A Single-Site Iron(III)-Salan Catalyst for Converting COS to Sulfur-Containing Polymers
Abstract
:1. Introduction
2. Materials and Methods
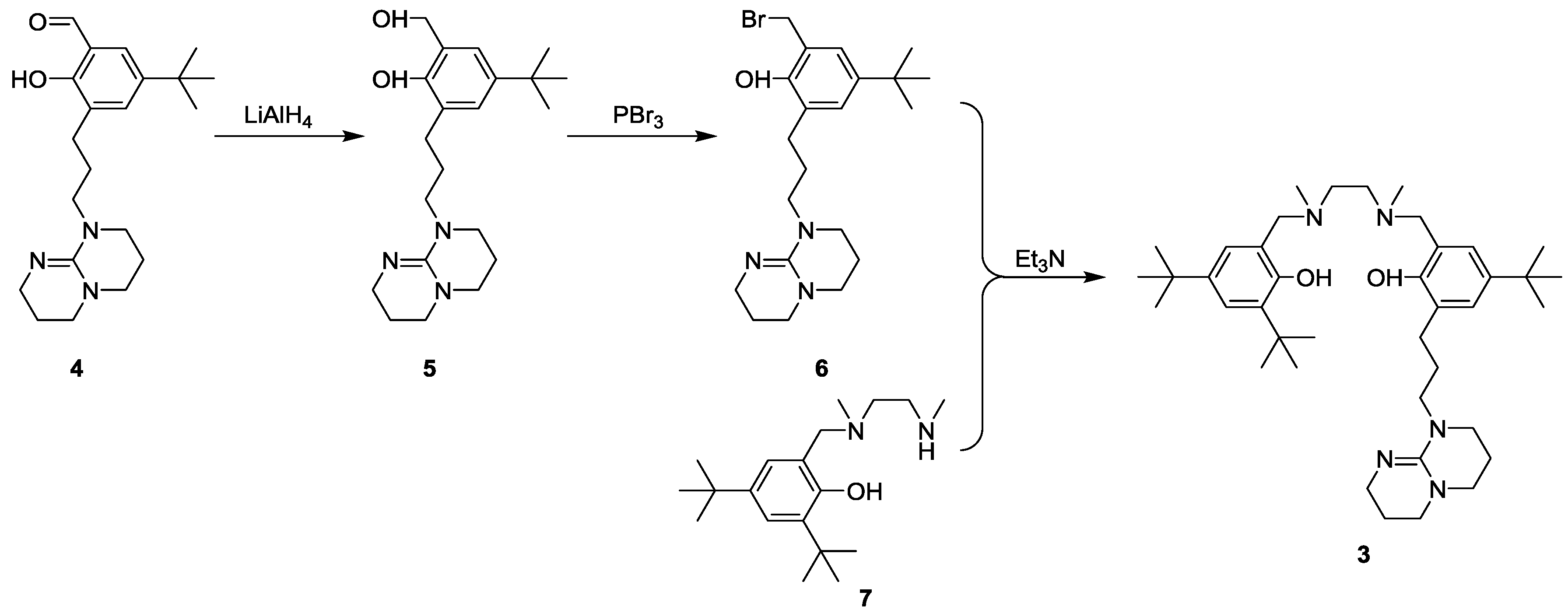
2.1. Synthesis of the Ligand
2.1.1. Synthesis of Compound 5
2.1.2. Synthesis of Compound 6
2.1.3. Synthesis of the Ligand 3
2.2. Synthesis of Complexes
2.3. Representative Procedures for COS/Epoxides Copolymerization
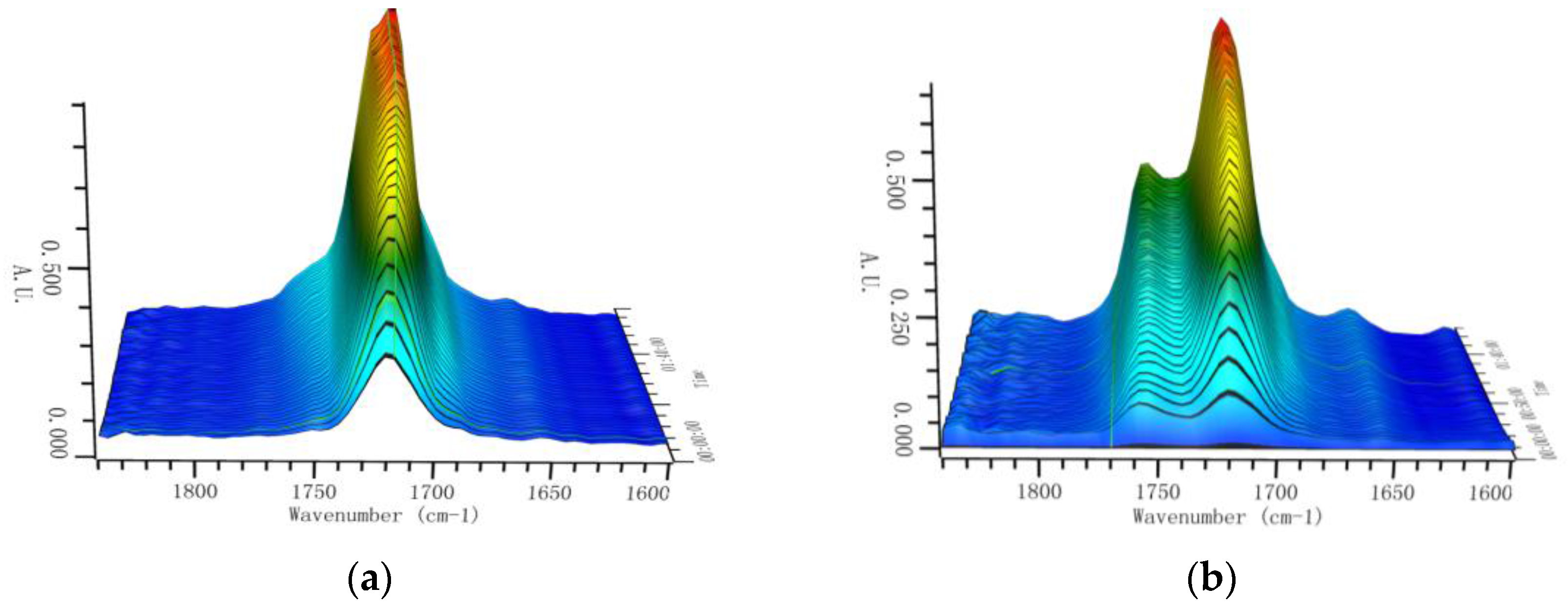
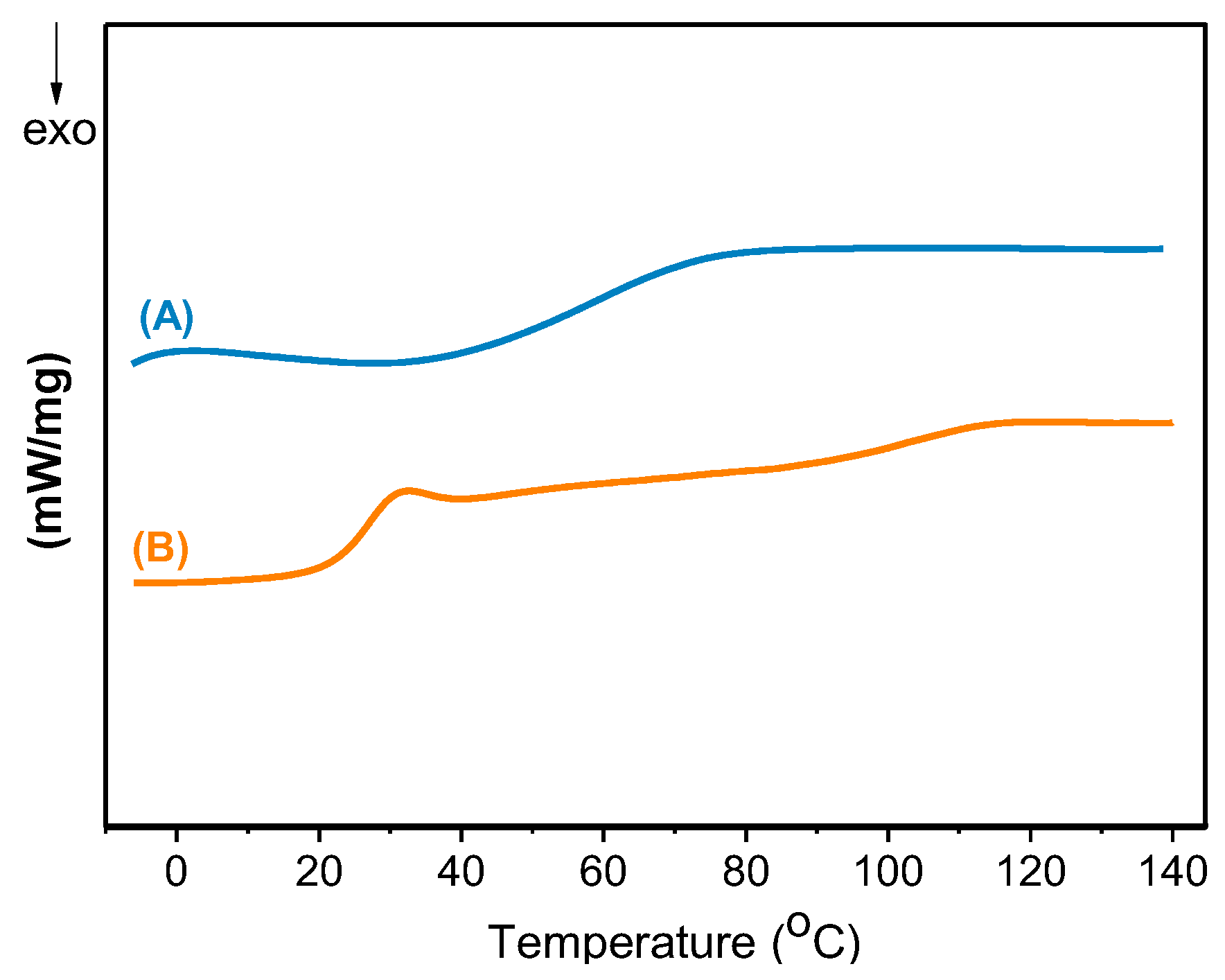
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Svoronost, P.D.N.; Bruno, T.J. Carbonyl sulfide: A review of its chemistry and properties. Ind. Eng. Chem. Res. 2002, 41, 5321–5336. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Mu, Y. Heterogeneous reactivity of carbonyl sulfide on alpha-Al2O3 and gamma-Al2O3. Atmos. Environ. 2008, 42, 960–969. [Google Scholar] [CrossRef]
- Liu, Y.; He, H. Experimental and Theoretical Study of Hydrogen Thiocarbonate for Heterogeneous Reaction of Carbonyl Sulfide on Magnesium Oxide. J. Phys. Chem. 2009, 113, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Saheb, V.; Alizadeh, M.; Rezaei, F.; Shahidi, S. Quantum chemical and theoretical kinetics studies on the reaction of carbonyl sulfide with H, OH and O(P-3). Comput. Theor. Chem. 2012, 994, 25–33. [Google Scholar] [CrossRef]
- Bartholomaeus, A.R.; Haritos, V.S. Review of the toxicology of carbonyl sulfide, a new grain fumigant. Food Chem. Toxicol. 2005, 4, 1687–1701. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, P.D.; Kenig, E.Y. Kinetics of carbonyl sulfide reaction with alkanolamines: A review. Chem. Eng. J. 2009, 148, 207–211. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Hulea, V.; Tichit, D. Methyl mercaptan and carbonyl sulfide traces removal through adsorption and catalysis on zeolites and layered double hydroxides. Appl. Catal. A Gen. 2011, 397, 218–224. [Google Scholar] [CrossRef]
