Electrosynthesis of Copolymers Based on 1,3,5-Tris(N-Carbazolyl)Benzene and 2,2′-Bithiophene and Their Applications in Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 1,3,5-Tris(N-Carbazolyl)Benzene (tnCz)
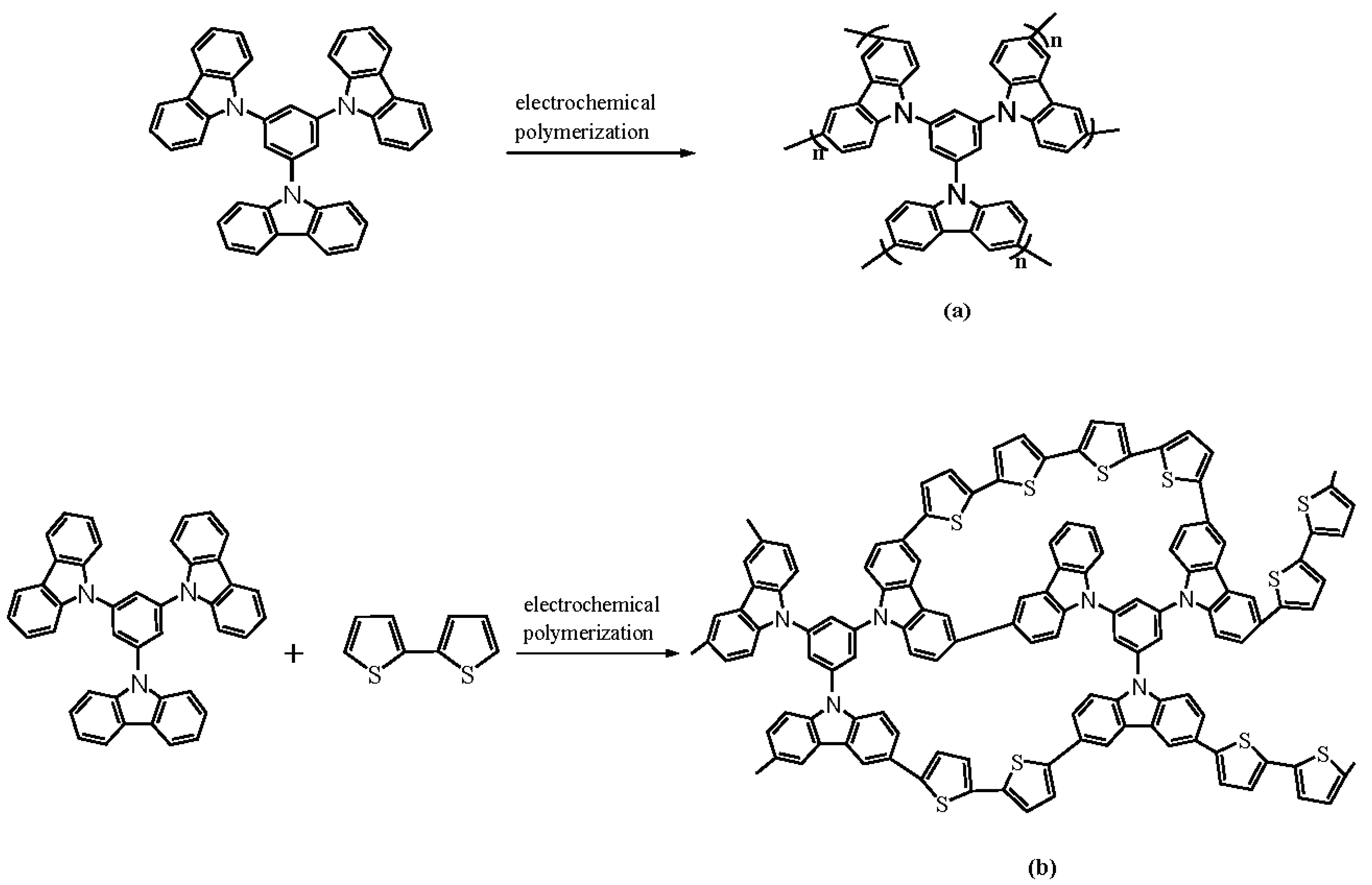
2.3. Electrochemical Polymerization
2.4. Construction of Dual-Type ECDs
2.5. Spectroelectrochemical and Electrochemical Characterization
3. Results and Discussion
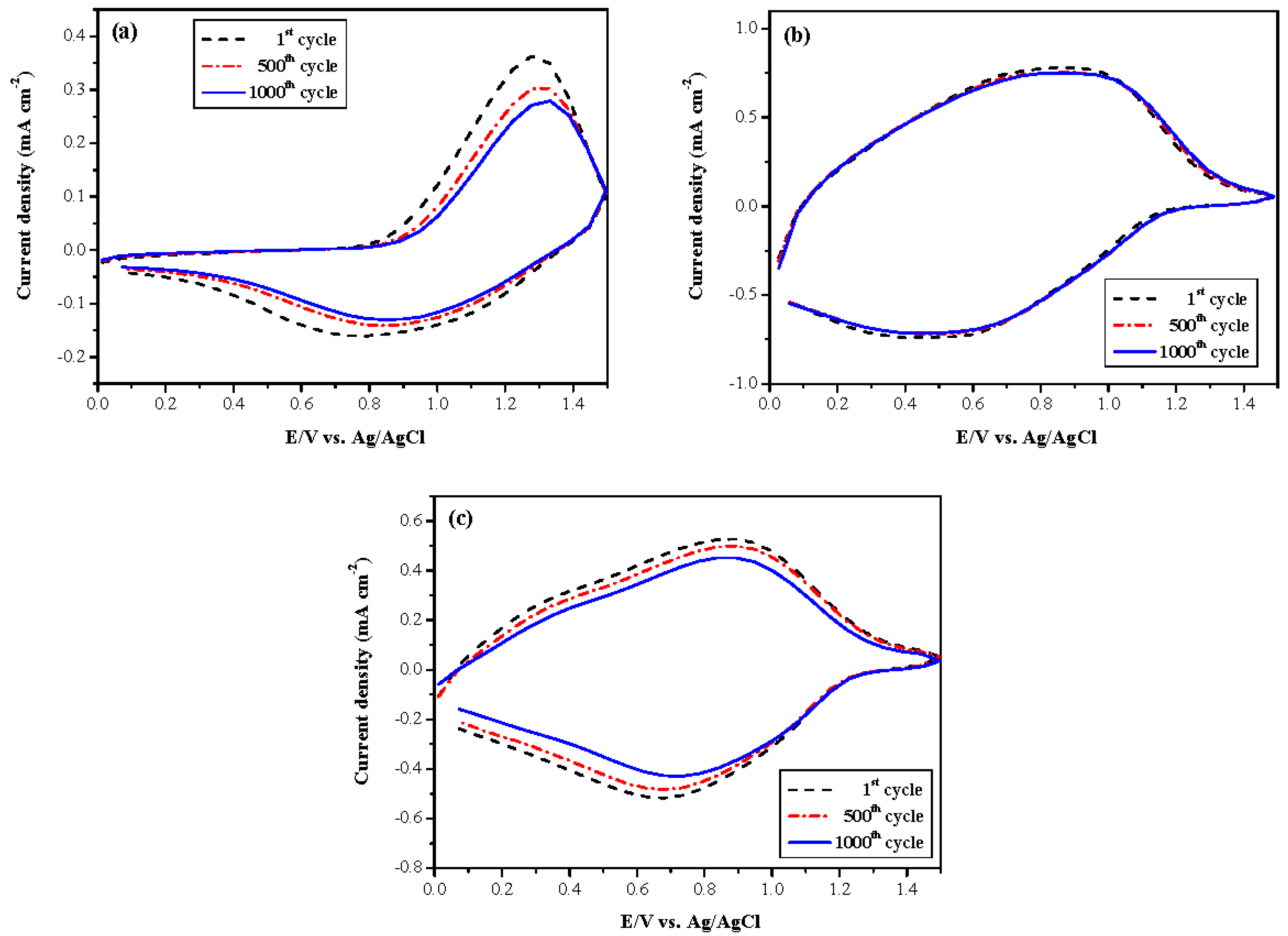
3.1. Electrochemical Polymerizations
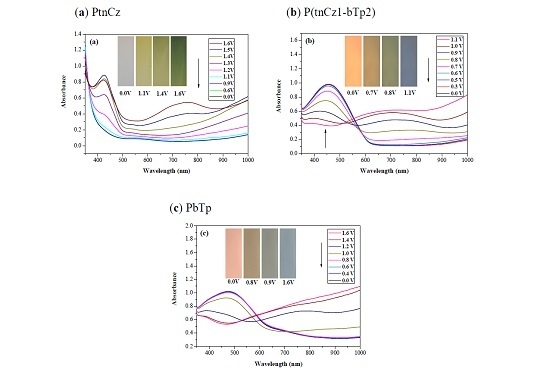
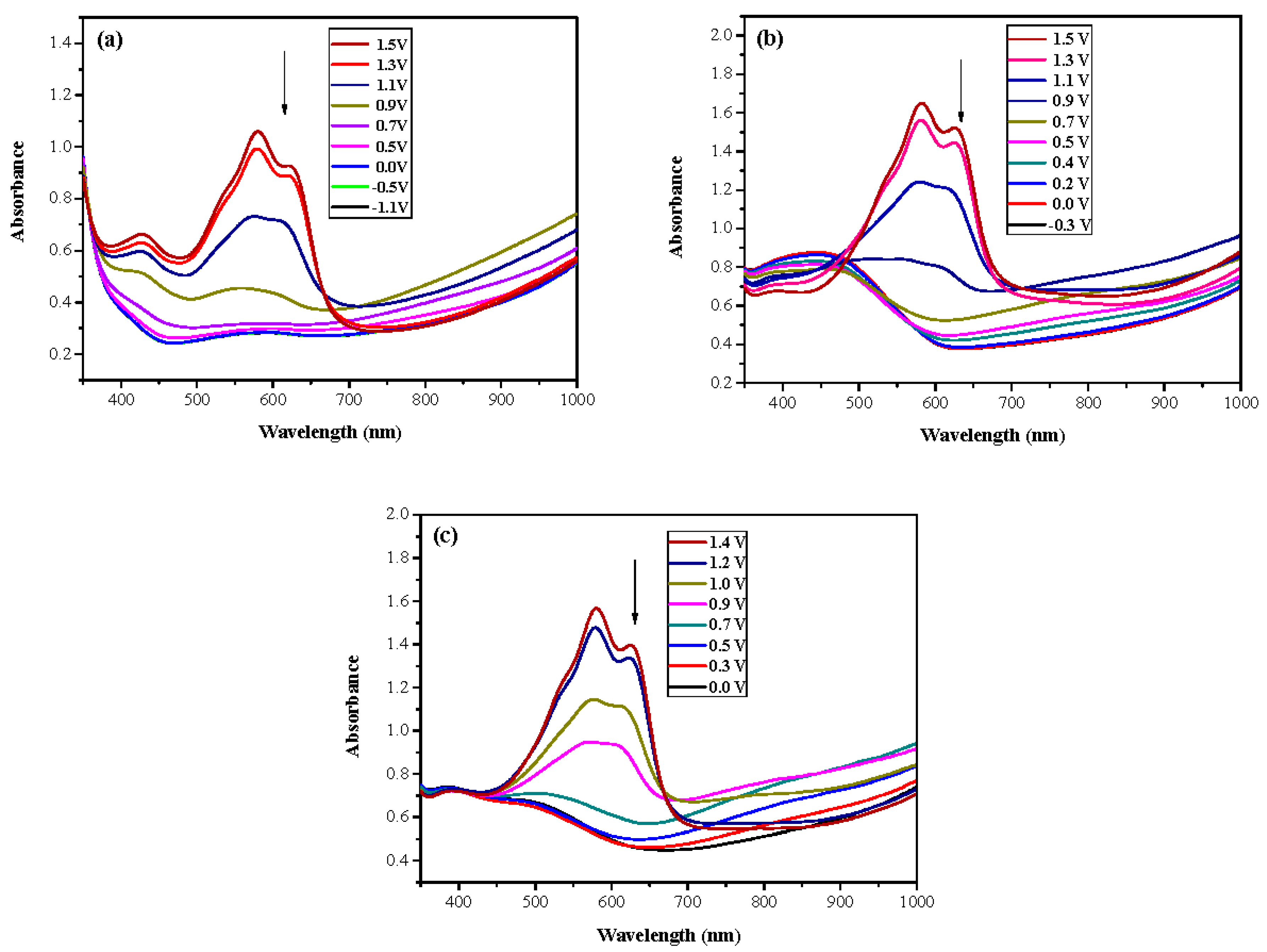
3.2. Spectroelectrochemical Characterizations of PtnCz, P(tnCz-bTp), and PbTp Films
3.3. Spectroelectrochemistry of ECDs
