Medium Bandgap D-A Type Photovoltaic Polymers Based on an Asymmetric Dithienopyran Donor and a Benzotriazole Acceptor
Abstract
:1. Introduction
2. Experimental Section
2.1. Measurements and Characterization
2.2. Fabrication of the Photovoltaic Devices
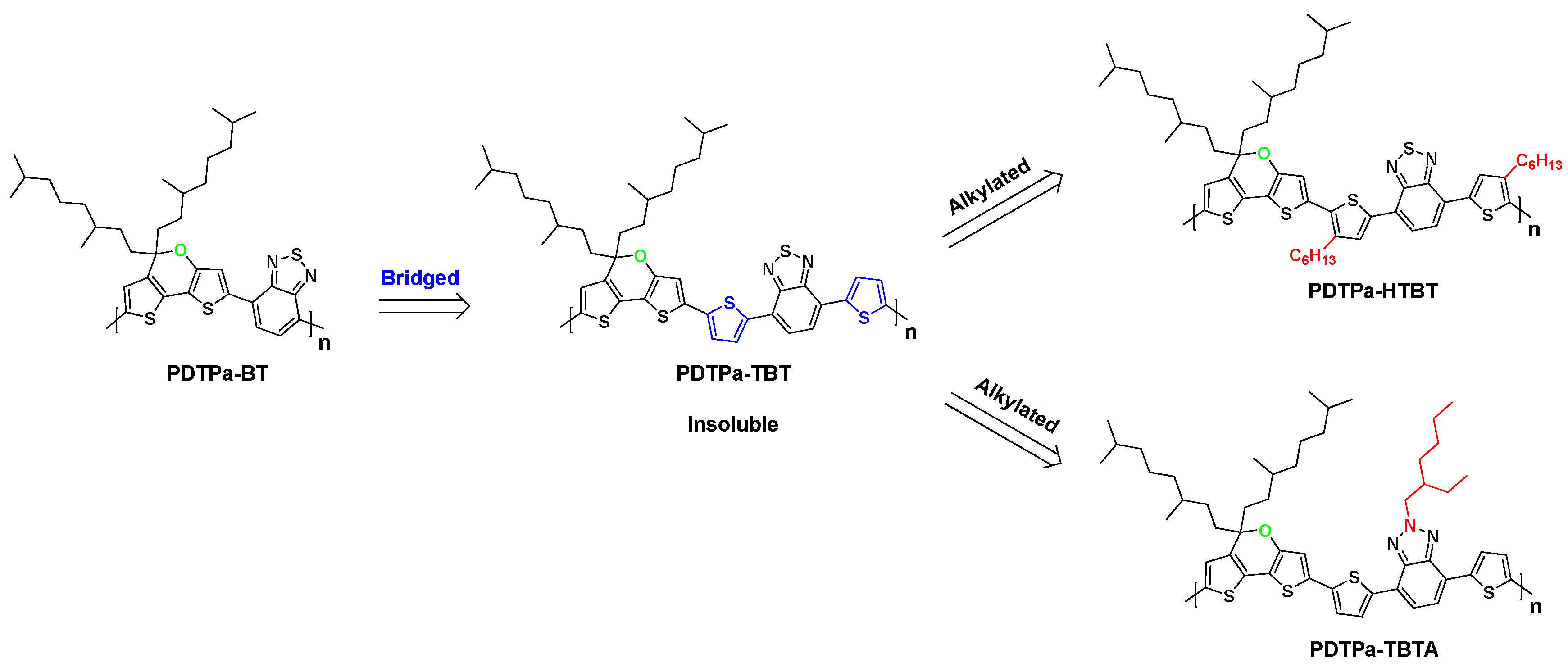
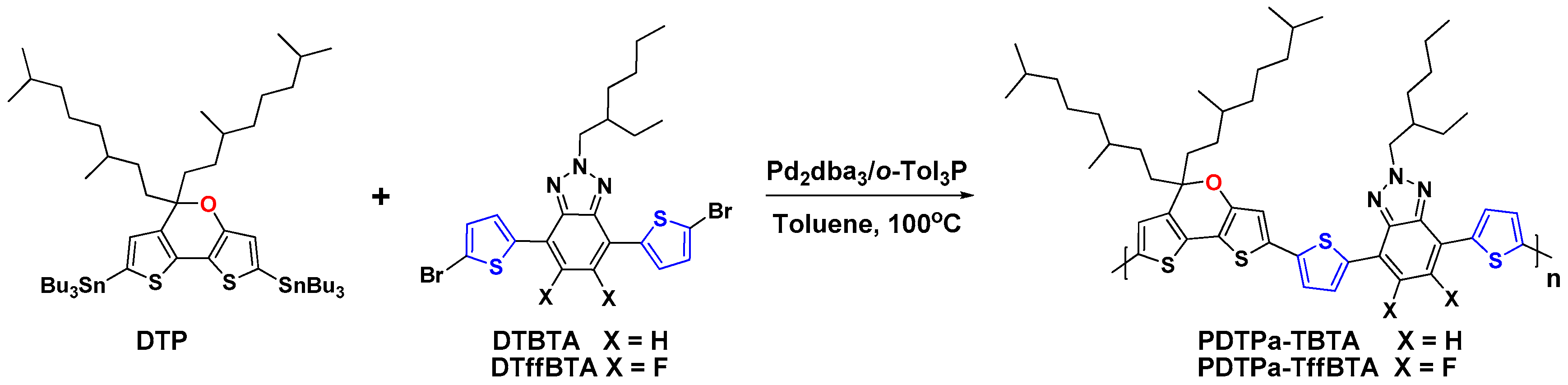
2.3. Synthesis of the Polymers
3. Results and Discussion
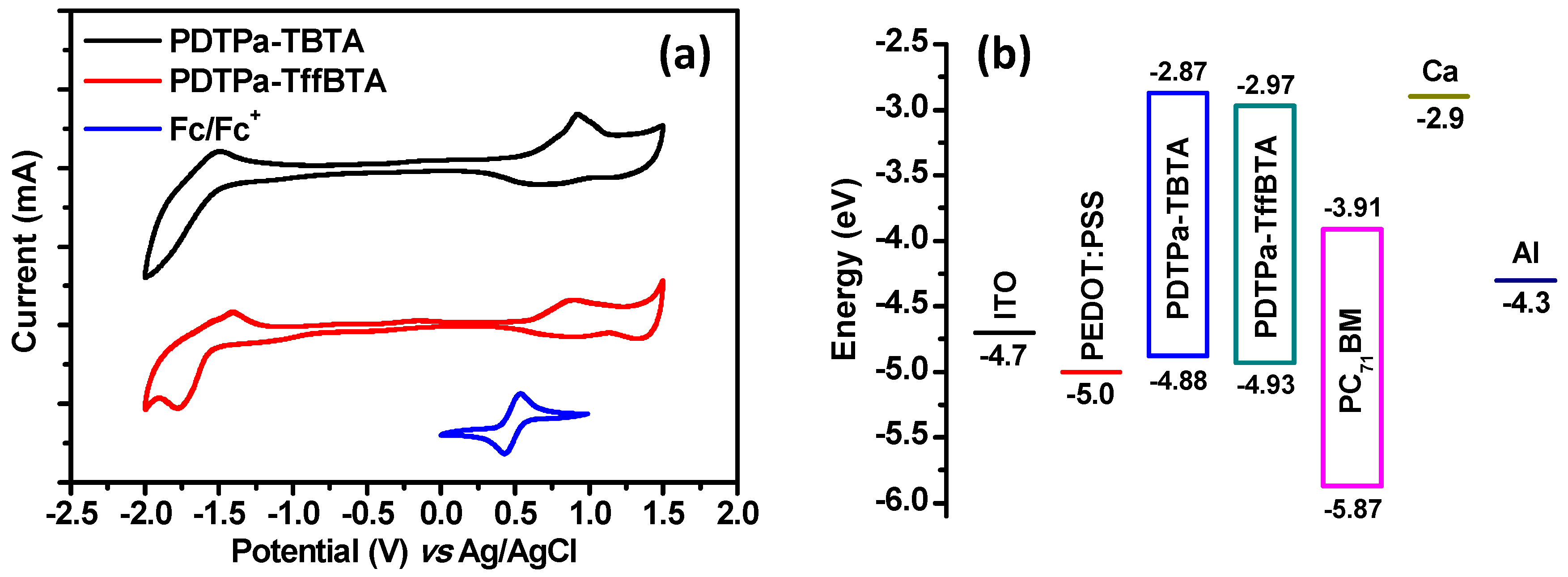
3.1. Optical and Electronic Properties
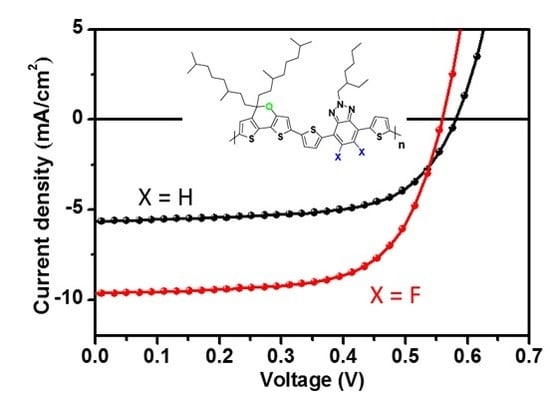
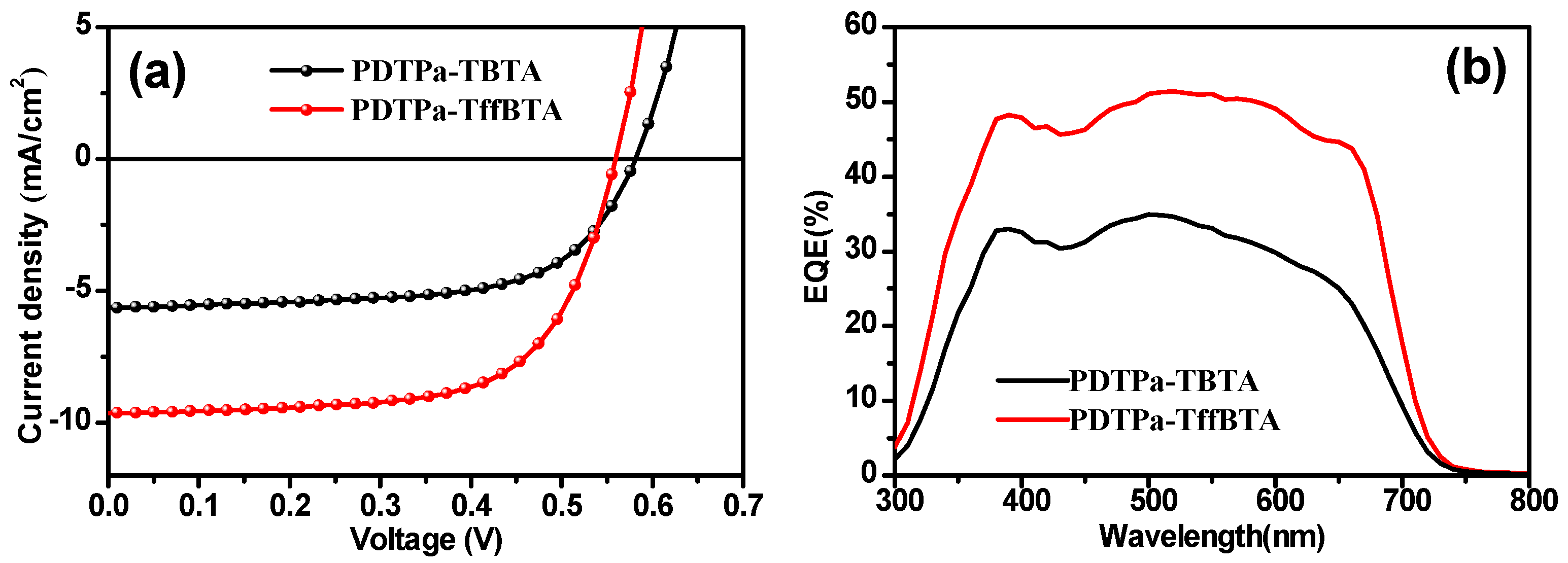
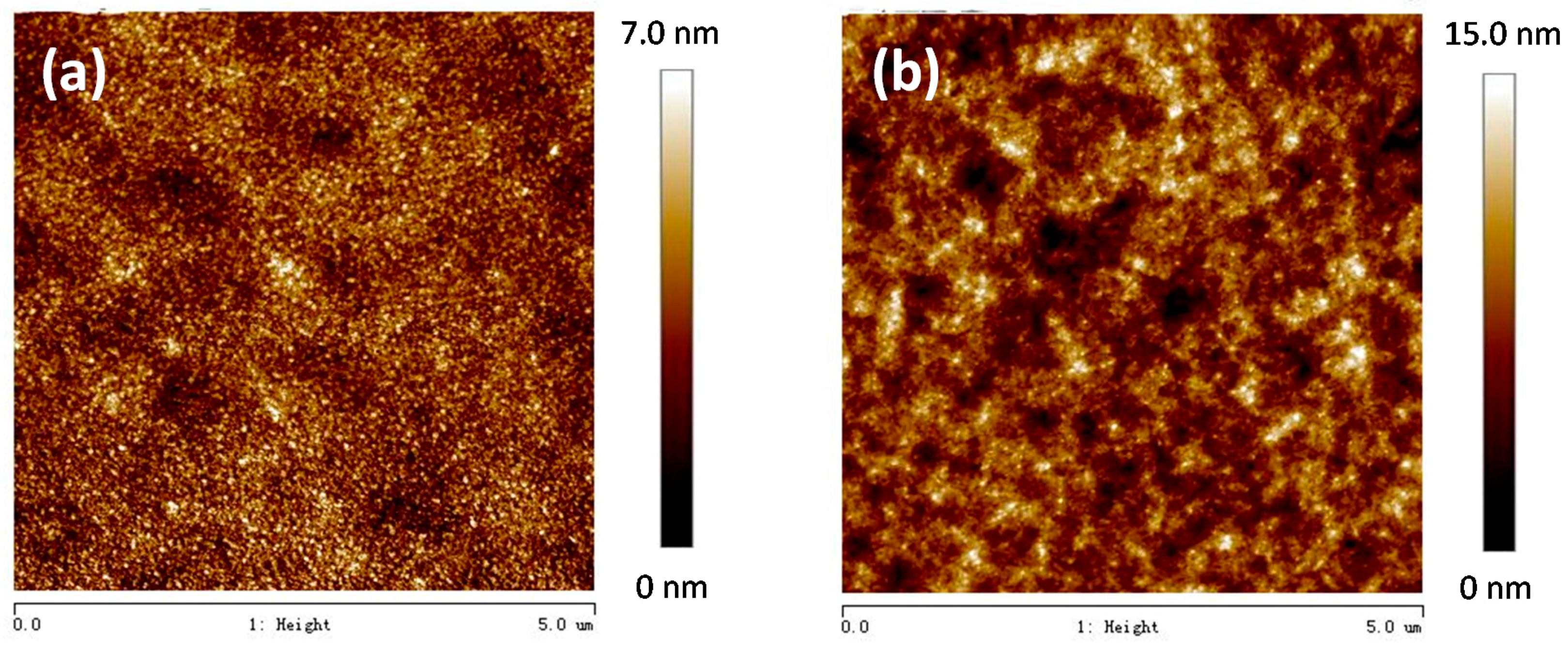
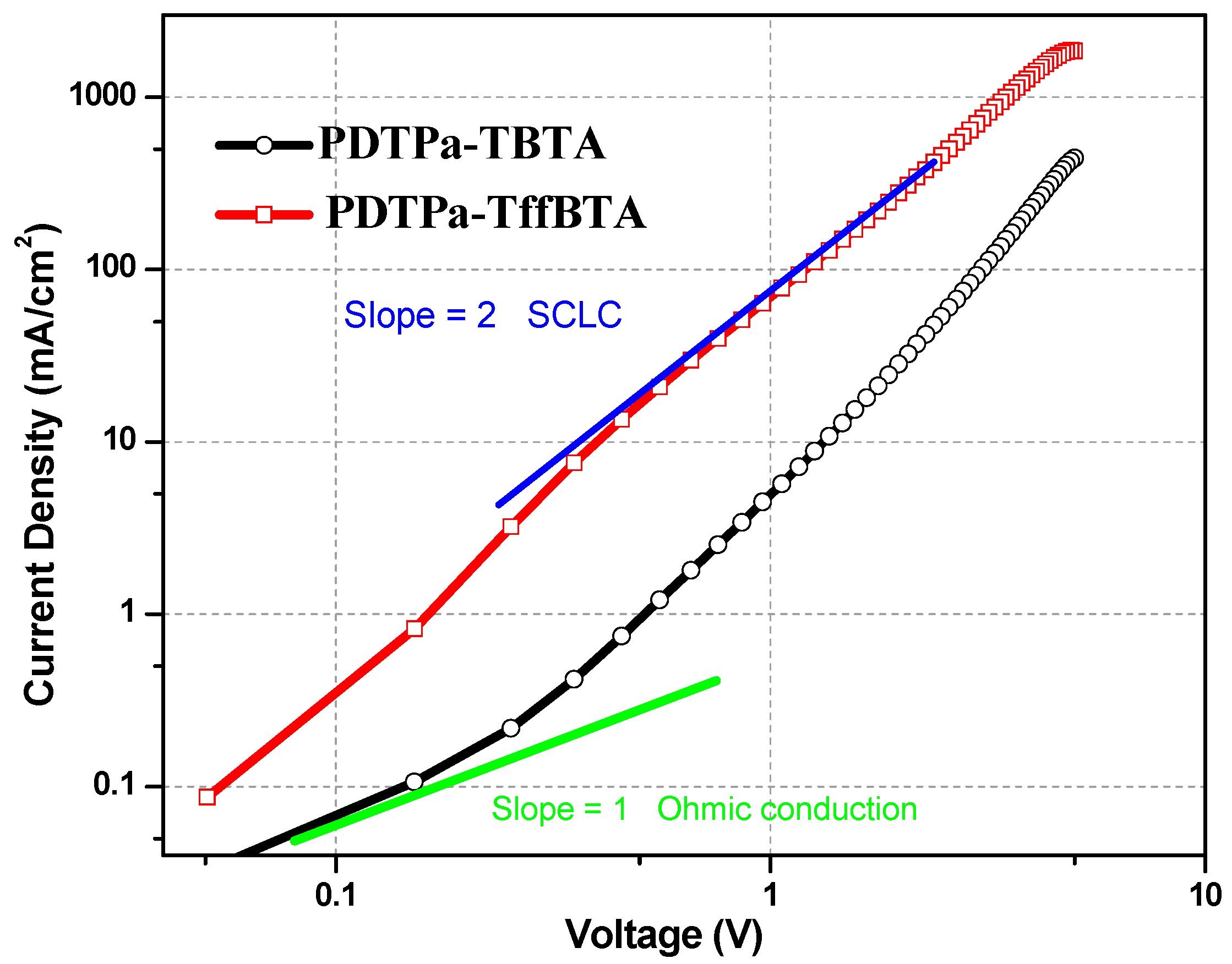
3.2. Photovoltaic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Thompson, B.C.; Frechet, J.M. Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. Engl. 2008, 47, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 153–161. [Google Scholar] [CrossRef]
- Huang, Y.; Kramer, E.J.; Heeger, A.J.; Bazan, G.C. Bulk Heterojunction Solar Cells: Morphology and Performance Relationships. Chem. Rev. 2014, 114, 7006–7043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, M.Y.; Pschirer, N.G.; Baumgarten, M.; Mullen, K. Polyphenylene-Based Materials for Organic Photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, L.; Hou, J. Breaking the 10% Efficiency Barrier in Organic Photovoltaics: Morphology and Device Optimization of Well-Known PBDTTT Polymers. Adv. Energy Mater. 2016, 6, 1502529. [Google Scholar] [CrossRef]