- Rhodesa, C.; Riddelb, S.A.; West, J.; Williams, B.P.; Hutchings, G.J. The low-temperature hydrolysis of carbonyl sulfide and carbon disulfide: A review. Catal. Today 2000, 59, 443–464. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Hrabar, A.; Zhu, Y.-Z.; Lercher, J.A. Synthesis of methyl mercaptan from carbonyl sulfide over sulfide K2MoO4/SiO2. J. Catal. 2011, 280, 264–273. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Lercher, J.A. Effect of the support on the high activity of the (Ni)Mo/ZrO2-SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4,6-DMDBT. ACS Catal. 2011, 1, 1595–1603. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Du, B.-Y.; Wang, Q.; Fan, Z.-Q. Regioselective and Alternating Copolymerization of Carbonyl Sulfide with Racemic Propylene Oxide. Macromolecules 2013, 46, 5899–5904. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Darensbourg, D.J. An Examination of the Steric and Electronic Effects in the Copolymerization of Carbonyl Sulfide and Styrene Oxide. Macromolecules 2015, 48, 6057–6062. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Du, B.-Y.; Wang, Q.; Fan, Z.-Q. Alternating copolymerization of carbonyl sulfide and Cyclohexene Oxide catalyzed by zinc-cobalt double metal cyanide complex. Polymer 2014, 55, 3688–3695. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Darensbourg, D.J. Highly regioselective and alternating copolymerization of carbonyl sulfide with phenyl glycidyl ether. Polym. Chem. 2015, 6, 6955–6958. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.-H.; Du, B.-Y.; Wang, Q.; Fan, Z.-Q. Well-defined high refractive index poly(monothiocarbonate) with tunable Abbe’s numbers and glass-transition temperatures via terpolymerization. Polym. Chem. 2015, 6, 4978–4983. [Google Scholar] [CrossRef]
- Luo, M.; Li, Y.; Zhang, Y.-Y.; Zhang, X.-H. Using carbon dioxide and its sulfur analogues as monomers in polymer synthesis. Polymer 2016, 82, 406–431. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, X.H.; Darensbourg, D.J. Poly(monothiocarbonate)s from the Alternating and Regioselective Copolymerization of Carbonyl Sulfide with Epoxides. Acc. Chem. Res. 2016, 49, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.-M.; Liu, Y.; Xin, A.-X.; Fu, S.; Lu, X.-B. Single-Site Bifunctional Catalysts for COX (X = O or S)/Epoxides Copolymerization: Combining High Activity, Selectivity, and Durability. Macromolecules 2015, 48, 8445–8450. [Google Scholar] [CrossRef]
- Yue, T.-J.; Ren, W.-M.; Liu, Y.; Wan, Z.-Q.; Lu, X.-B. Crystalline Polythiocarbonate from Stereoregular Copolymerization of Carbonyl Sulfide and Epichlorohydrin. Macromolecules 2016, 49, 2971–2976. [Google Scholar] [CrossRef]
- Wu, H.-L.; Yang, J.-L.; Luo, M.; Wang, R.-Y.; Xu, J.-T.; Du, B.-Y.; Zhang, X.-H.; Darensbourg, D.J. Poly(trimethylene monothiocarbonate) from the Alternating Copolymerization of COS and Oxetane: A Semicrystalline Copolymer. Macromolecules 2016, 49, 8863–8869. [Google Scholar] [CrossRef]
