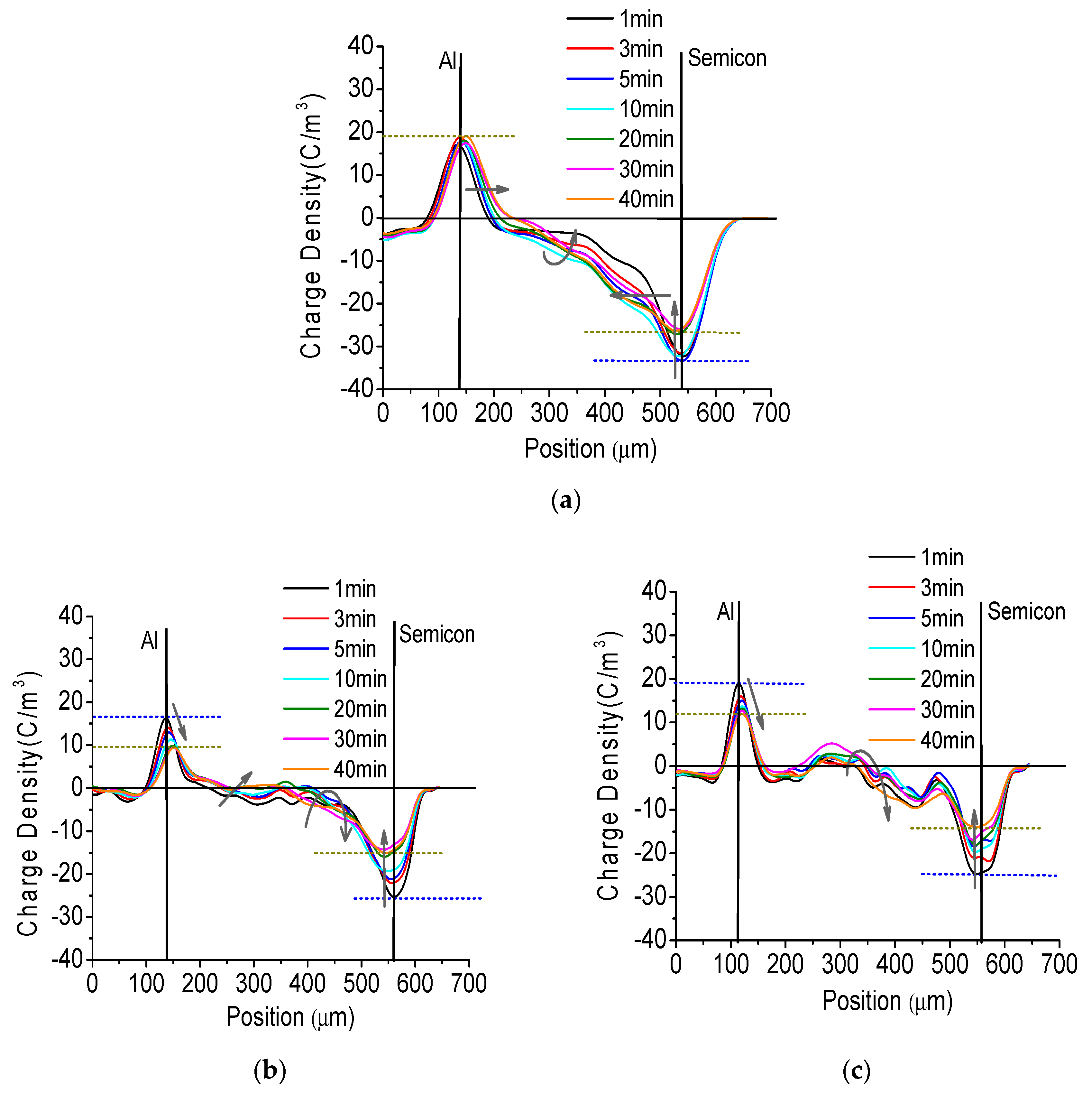
3.5. Space Charge Distribution in Sputtered Pressboard with Nano-Structured Al2O3 Film
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, J.L.; Chen, G. Insulation materials for HVDC polymeric Cables. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1307. [Google Scholar] [CrossRef]
- Tang, C.; Chen, G.; Fu, M.; Liao, R.J. Space charge behaviour in multi-layer oil-paper insulation under different DC voltages and temperatures. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 778–788. [Google Scholar] [CrossRef]
- Chen, G.; Tanaka, Y.; Takada, T.; Zhong, L. Effect of polyethylene interface on space charge formation. IEEE Trans. Dielectr. Electr. Insul. 2015, 11, 113–121. [Google Scholar] [CrossRef]
- Hao, M.; Zhou, Y.; Chen, G.; Wilson, G.; Jarman, P. Space charge behaviour in oil gap and impregnated pressboard combined system under HVDC stresses. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 848–858. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Z.Q. Charge trapping and de-trapping in polymeric materials. Appl. Phys. Lett. 2009, 106, 123707. [Google Scholar]
- Lewis, T.J. Nanometric dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 812–825. [Google Scholar] [CrossRef]
- Tanaka, T. Interpretation of several key phenomena peculiar to nano-dielectrics in terms of a multi-core model. IEEE Conf. Electr. Insul. Dielectr. Phenom. 2006, 298–301. [Google Scholar]
- Wang, S.J.; Zha, J.W.; Li, W.K.; Dang, Z.M. Distinctive electrical properties in sandwich-structured Al2O3/low density polyethylene nanocomposites. Appl. Phys. Lett. 2016, 108, 031605. [Google Scholar] [CrossRef]
- Fleming, R.J.; Ammala, A.; Casey, P.S. Conductivity and space charge in LDPE containing nano- and micro-sized ZnO particles. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 118–126. [Google Scholar] [CrossRef]
- Okuzumi, S.; Murakami, Y.; Naga, M.; Sekiguchi, Y.; Reddy, C.C.; Murata, Y. DC breakdown strength and conduction current of MgO/LDPE composite influenced by filler size. IEEE Conf. Electr. Insul. Dielectr. Phenom. 2008, 722–725. [Google Scholar]
- Lv, Z.P.; Wang, X.; Wu, K.; Chen, X.; Cheng, Y.H.; Dissado, L.A. Dependence of charge accumulation on sample thickness in nano-SiO2, doped LDPE. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 337–345. [Google Scholar]
- Katayama, J.; Ohki, Y.; Fuse, N.; Kozako, M.; Tanaka, T. Effects of nano-filler materials on the dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 157–165. [Google Scholar] [CrossRef]
- Pandey, J.C.; Gupta, N. Thermal aging assessment of epoxy-based nanocomposites by space charge and conduction current measurements. IEEE Electr. Insul. Conf. 2014, 59–63. [Google Scholar]
- Andritsch, T.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Dielectric properties and space charge behaviour of MgO epoxy nanocomposites. IEEE Int. Conf. Solid Dielectr. 2010, 1–4. [Google Scholar]
- Yan, S.; Liao, R.J.; Yang, L.J.; Zhao, X.T.; Yuan, Y.; He, L.H. Influence of nano-Al2O3 on electrical properties of insulation paper under thermal aging. IEEE Int. Conf. High Volt. Eng. Appl. 2016, 1–4. [Google Scholar]