- Li, H.; He, D.; Mao, P.; Wei, Y.; Ding, L.; Wang, J. Additive-Free Organic Solar Cells with Power Conversion Efficiency over 10%. Adv. Energy Mater. 2017, 7, 1602663. [Google Scholar] [CrossRef]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Osaka, I.; McCullough, R.D. Advances in molecular design and synthesis of regioregular polythiophenes. Acc. Chem. Res. 2008, 41, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Marrocchi, A.; Lanari, D.; Facchetti, A.; Vaccaro, L. Poly(3-hexylthiophene): Synthetic methodologies and properties in bulk heterojunction solar cells. Energy Environ. Sci. 2012, 5, 8457. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the Morphology and Performance of Bulk Heterojunctions in Solar Cells. Lessons Learned from the Benchmark Poly(3-hexylthiophene): 6,6 -Phenyl-C-61-butyric Acid Methyl Ester System. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Jia, H.; He, W.; Zhang, Y.; Mi, L.; Hou, H.; Zhu, G.; Zheng, Z. Hybrid solar cells with outstanding short-circuit currents based on a room temperature soft-chemical strategy: The case of P3HT:Ag2S. J. Am. Chem. Soc. 2012, 134, 17392–17395. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yi, C.; Wang, K.; Yang, Y.; Bhatta, R.S.; Tsige, M.; Xiao, S.; Gong, X. Single-junction polymer solar cells with over 10% efficiency by a novel two-dimensional donor-acceptor conjugated copolymer. ACS Appl. Mater. Interfaces 2015, 7, 4928–4935. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Keum, J.K.; Browning, J.F.; Gu, G.; Yang, B.; Dyck, O.; Do, C.; Chen, W.; Chen, J.; Ivanov, I.N.; et al. Correlating high power conversion efficiency of PTB7:PC71BM inverted organic solar cells with nanoscale structures. Nanoscale 2015, 7, 15576–15583. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, A.; Chen, F.; Zhou, E. The effect of conjugated π-bridge and fluorination on the properties of asymmetric-building-block-containing polymers (ABC polymers) based on dithienopyran donor and benzothiadiazole acceptors. Polym. Chem. 2017, 8, 5396–5406. [Google Scholar] [CrossRef]
- Wang, X.; Tang, A.; Chen, Y.; Mahmood, A.; Hou, J.; Wei, Z.; Zhou, E. Effect of fluorination and symmetry on the properties of polymeric photovoltaic materials based on an asymmetric building block. RSC Adv. 2016, 6, 90051–90060. [Google Scholar] [CrossRef]
- Xiao, B.; Tang, A.; Zhang, J.; Mahmood, A.; Wei, Z.; Zhou, E. Achievement of high Voc of 1.02 V for P3HT-based Organic Solar Cell using a Benzotriazole-containing Non-fullerene Acceptor. Adv. Energy Mater. 2017, 7, 1602229. [Google Scholar] [CrossRef]