- Ren, W.-M.; Yue, T.-J.; Li, M.-R.; Wan, Z.-Q.; Lu, X.-B. Crystalline and Elastomeric Poly(monothiocarbonate)s Prepared from Copolymerization of COS and Achiral Epoxide. Macromolecules 2017, 50, 63–68. [Google Scholar] [CrossRef]
- Bolm, C.; Legros, J.; Le Paih, J.; Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.C.; Brandstetter, H.; Whittington, D.A.; Nordlund, P.; Lippard, S.J.; Frederick, C.A. Crystal structures of the methane monooxygenase hydroxylase from Methylococcus capsulatus (Bath): Implications for substrate gating and component interactions. Proteins 1997, 29, 141–152. [Google Scholar] [CrossRef]
- Buchard, A.; Kember, M.R.; Sandemanb, K.G.; Williams, C.K. A bimetallic iron(III) catalyst for CO2/epoxide coupling. Chem. Commun. 2011, 47, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Kobayashi, K.; Ohkawara, T.; Imoto, H.; Nozaki, K. Copolymerization of Epoxides with Carbon Dioxide Catalyzed by Iron-Corrole Complexes: Synthesis of a Crystalline Copolymer. J. Am. Chem. Soc. 2013, 135, 8456–8459. [Google Scholar] [CrossRef] [PubMed]
- Taherimehr, M.; Sertã, P.C.; Kleij, A.W.; Whiteoak, C.J.; Pescarmona, P.P. New Iron Pyridylamino-Bis(Phenolate) Catalyst for Converting CO2 into Cyclic Carbonates and Cross-Linked Polycarbonates. Chemsuschem 2015, 8, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, A.; De Nisi, A.; Grassi, A.; Milione, S.; Capacchione, C.; Vaginc, S.; Rieger, B. Novel iron(III) catalyst for the efficient and selective coupling of carbon dioxide and epoxides to form cyclic carbonates. Catal. Sci. Technol. 2015, 5, 118–123. [Google Scholar] [CrossRef]
- Feda’a, A.-Q.; Nevil, G.; Martin, N.; Timo, R. Synthesis, structure and catalytic activity of bis(phenoxyiminato)iron (III) complexes in coupling reaction of CO2 and epoxides. Inorg. Chim. Acta 2016, 442, 81–85. [Google Scholar] [CrossRef]