- He, L.H.; Liao, R.J.; Lv, Y.D.; Yang, L.J.; Zhao, X.T.; Yan, S.Q. Effect of nano-Al2O3 on the thermal aging physicochemical properties of insulating paper. Int. Conf. Cond. Monit. Diagn. 2016, 254–257. [Google Scholar]
- Liao, R.J.; Lv, C.; Wu, W.Q.; Liang, N.C.; Yang, L.J. Insulating properties of insulation paper modified by nano-Al2O3 for power transformer. J. Electr. Power Sci. Technol. 2014, 29, 3–7. [Google Scholar]
- Zhang, S.; Tang, C.; Chen, G.; Zhou, Q.; Lv, C.; Xu, L.I. The influence and mechanism of nano-Al2O3 to the thermal stability of cellulose insulation paper. Sci. Sin. 2015, 45, 1167–1179. [Google Scholar]
- Wang, X.; Lv, Z.; Wu, K.; Chen, X.; Tu, D.; Dissado, L.A. Study of the factors that suppress space charge accumulation in LDPE nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1670–1679. [Google Scholar] [CrossRef]
- Liu, L.; Xu, F.; Yu, Z.; Dong, P. Facile fabrication of non-sticking super-hydrophobic boehmite film on Al foil. Appl. Surf. Sci. 2012, 258, 8928–8933. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; She, Z.; Chen, F.; Li, L.; Zhang, X.; Zhang, P. Facile and fast fabrication of superhydrophobic surface on magnesium alloy. Appl. Surf. Sci. 2013, 271, 182–192. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Wang, B.; Deng, B.; Ma, F.; Yu, Z. Preparation and anti-icing behavior of superhydrophobic surfaces on aluminum alloy substrates. Langmuir 2013, 29, 8482–8491. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.; Cannavale, A.; De Marco, L.; Arico, A.S.; Cingolani, R.; Gigli, G. Durable superhydrophobic and antireflective surfaces by trimethylsilanized silica nanoparticles-based sol-gel processing. Langmuir 2009, 25, 6357–6362. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Latthe, S.S.; Mahadik, S.A.; Kappenstein, C. Mechanically stable and corrosion resistant superhydrophobic sol–gel coatings on copper substrate. Appl. Surf. Sci. 2011, 257, 5772–5776. [Google Scholar] [CrossRef]
- Crick, C.R.; Bear, J.C.; Kafizas, A.; Parkin, I.P. Superhydrophobic photocatalytic surfaces through direct incorporation of Titania nanoparticles into a polymer matrix by aerosol assisted chemical vapor deposition. Adv. Mater. 2012, 24, 3505–3508. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, F.; Liu, X.; Jiang, Z.; Ren, L. Superhydrophobicity of bionic alumina surfaces fabricated by hard anodizing. J. Bionic Eng. 2011, 8, 369–374. [Google Scholar] [CrossRef]
- Wang, D.; Dai, X. Fabrication of superhydrophobic surfaces on aluminum. Appl. Surf. Sci. 2008, 254, 5599–5601. [Google Scholar] [CrossRef]
- Song, J.; Xu, W.; Lu, Y. One-step electrochemical machining of superhydrophobic surfaces on aluminum substrates. J. Mater. Sci. 2012, 47, 162–168. [Google Scholar] [CrossRef]
- Liao, R.J.; Zuo, Z.P.; Guo, C.; Zhuang, A.Y.; Zhao, X.T.; Yuan, Y. Anti-icing performance in glaze ice of nanostructured film prepared by RF magnetron sputtering. Appl. Surf. Sci. 2015, 356, 539–545. [Google Scholar] [CrossRef]