- Xiao, B.; Tang, A.; Yang, J.; Wei, Z.; Zhou, E. P3HT-Based Photovoltaic Cells with a High Voc of 1.22 V by Using a Benzotriazole-Containing Nonfullerene Acceptor End-Capped with Thiazolidine-2,4-dione. ACS Macro. Lett. 2017, 6, 410–414. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J. Am. Chem Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Zhang, Z.-G.; Zhang, S.; Zhang, M.; Zhang, J.; Li, Y. Synthesis and Photovoltaic Properties of D–A Copolymers Based on Dithienosilole and Benzotriazole. Macromolecules 2011, 44, 7632–7638. [Google Scholar] [CrossRef]
- Bin, H.; Zhang, Z.G.; Gao, L.; Chen, S.; Zhong, L.; Xue, L.; Yang, C.; Li, Y. Non-Fullerene Polymer Solar Cells Based on Alkylthio and Fluorine Substituted 2D-Conjugated Polymers Reach 9.5% Efficiency. J. Am. Chem Soc. 2016, 138, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Z.G.; Bin, H.; Xue, L.; Yang, Y.; Wang, C.; Liu, F.; Russell, T.P.; Li, Y. High-Efficiency Nonfullerene Polymer Solar Cells with Medium Bandgap Polymer Donor and Narrow Bandgap Organic Semiconductor Acceptor. Adv. Mater. 2016, 28, 8288–8295. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Z.G.; Xue, L.; Min, J.; Zhang, J.; Wei, Z.; Li, Y. All-Polymer Solar Cells Based on Absorption-Complementary Polymer Donor and Acceptor with High Power Conversion Efficiency of 8.27%. Adv. Mater. 