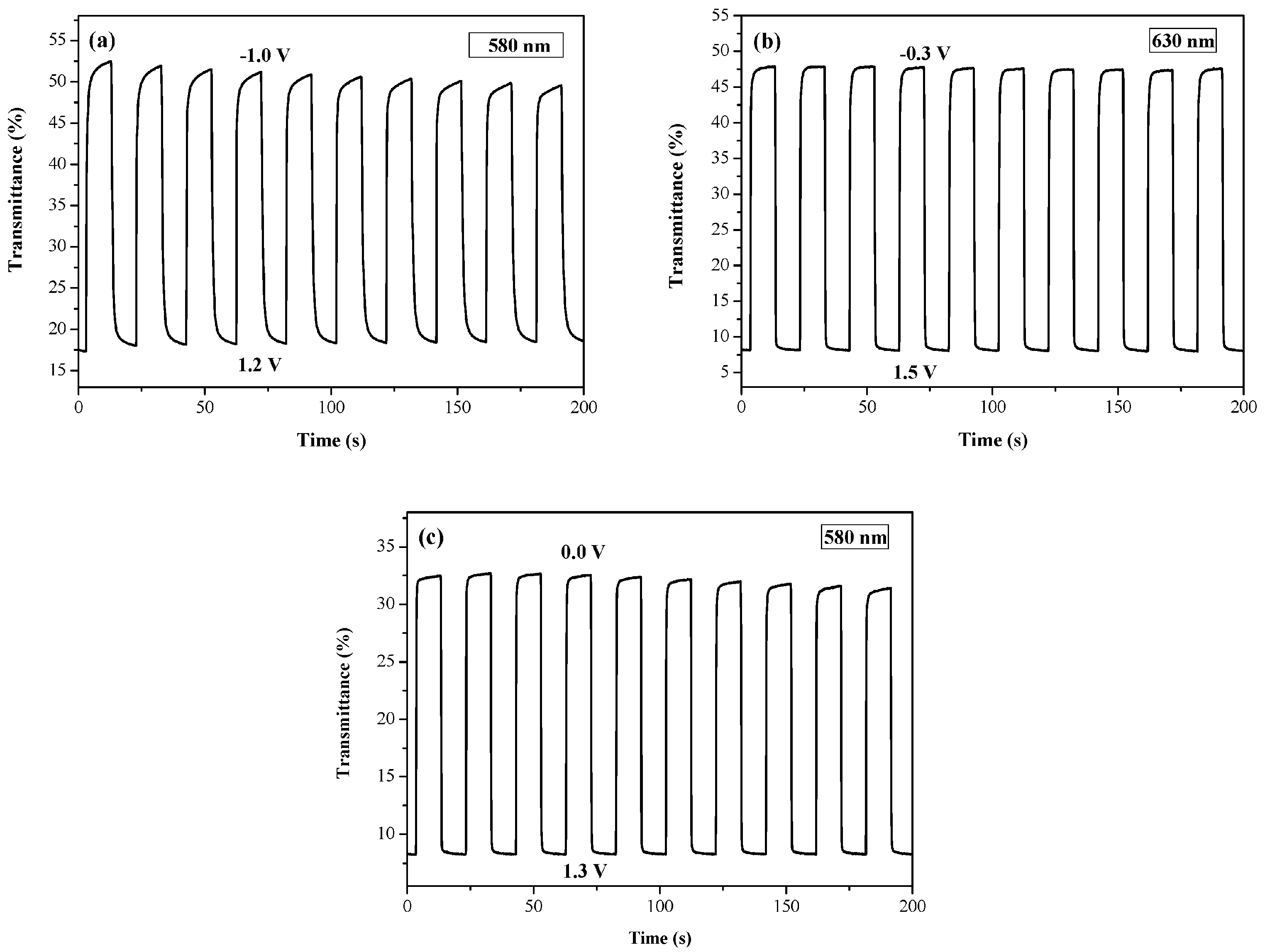
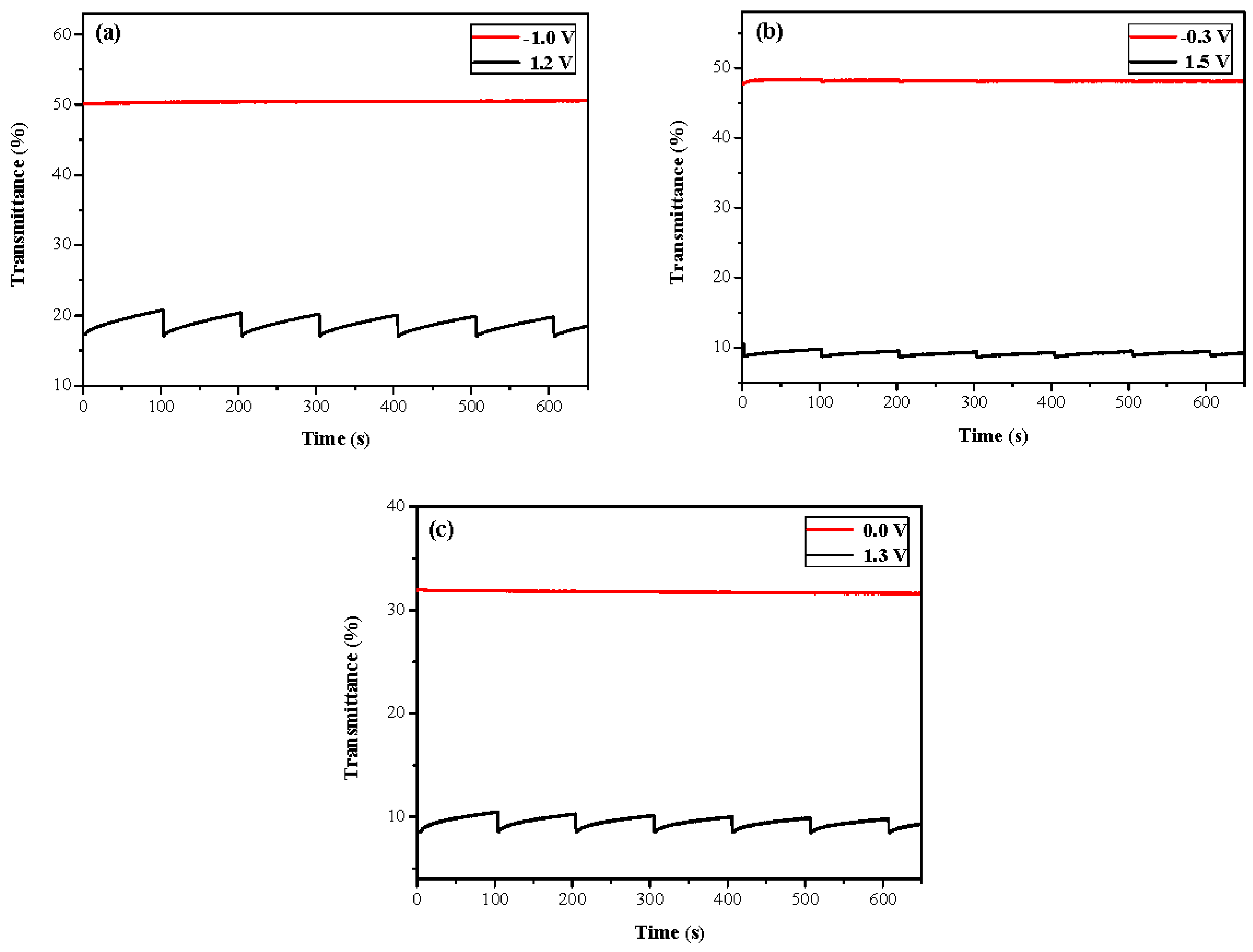
3.4. Open Circuit Memory of Electrochromic Devices
3.5. Switching Stability of Electrochromic Devices
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, S.; Li, X.; Chu, D. An organic electroactive material for flow batteries. Electrochim. Acta 2016, 190, 737–743. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, C.N. Electrochemical and electrochromic studies of redox-active aromatic polyamides with 3,5-dimethyltriphenylamine units. J. Electroanal. Chem. 2016, 776, 139–147. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J. Review-advanced carbon-supported organic electrode materials for lithium (sodium)-ion batteries. J. Electrochem. Soc. 2015, 162, A2393–A2405. [Google Scholar] [CrossRef]
