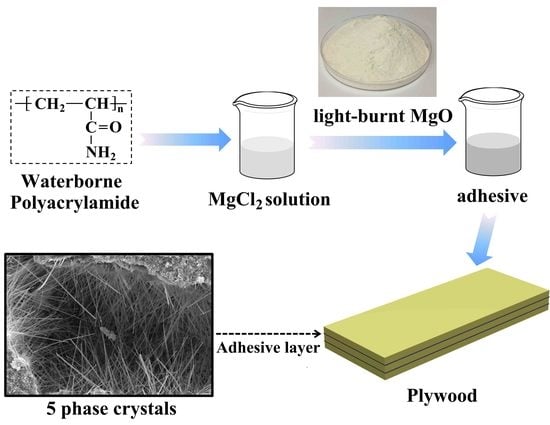
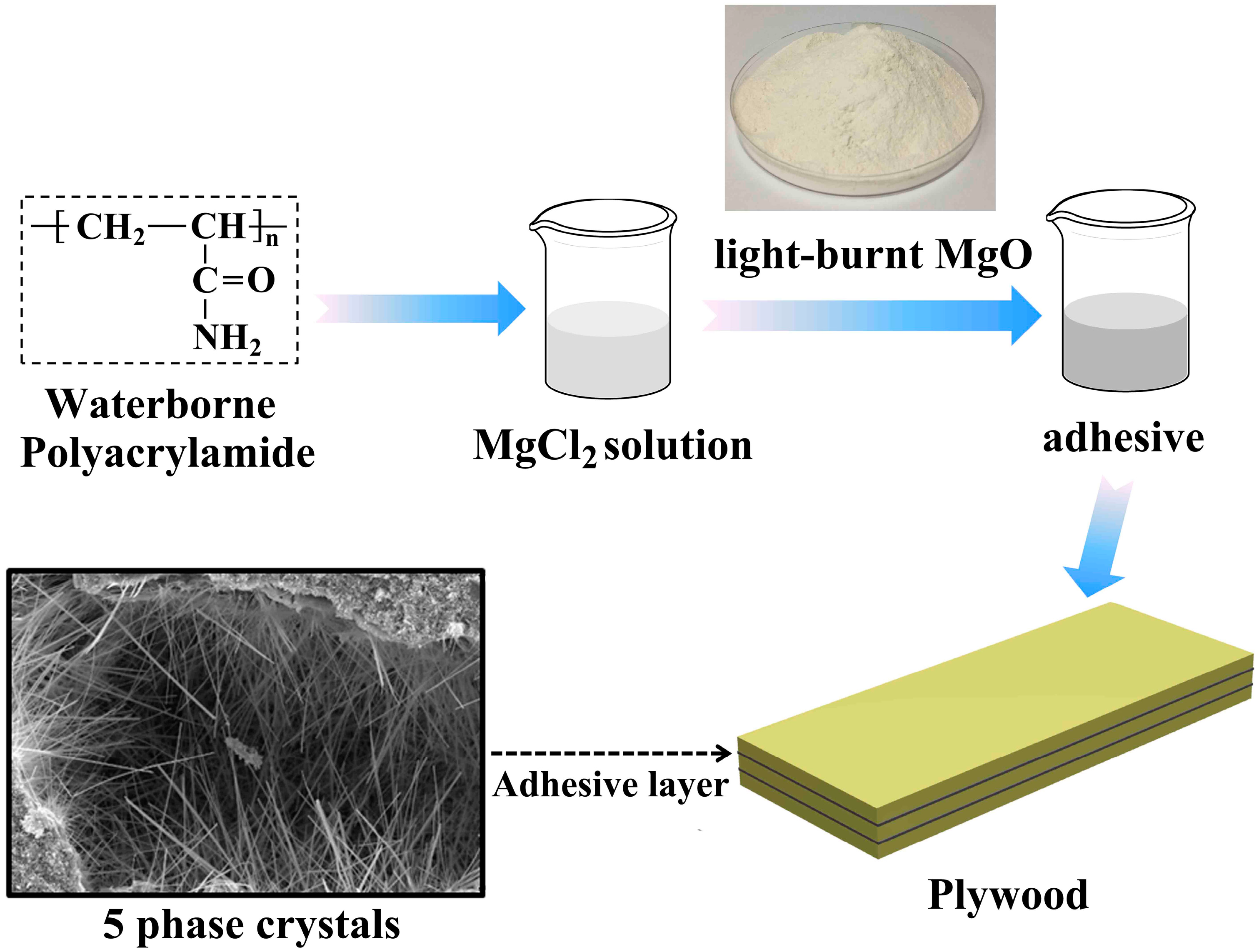
A Low-Cost, Formaldehyde-Free and High Flame Retardancy Wood Adhesive from Inorganic Adhesives: Properties and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MOC Adhesives and Plywood Samples
2.3. Characterization
2.3.1. Viscoelastic Measurement
2.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.3. X-ray Diffraction (XRD)
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Thermogravimetric Analysis
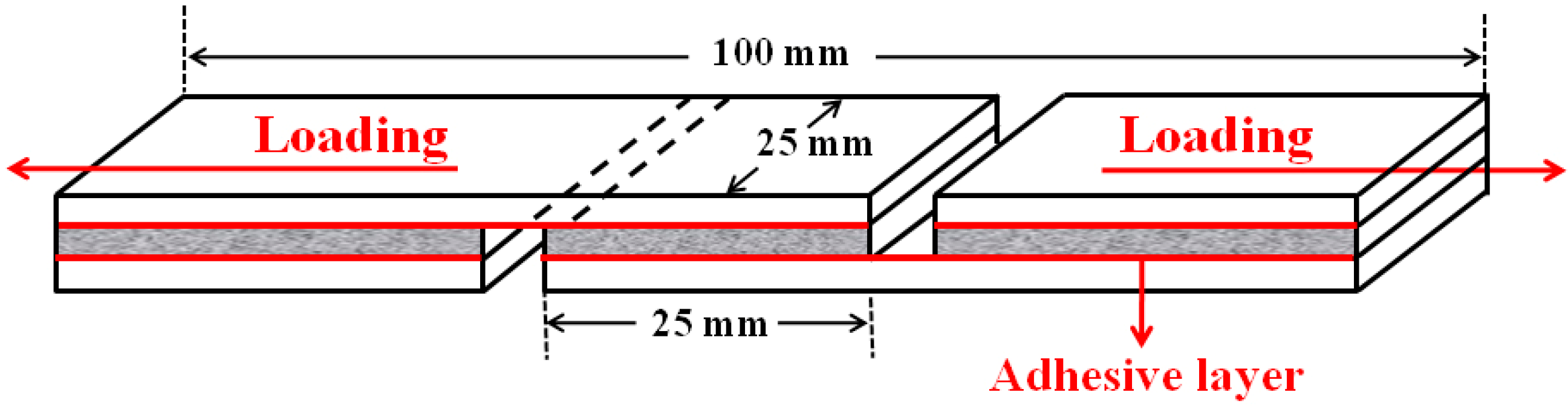
2.3.6. Shear Strength
2.3.7. Limiting Oxygen Index
3. Results and Discussion
3.1. Viscoelastic Measurement
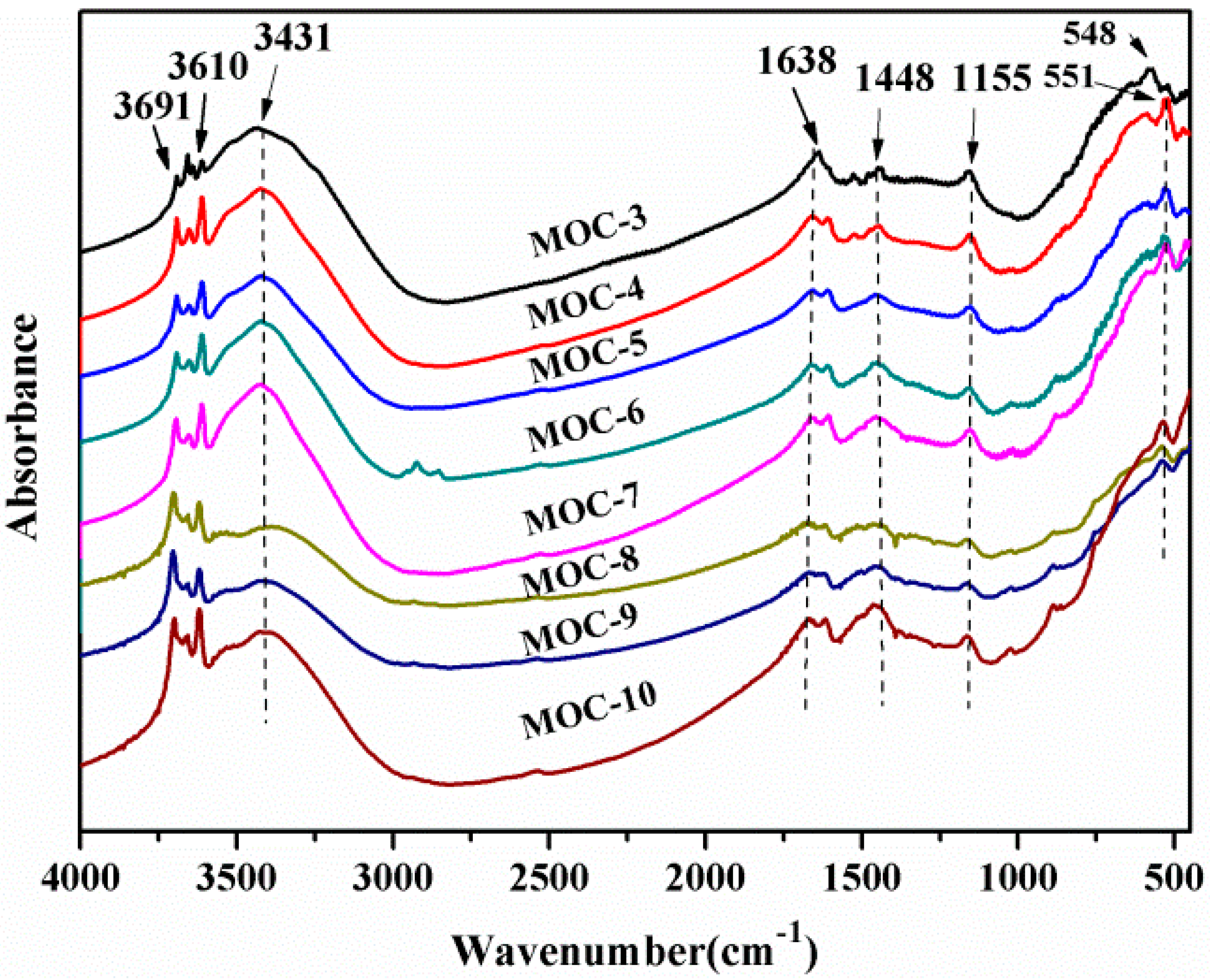
3.2. FTIR Spectroscopic Analysis
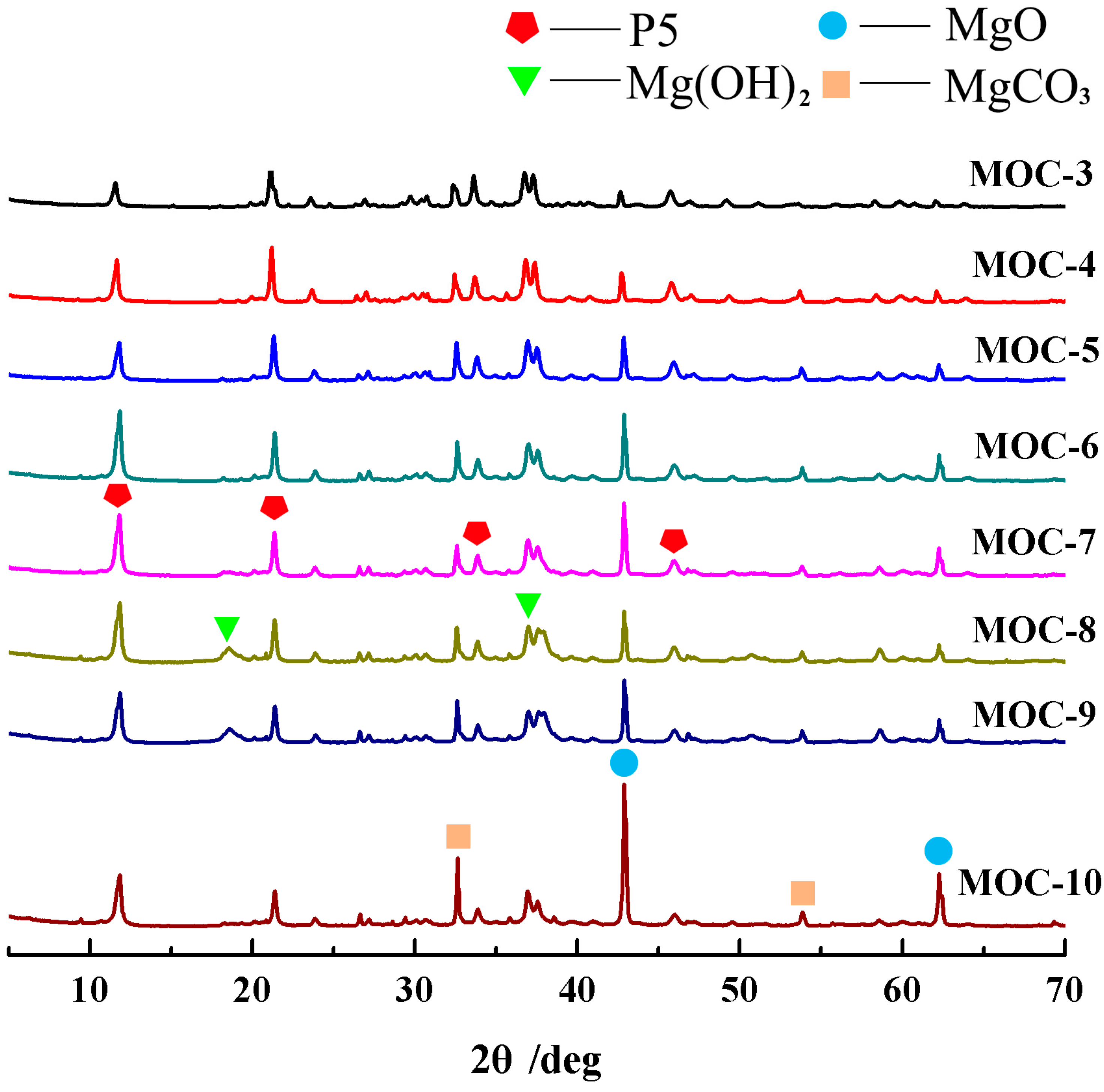
3.3. X-ray Diffraction
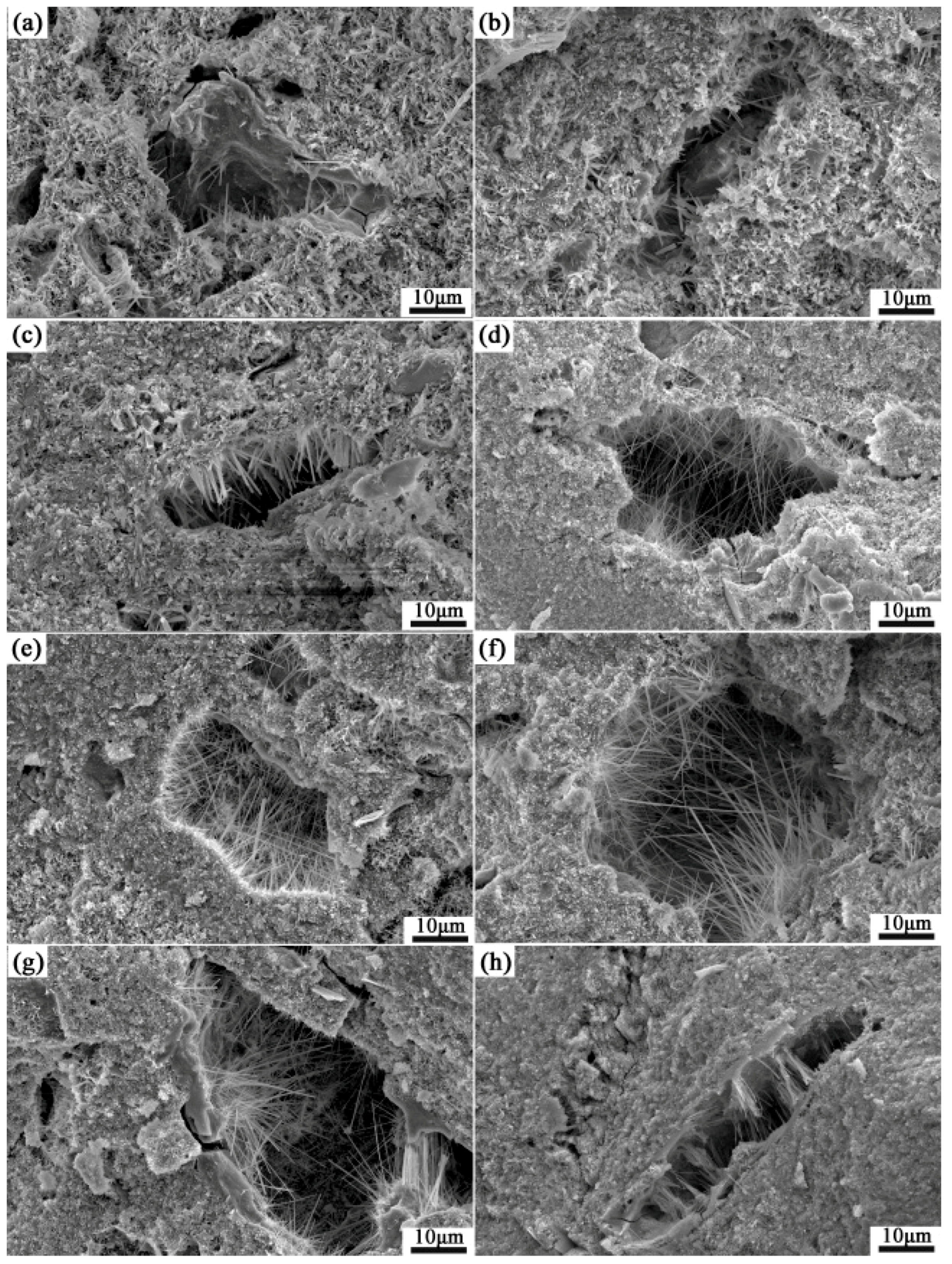
3.4. Microstructure
3.5. Thermal Stability
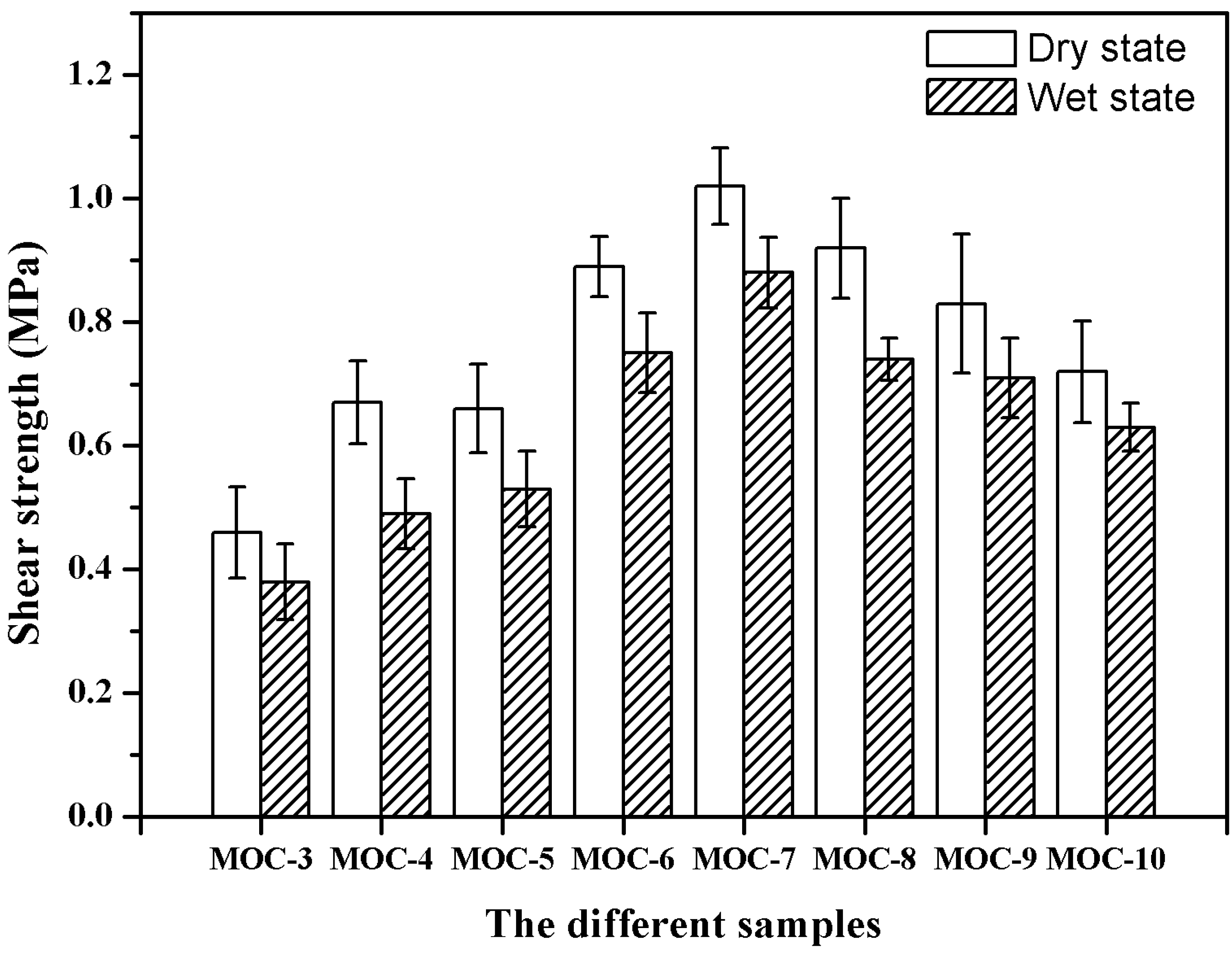
3.6. Plywood Performance
3.7. Flame Retardancy of Plywood
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crop. Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- Li, K.; Chen, H.; Li, Y.; Li, J.; He, J. Endogenous Cu and Zn nanocluster-regulated soy protein isolate films: Excellent hydrophobicity and flexibility. RSC Adv. 2015, 5, 66543–66548. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. A high-performance bio-adhesive derived from soy protein isolate and condensed tannins. RSC Adv. 2017, 7, 21226–21233. [Google Scholar] [CrossRef]