- Sproulesand, S.; Wieghardt, K. o-Dithiolene and o-aminothiolate chemistry of iron: Synthesis, structure and reactivity. Coord. Chem. Rev. 2010, 254, 1358–1382. [Google Scholar] [CrossRef]
- Li, B.; Wu, G.-P.; Ren, W.-M.; Wang, Y.-M.; Rao, D.-Y.; Lu, X.-B.J. Asymmetric, regio- and stereo-selective alternating copolymerization of CO2 and propylene oxide catalyzed by chiral chromium Salan complexes. Polym. Sci. Part A Polym. Chem. 2008, 46, 6102–6113. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Ulusoy, M.; Karroonnirum, O.; Poland, R.R.; Reibenspies, J.H.; Cetinkaya, B. Highly Selective and Reactive (salan)CrCl Catalyst for the Copolymerization and Block Copolymerization of Epoxides with Carbon Dioxide. Macromolecules 2009, 42, 6992–6998. [Google Scholar] [CrossRef]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistic Aspects of the Copolymerization of CO2 with Epoxides Using a Thermally Stable Single-Site Cobalt(III) Catalyst. J. Am. Chem. Soc. 2009, 131, 11509–11518. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bao, Y.-Y.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Binuclear chromium-salan complex catalyzed alternating copolymerization of epoxides and cyclic anhydrides. Polym. Chem. 2013, 4, 1439–1444. [Google Scholar] [CrossRef]

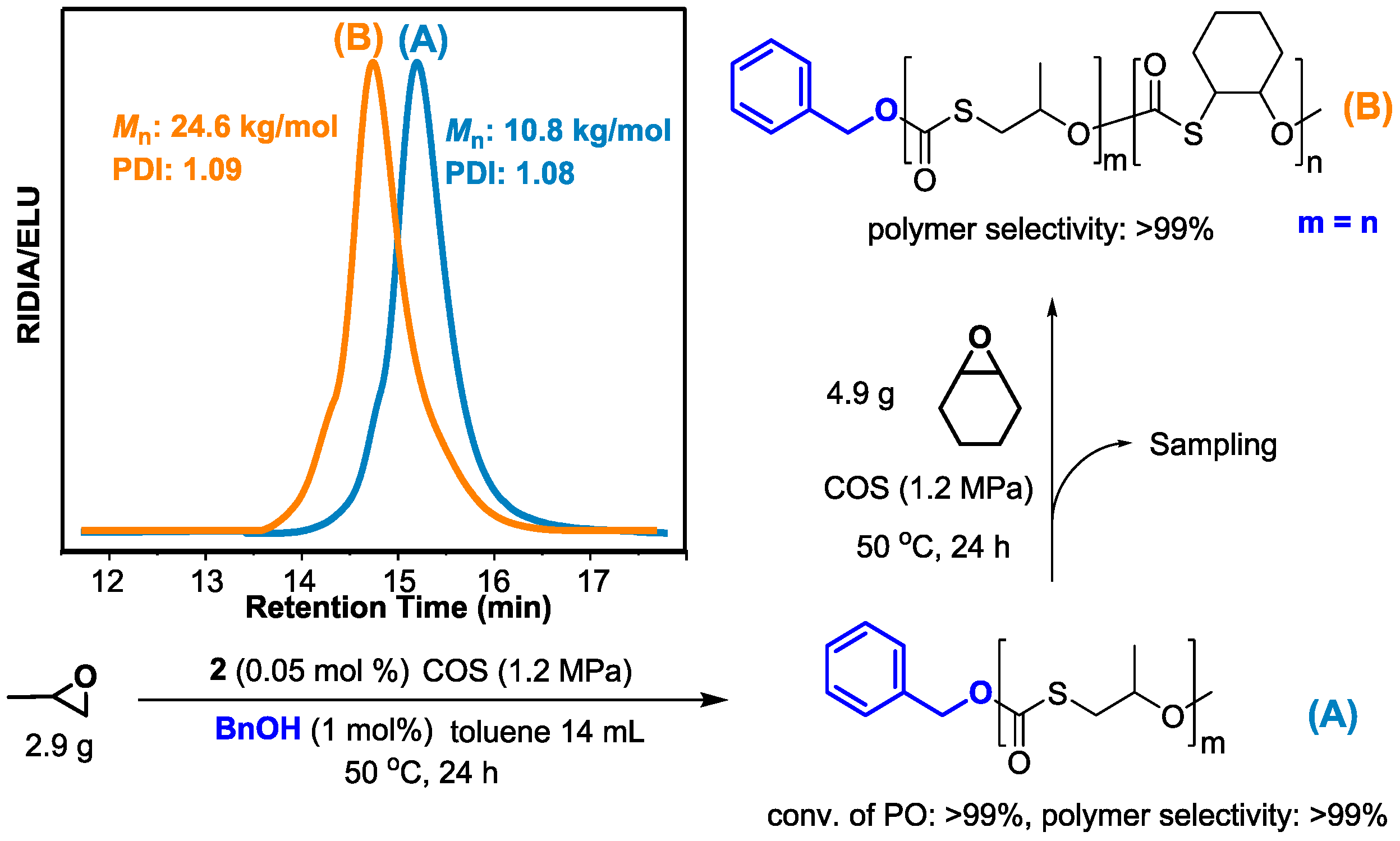

| Entry | Catalyst | [PO]/[cat] | Temperature (°C) | Time (h) | TOF b (h−1) | Selectivity c (% Polymer) | Mn d (kg/mol) | PDI d |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2000 | 25 | 1.0 | 1040 | 94 | 33.3 | 1.15 |