2016, 28, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, L.; Gautam, B.; Bin, H.-J.; Lin, J.-D.; Wu, F.-P.; Zhang, Z.; Jiang, Z.-Q.; Zhang, Z.-G.; Gundogdu, K.; et al. A near-infrared non-fullerene electron acceptor for high performance polymer solar cells. Energy Environ. Sci. 2017, 10, 1610–1620. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.G.; Bin, H.; Chen, S.; Gao, L.; Xue, L.; Yang, C.; Li, Y. Side-Chain Isomerization on an n-type Organic Semiconductor ITIC Acceptor Makes 11.77% High Efficiency Polymer Solar Cells. J. Am. Chem. Soc. 2016, 138, 15011–15018. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.N.; Bisen, M.D.; Stemer, D.M.; McKinnon, S.; Luscombe, C.K. Direct Arylation Polycondensation of 2,5-Dithienylsilole with a Series of Difluorobenzodiimine-Based Electron Acceptors. Macromolecules 2017, 50, 4623–4628. [Google Scholar] [CrossRef]
- Zhang, L.; He, C.; Chen, J.; Yuan, P.; Huang, L.; Zhang, C.; Cai, W.; Liu, Z.; Cao, Y. Bulk-Heterojunction Solar Cells with Benzotriazole-Based Copolymers as Electron Donors: Largely Improved Photovoltaic Parameters by Using PFN/Al Bilayer Cathode. Macromolecules 2010, 43, 9771–9778. [Google Scholar] [CrossRef]
- Meyer, F. Fluorinated conjugated polymers in organic bulk heterojunction photovoltaic solar cells. Prog. Polym. Sci. 2015, 47, 70–91. [Google Scholar] [CrossRef]