- Miyashita, S.; Toyota, H. Effect of ion irradiation for Ni films prepared on a flexible substrate material using magnetron sputtering with multipolar magnetic plasma confinement assisted by inductively coupled plasma. J. Vac. Soc. Jpn. 2015, 58, 319–323. [Google Scholar] [CrossRef]
- Kylián, O.; Drábik, M.; Polonskyi, O.; Čechvala, J.; Artemenko, A.; Gordeev, I.; Choukourov, A.; Matolínová, I.; Slavínská, D.; Biederman, H. Deposition of nanostructured fluorocarbon plasma polymer films by RF magnetron sputtering of polytetrafluoroethylene. Thin Solid Films 2011, 519, 6426–6431. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Qian, L.Q.; Guo, C.; Jia, X.; Wang, J.W.; Tang, W.H. Natural superhydrophilic TiO2/SiO2 composite thin films deposited by radio frequency magnetron sputtering. J. Alloy. Compd. 2009, 479, 532–535. [Google Scholar] [CrossRef]
- Bobzin, K.; Lugscheider, E.; Maes, M.; Piñero, C. Relation of hardness and oxygen flow of Al2O3 coatings deposited by reactive bipolar pulsed magnetron sputtering. Thin Solid Films 2009, 494, 255–262. [Google Scholar] [CrossRef]
- Beysens, D.; Chatain, D.; Evesque, P.; Garrabos, Y. Synthesis of α-Al2O3 thin films using reactive high-power impulse magnetron sputtering. Euro Phys. Lett. 2006, 3, 36002–36005. [Google Scholar]
- Kohara, T.; Tamagaki, H.; Ikari, Y.; Fujii, H. Deposition of α-Al2O3 hard coatings by reactive magnetron sputtering. Surf. Coat. Technol. 2004, 185, 166–171. [Google Scholar] [CrossRef]
- Xu, Z.Q. Space Charge Measurement and Analysis in Low Density Polyethylene Film. Ph.D. Thesis, University of Southampton, Southampton, UK, 2009. [Google Scholar]
- Sahari, S.K.; Kashif, M.; Sutan, N.M.; Embong, Z.; Nik, N.A.F.; Hamzah, A.A.; Majlis, B.Y.; Ahmad, I. Growth kinetic and composition of the interfacial layer for RF Sputtering Al2O3 Layer on Germanium. Microelectron. Int. 2017, 34, 64. [Google Scholar] [CrossRef]
- Nowicki, R.S. Properties of RF sputtered Al2O3 films deposited by planar magnetron. J. Vac. Sci. Technol. 1977, 14, 127. [Google Scholar] [CrossRef]
- Liao, R.J.; Tang, C.; Yang, L.J.; Grzybowski, S. Thermal aging micro-scale analysis of power transformer pressboard. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1281–1287. [Google Scholar] [CrossRef]
- Yang, L.J.; Liao, R.J.; Sun, C.X.; Zhu, M.Z. Influence of vegetable oil on the thermal aging of transformer paper and its mechanism. IEEE Trans. Dielectr. Electr. Insul. 2012, 18, 2059. [Google Scholar]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Ałęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS characterization of ALD Al2O3 coated porous silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Lee, W.J.; Ma, J.W.; Bae, J.M. The diffusion of silicon atoms in stack structures of La2O3 and Al2O3. Curr. Appl. Phys. 2013, 13, 633–639. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.H.; Lee, S.C.; Chung, Y.W. Tribological and optical properties of crystalline and amorphous alumina thin films grown by low-temperature reactive magnetron sputter-deposition. Surf. Coat. Technol. 2001, 146, 189–194. [Google Scholar] [CrossRef]