- Kerszulis, J.A.; Johnson, K.E.; Kuepfert, M.; Khoshabo, D.; Dyerac, A.L.; Reynolds, J.R. Tuning the painter’s palette: Subtle steric effects on spectra and colour in conjugated electrochromic polymers. J. Mater. Chem. C 2015, 3, 3211–3218. [Google Scholar] [CrossRef]
- Chou, J.C.; Huang, Y.C.; Wu, T.Y.; Liao, Y.H.; Lai, C.H.; Chu, C.M.; Lin, Y.J. Poly(3,3-dibenzyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine)/platinum composite films as potential counter electrodes for dye-sensitized solar cells. Polymers 2017, 9, 271. [Google Scholar] [CrossRef]
- Che Balian, S.R.; Ahmad, A.; Mohamed, N.S. The effect of lithium iodide to the properties of carboxymethyl κ-carrageenan/carboxymethyl cellulose polymer electrolyte and dye-sensitized solar cell performance. Polymers 2016, 8, 163. [Google Scholar] [CrossRef]
- Selvaraj, V.; Alagar, M.; Hamerton, I. Electrocatalytic properties of monometallic and bimetallic nanoparticles-incorporated polypyrrole films for electro-oxidation of methanol. J. Power Sources 2006, 160, 940–948. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Chang, J.K.; Chen, P.R.; Kuo, C.W. Nanostructured poly(aniline-co-metanilic acid) as platinum catalyst support for electro-oxidation of methanol. Int. J. Hydrog. Energy 2015, 40, 2631–2640. [Google Scholar] [CrossRef]