- Quiroga, A.; Marzocchi, V.; Rintoul, I. Influence of wood treatments on mechanical properties of wood–cement composites and of Populus Euroamericana wood fibers. Compos. Part B Eng. 2016, 84, 25–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Lu, Y.; Gao, Z.; Gu, J. Nano-scale blocking mechanism of MMT and its effects on the properties of polyisocyanate-modified soybean protein adhesive. Ind. Crop. Prod. 2014, 57, 35–42. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A high-performance soy protein isolate-based nanocomposite film modified with microcrystalline cellulose and Cu and Zn nanoclusters. Polymers 2017, 9, 167. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Synthesis and mechanism of metal-mediated polymerization of phenolic resins. Polymers 2016, 8, 159. [Google Scholar] [CrossRef]
- Song, Y.H.; Seo, J.H.; Choi, Y.S.; Kim, D.H.; Choi, B.-H.; Cha, H.J. Mussel adhesive protein as an environmentally-friendly harmless wood furniture adhesive. Int. J. Adhes. Adhes. 2016, 70, 260–264. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z. Light-weight wood-magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Liu, X.; Chen, H.; He, J.; Li, J. Preparation and characterization of chitosan/soy protein isolate nanocomposite film reinforced by Cu nanoclusters. Polymers 2017, 9, 247. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xu, B.; Ma, H.; Chen, B.; Li, Z. Micromechanical investigation of magnesium oxychloride cement paste. Constr. Build. Mater. 2016, 105, 496–502. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Using incinerated sewage sludge ash to improve the water resistance of magnesium oxychloride cement (MOC). Constr. Build. Mater. 2017, 147, 519–524. [Google Scholar] [CrossRef]