- Kot, M.; Das, C.; Wang, Z.; Henkel, K.; Rouissi, Z.; Wojciechowski, K. Room-temperature atomic layer deposition of Al2O3: Impact on efficiency, stability and surface properties in perovskite solar cells. ChemSusChem 2016, 9, 3401. [Google Scholar] [CrossRef] [PubMed]
- Naumann, V.; Otto, M.; Wehrspohn, R.B.; Werner, M.; Hagendorf, C. Interface and material characterization of thin ALD-Al2O3 layers on crystalline silicon. Energy Procedia 2012, 27, 312. [Google Scholar] [CrossRef]
- Thornton, J.A. Structure and heat treatment characteristics of sputter-deposited alumina. J. Am. Ceram. Soc. Bull. 1977, 56, 504. [Google Scholar]
- Chou, T.C.; Adamson, D.; Mardinly, J.; Nieh, T.G. Microstructural evolution and properties of nanocrystalline alumina made by reactive sputtering deposition. Thin Solid Films 1991, 205, 131. [Google Scholar]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the interpretation of X-Ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol. Chem. Phys. 2005, 206, 1568. [Google Scholar] [CrossRef]
- Nelson, J.K.; Fothergill, J.C. Internal charge behavior of nanocomposites. Nanotechnology 2004, 15, 586–595. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric Nanocomposites with Insulating Properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Takada, T.; Hayase, Y.; Tanaka, Y.; Okamoto, T. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Milliere, L.; Maskasheva, K.; Laurent, C.; Despax, B.; Boudou, L.; Teyssedre, G. Silver nanoparticles as a key feature of a plasma polymer composite layer in mitigation of charge injection into polyethylene under dc stress. J. Phys. D Appl. Phys. 2016, 49, 015304. [Google Scholar] [CrossRef]
- Min, D.; Wang, W.; Li, S.T. Numerical analysis of space charge accumulation and conduction properties in LDPE nano-dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1483–1491. [Google Scholar]
- Nichols, M.T.; Li, W.; Pei, D.; Antonelli, G.A.; Lin, Q.; Banna, S.; Nishi, Y.; Shohet, J.L. Measurement of band gap energies in low-k organosilicates. J. Appl. Phys. 2014, 115, 094105. [Google Scholar] [CrossRef]
- Sampath, S.; Maydannik, P.; Ivanova, T.; Homola, T.; Sillanpää, M.; Nagumothu, R.; Alagan, V. Structural and morphological characterization of Al2O3 coated macro-porous silicon by atomic layer deposition. Thin Solid Films 2016, 616, 628. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Li, Y.; Liao, R.; Liu, G.; Liao, Q.; Tang, C. Fabrication of Al2O3 Nano-Structure Functional Film on a Cellulose Insulation Polymer Surface and Its Space Charge Suppression Effect. Polymers 2017, 9, 502. https://doi.org/10.3390/polym9100502
Hao J, Li Y, Liao R, Liu G, Liao Q, Tang C. Fabrication of Al2O3 Nano-Structure Functional Film on a Cellulose Insulation Polymer Surface and Its Space Charge Suppression Effect. Polymers. 2017; 9(10):502. https://doi.org/10.3390/polym9100502
Chicago/Turabian StyleHao, Jian, Yanqing Li, Ruijin Liao, Guoyong Liu, Qiang Liao, and Chao Tang. 2017. "Fabrication of Al2O3 Nano-Structure Functional Film on a Cellulose Insulation Polymer Surface and Its Space Charge Suppression Effect" Polymers 9, no. 10: 502. https://doi.org/10.3390/polym9100502