| 2 | 2 | 2000 | 25 | 1.0 | 700 | >99 | 22.3 | 1.14 |
| 3 | 2 | 5000 | 50 | 0.5 | 2820 | >99 | 33.6 | 1.17 |
| 4 | 2 | 10,000 | 80 | 0.5 | 5400 | 99 | 56.9 | 1.23 |
| 5 | 1 | 10,000 | 80 | 0.5 | 7290 | 67 | 48.9 | 1.28 |
| 6 e | 2 | 2000 | 25 | 10.0 | 136 | >99 | 57.4 | 1.17 |
| 7 | 2 | 2000 | 50 | 24.0 | 83 | >99 | 65.6 | 1.18 |
| 8 f | 2 | 2000 | 50 | 24.0 | 83 | >99 | 3.0 | 1.09 |

| Entry | Epoxide | Time (h) | TOF b (h−1) | Mn c (kg/mol) | PDI c |
|---|---|---|---|---|---|
| 1 | BO | 3.0 | 343 | 43.6 | 1.12 |
| 2 | HO | 5.0 | 216 | 49.0 | 1.15 |
| 3 | SO | 24.0 | 39 | 14.6 | 1.18 |
| 4 | CMO | 24.0 | 75 | 5.3 | 1.53 |
| 5 | OMA | 2.0 | 490 | 25.8 | 1.19 |
| 6 | iPMO | 3.0 | 368 | 35.3 | 1.25 |
| 7 | BMO | 3.0 | 208 | 24.6 | 1.12 |
| 8 | PMO | 3.0 | 268 | 31.2 | 1.18 |
| 9 | CHO | 3.0 | 442 | 50.5 | 1.10 |
| 10 | CPO | 24.0 | 23 | 20.0 | 1.21 |
| 11 | VCHO | 3.0 | 267 | 35.8 | 1.11 |
| 12 | CXO | 24.0 | 10 | 12.5 | 1.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, G.-G.; Yue, T.-J.; Wan, Z.-Q.; Zhang, R.; Lu, X.-B.; Ren, W.-M. A Single-Site Iron(III)-Salan Catalyst for Converting COS to Sulfur-Containing Polymers. Polymers 2017, 9, 515. https://doi.org/10.3390/polym9100515
Gu G-G, Yue T-J, Wan Z-Q, Zhang R, Lu X-B, Ren W-M. A Single-Site Iron(III)-Salan Catalyst for Converting COS to Sulfur-Containing Polymers. Polymers. 2017; 9(10):515. https://doi.org/10.3390/polym9100515
Chicago/Turabian StyleGu, Ge-Ge, Tian-Jun Yue, Zhao-Qian Wan, Rong Zhang, Xiao-Bing Lu, and Wei-Min Ren. 2017. "A Single-Site Iron(III)-Salan Catalyst for Converting COS to Sulfur-Containing Polymers" Polymers 9, no. 10: 515. https://doi.org/10.3390/polym9100515