- Lu, C.; Lu, Z.; Li, Z.; Leung, C.K.Y. Effect of graphene oxide on the mechanical behavior of strain hardening cementitious composites. Constr. Build. Mater. 2016, 120, 457–464. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Li, Z.; Li, H. Simulation of the properties of MgO-MgCl2-H2O system by thermodynamic method. Cement Concr. Res. 2015, 68, 105–111. [Google Scholar] [CrossRef]
- Plekhanova, T.A.; Keriene, J.; Gailius, A.; Yakovlev, G.I. Structural, physical and mechanical properties of modified wood–magnesia composite. Constr. Build. Mater. 2007, 21, 1833–1838. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Rallini, M.; Ubertini, F.; Materazzi, A.L.; Kenny, J.M. Investigations on scalable fabrication procedures for self-sensing carbon nanotube cement-matrix composites for SHM applications. Cement Concr. Compos. 2016, 65, 200–213. [Google Scholar] [CrossRef]
- Al-Dahawi, A.; Öztürk, O.; Emami, F.; Yıldırım, G.; Şahmaran, M. Effect of mixing methods on the electrical properties of cementitious composites incorporating different carbon-based materials. Constr. Build. Mater. 2016, 104, 160–168. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Halasz, I.; Freyer, D.; Hanson, J.C. The crystal structures of two anhydrous magnesium hydroxychloride phases from in situ synchrotron powder diffraction data. Z. Anorg. Allg. Chem. 2011, 637, 1458–1462. [Google Scholar] [CrossRef]