- Leclerc, N.; Chávez, P.; Ibraikulov, O.; Heiser, T.; Lévêque, P. Impact of Backbone Fluorination on π-Conjugated Polymers in Organic Photovoltaic Devices: A Review. Polymers 2016, 8, 11. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.-G.; Luo, H.; Chen, S.; Yu, S.; Wang, H.; Li, X.; Yu, G.; Li, Y. Effects of fluorination on the properties of thieno[3,2-b]thiophene-bridged donor–π–acceptor polymer semiconductors. Polym. Chem. 2014, 5, 502–511. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Huang, Z.; Ashraf, R.S.; Smith, J.; D’Angelo, P.; Watkins, S.E.; Anthopoulos, T.D.; Durrant, J.R.; McCulloch, I. Silaindacenodithiophene-Based Low Band Gap Polymers—The Effect of Fluorine Substitution on Device Performances and Film Morphologies. Adv. Funct. Mater. 2012, 22, 1663–1670. [Google Scholar] [CrossRef]
- Jo, J.W.; Bae, S.; Liu, F.; Russell, T.P.; Jo, W.H. Comparison of Two D−A Type Polymers with Each Being Fluorinated on D and A Unit for High Performance Solar Cells. Adv. Funct. Mater. 2015, 25, 120–125. [Google Scholar] [CrossRef]
- Cartwright, L.; Iraqi, A.; Zhang, Y.; Wang, T.; Lidzey, D.G. Impact of fluorine substitution upon the photovoltaic properties of benzothiadiazole-fluorene alternate copolymers. RSC Adv. 2015, 5, 46386–46394. [Google Scholar] [CrossRef]