- Li, Z.J.; Qiao, F.; Chau, C.K. Recent development of magnesium-based cements—Magnesium phosphate cement and magnesium oxychloride cement. Adv. Sci. Technol. 2010, 69, 21–30. [Google Scholar] [CrossRef]
- Qiao, H.X.; Gong, W.; Shi, Y.Y.; Wanjiru, M.E.; Dong, J.M. Experimental study on magnesium oxychloride cement concrete. Emerg. Mater. Res. 2016, 5, 248–255. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Pei, H.; Yu, H. The influence of FeSO4 and KH2PO4 on the performance of magnesium oxychloride cement. Constr. Build. Mater. 2016, 102, 233–238. [Google Scholar] [CrossRef]
- Xu, K.; Xi, J.; Guo, Y.; Dong, S. Effects of a new modifier on the water-resistance of magnesite cement tiles. Solid State Sci. 2012, 14, 10–14. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Guan, L.; Hu, S. Microstructure and properties of cement foams prepared by magnesium oxychloride cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 331–337. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, Y.; Grover, L. Effect of phosphoric acid on the properties of magnesium oxychloride cement as a biomaterial. Cement Concr. Res. 2014, 56, 69–74. [Google Scholar] [CrossRef]
- Li, Z.; Chau, C.K. Influence of molar ratios on properties of magnesium oxychloride cement. Cement Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Sun, X.S. Soy-oil-based waterborne polyurethane improved wet strength of soy protein adhesives on wood. Int. J. Adhes. Adhes. 2017, 73, 66–74. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zia Ud, D.; Fei, P.; Jin, W.; Xiong, H.; Wang, Z. Enhancing the performance of starch-based wood adhesive by silane coupling agent (KH570). Int. J. Biol. Macromol. 2017, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhang, Y.; Weng, X. Preparation of the plywood using starch-based adhesives modified with blocked isocyanates. Procedia Eng. 2011, 15, 1171–1175. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Li, Z. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater. Struct. 2015, 49, 1319–1326. [Google Scholar] [CrossRef]
- Karimi, Y.; Monshi, A. Effect of magnesium chloride concentrations on the properties of magnesium oxychloride cement for nano SiC composite purposes. Ceram. Int. 2011, 37, 2405–2410. [Google Scholar] [CrossRef]
- Góchez, R.; Wambaugh, J.; Rochner, B.; Kitchens, C.L. Kinetic study of the magnesium oxychloride cement cure reaction. J. Mater. Sci. 2017, 52, 7637–7646. [Google Scholar] [CrossRef]
- Wang, F.; Sun, G.; Zhang, W.; Yang, L.; Liu, P. Performance of photocatalytic cementitious material: Influence of substrate surface microstructure. Constr. Build. Mater. 2016, 110, 175–181. [Google Scholar] [CrossRef]
- Tian, L.; Tahmasebi, A.; Yu, J. An experimental study on thermal decomposition behavior of magnesite. J. Therm. Anal. Calorim. 2014, 118, 1577–1584. [Google Scholar] [CrossRef]