- Umeyama, T.; Watanabe, Y.; Douvogianni, E.; Imahori, H. Effect of Fluorine Substitution on Photovoltaic Properties of Benzothiadiazole–Carbazole Alternating Copolymers. J. Phys. Chem. C 2013, 117, 21148–21157. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Tse, S.-C.; Zhou, J.; Du, X.; Tao, Y.; Ding, J. Synthesis and applications of difluorobenzothiadiazole based conjugated polymers for organic photovoltaics. J. Mater. Chem. 2011, 21, 3226. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J. Open-circuit voltage in organic solar cells. J. Mater. Chem. 2012, 22, 24315. [Google Scholar] [CrossRef]
- Wang, X.; Gao, C.; Wang, K.; Fan, X.; Wang, H.; Li, X.; Zhang, Z.-G.; Li, Y. Synthesis and electronic energy-level regulation of imide-fused poly(thienylene vinylene) derivatives. J. Polym. Sci. Part A Chemistry 2013, 51, 4975–4982. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open circuit voltage of organic solar cells: An in-depth review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Shen, Y.; Hosseini, A.R.; Wong, M.H.; Malliaras, G.G. How to make ohmic contacts to organic semiconductors. Chemphyschem 2004, 5, 16–25. [Google Scholar] [CrossRef] [PubMed]

| Polymer | λabs in CHCl3 (nm) | λabs in Films (nm) | λonset in Films (nm) | Egopt (eV) |
|---|---|---|---|---|
| PDTPa-TBTA | 593 | 622 | 715 | 1.73 |
| PDTPa-TffBTA | 601, 645 | 604, 656 | 700 | 1.77 |
| Polymer | EOX (V) | HOMO (eV) | Ered (V) | LUMO (eV) | EgCV (eV) |
|---|---|---|---|---|---|
| PDTPa-TBTA | 0.56 | −4.88 | −1.45 | −2.87 | 1.96 |
| PDTPa-TffBTA | 0.61 | −4.93 | −1.55 | −2.97 | 2.01 |
| Polymer | Voc [v] | Jsc [mA cm−2] | FF | PCEmax (PCEave a) [%] | Hole Mobility b [cm2 v−1 s−1] |
|---|---|---|---|---|---|
| PDTPa-TBTA | 0.58 | 6.04 | 0.63 | 2.22 (2.17) | 1.61 × 10−5 |
| PDTPa-TffBTA | 0.52 | 10.23 | 0.64 | 3.43 (3.20) | 2.66 × 10−4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wang, X.; Chen, F.; Xiao, B.; Tang, A.; Zhou, E. Medium Bandgap D-A Type Photovoltaic Polymers Based on an Asymmetric Dithienopyran Donor and a Benzotriazole Acceptor. Polymers 2017, 9, 516. https://doi.org/10.3390/polym9100516
Hu J, Wang X, Chen F, Xiao B, Tang A, Zhou E. Medium Bandgap D-A Type Photovoltaic Polymers Based on an Asymmetric Dithienopyran Donor and a Benzotriazole Acceptor. Polymers. 2017; 9(10):516. https://doi.org/10.3390/polym9100516
Chicago/Turabian StyleHu, Junyi, Xiaochen Wang, Fan Chen, Bo Xiao, Ailing Tang, and Erjun Zhou. 2017. "Medium Bandgap D-A Type Photovoltaic Polymers Based on an Asymmetric Dithienopyran Donor and a Benzotriazole Acceptor" Polymers 9, no. 10: 516. https://doi.org/10.3390/polym9100516