- Chau, C.K.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cement Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Dehua, D.; Chuanmei, Z. The formation mechanism of the hydrate phases in magnesium oxychloride cement. Cement Concr. Res. 1998, 29, 1365–1371. [Google Scholar] [CrossRef]
- Yuan, C.; Luo, J.; Luo, J.; Gao, Q.; Li, J. A soybean meal-based wood adhesive improved by a diethylene glycol diglycidyl ether: Properties and performance. RSC Adv. 2016, 6, 74186–74194. [Google Scholar] [CrossRef]
- Zhao, M.; Jing, J.; Zhu, Y.; Yang, X.; Wang, X.; Wang, Z. Preparation and performance of lignin–phenol–formaldehyde adhesives. Int. J. Adhes. Adhes. 2016, 64, 163–167. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Zhang, X.; Zuo, Y. Study on the effect of organic additives and inorganic fillers on properties of sodium silicate wood adhesive modified by polyvinyl alcohol. Bioresources 2015, 10, 1528–1542. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Bernard, G.M. Preparation and characterization of canola protein isolate-poly(glycidyl methacrylate) conjugates: A bio-based adhesive. Ind. Crop. Prod. 2014, 57, 124–131. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Jiang, X.; Li, F.; Li, X.; Zhang, W.; Jiang, J.S.; Liu, J.; Ariga, K.; Hu, M. Coordination polymer nanoglue: Robust adhesion based on collective lamellar stacking of nanoplates. ACS Nano 2017, 11, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, G. Influence of molecular weight of PEG on thermal and fire protection properties of PEPA-containing polyether flame retardants with high water solubility. Prog. Org. Coat. 2016, 90, 390–398. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.; Wang, H.; Chen, R. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.Q.; Chen, Z.; Chi, L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef] [PubMed]


| Material | MgO | SiO2 | Fe2O3 | CaO | Eu2O3 |
|---|---|---|---|---|---|
| Magnesia | 94.38% | 1.54% | 2.84% | 1.06% | 0.18% |
| Adhesives | MOC-3 | MOC-4 | MOC-5 | MOC-6 | MOC-7 | MOC-8 | MOC-9 | MOC-10 |
|---|---|---|---|---|---|---|---|---|
| Viscosity (mPa·s) | 1653 | 1821 | 3463 | 5971 | 7126 | 7930 | 11,410 | 15,749 |
| Samples | Tmax of Thermal Event (°C) | Mass Loss Ratio (%) at 700 °C | ||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | ||
| MOC-3 | 86.84 | 151.58 | - | 401.89 | 509.28 | 55.64 |
| MOC-4 | 83.52 | 160.65 | 309.63 | 387.52 | 517.59 | 49.07 |
| MOC-5 | 84.27 | 154.60 | 305.85 | 376.93 | 522.13 | 45.82 |
| MOC-6 | 85.79 | 152.33 | 303.58 | 373.15 | 523.64 | 43.18 |
| MOC-7 | 86.54 | 151.06 | 304.34 | 370.13 | 525.16 | 41.56 |
| MOC-8 | 85.03 | 147.04 | 308.87 | 373.91 | 509.28 | 42.32 |
| MOC-9 | 89.57 | 145.53 | 311.14 | 377.69 | 510.79 | 40.02 |
| MOC-10 | 94.10 | 144.77 | 307.36 | 371.64 | 515.33 | 35.13 |
| Adhesives | MOC-3 | MOC-4 | MOC-5 | MOC-6 | MOC-7 | MOC-8 | MOC-9 | MOC-10 |
|---|---|---|---|---|---|---|---|---|
| Dry strength (MPa) | 0.48 | 0.67 | 0.66 | 0.89 | 1.02 | 0.91 | 0.83 | 0.72 |
| Wet strength (MPa) | 0.38 | 0.49 | 0.53 | 0.75 | 0.88 | 0.74 | 0.71 | 0.63 |
| Structure | Wet Shear Strength (MPa) | Reference |
|---|---|---|
| Soy flour adhesive | 0.35 | [38] |
| Condensed tannins-SPI adhesive | 0.86 | [3] |
| blocked isocyanates-Starch adhesives | 0.73 | [29] |
| Lignin-phenol-formaldehyde adhesives | 0.89 | [39] |
| Polysaccharide-SPI adhesive | 0.85 | [11] |
| MOC inorganic adhesive | 0.88 | this work |
| Samples | Treatment | LOI (%) |
|---|---|---|
| A | Urea-formaldehyde resin | 24.8 ± 0.02 |
| MOC-3 | n(MgO):n(MgCl2) = 3:1 | 23.9 ± 0.02 |
| MOC-4 | n(MgO):n(MgCl2) = 4:1 | 24.5 ± 0.01 |
| MOC-5 | n(MgO):n(MgCl2) = 5:1 | 25.7 ± 0.01 |
| MOC-6 | n(MgO):n(MgCl2) = 6:1 | 26.2 ± 0.02 |
| MOC-7 | n(MgO):n(MgCl2) = 7:1 | 27.4 ± 0.01 |
| MOC-8 | n(MgO):n(MgCl2) = 8:1 | 27.2 ± 0.02 |
| MOC-9 | n(MgO):n(MgCl2) = 9:1 | 27.5 ± 0.02 |
| MOC-10 | n(MgO):n(MgCl2) = 10:1 | 27.8 ± 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Li, K.; Li, J.; Chen, H. A Low-Cost, Formaldehyde-Free and High Flame Retardancy Wood Adhesive from Inorganic Adhesives: Properties and Performance. Polymers 2017, 9, 513. https://doi.org/10.3390/polym9100513
Jin S, Li K, Li J, Chen H. A Low-Cost, Formaldehyde-Free and High Flame Retardancy Wood Adhesive from Inorganic Adhesives: Properties and Performance. Polymers. 2017; 9(10):513. https://doi.org/10.3390/polym9100513
Chicago/Turabian StyleJin, Shicun, Kuang Li, Jianzhang Li, and Hui Chen. 2017. "A Low-Cost, Formaldehyde-Free and High Flame Retardancy Wood Adhesive from Inorganic Adhesives: Properties and Performance" Polymers 9, no. 10: 513. https://doi.org/10.3390/polym9100513