- Nagashree, K.L.; Raviraj, N.H.; Ahmed, M.F. Carbon paste electrodes modified by Pt and Pt–Ni microparticles dispersed in polyindole film for electrocatalytic oxidation of methanol. Electrochim. Acta 2010, 55, 2629–2635. [Google Scholar] [CrossRef]
- Hsieh, S.N.; Hsiao, S.W.; Chen, T.Y.; Li, C.Y.; Lee, C.H.; Guo, T.F.; Hsu, Y.J.; Lin, T.L.; Wei, Y.; Wen, T.C. Self-assembled tetraoctylammonium bromide as an electron-injection layer for cathode-independent high-efficiency polymer light-emitting diodes. J. Mater. Chem. 2011, 21, 8715–8720. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Haji Jumali, M.H.; AlSalhi, M.S. Enhanced optoelectronic properties of PFO/fluorol 7GA hybrid light emitting diodes via additions of TiO2 nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef]
- Hsieh, S.N.; Chen, S.P.; Li, C.Y.; Wen, T.C.; Guo, T.F.; Hsu, Y.J. Surface modification of TiO2 by a self-assembly monolayer in inverted-type polymer light-emitting devices. Org. Electron. 2009, 10, 1626–1631. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A brief review on electrode materials for supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Fu, W.C.; Hsieh, Y.T.; Wu, T.Y.; Sun, I.W. Electrochemical preparation of porous poly(3,4-ethylenedioxythiophene) electrodes from room temperature ionic liquids for supercapacitors. J. Electrochem. Soc. 2016, 163, G61–G68. [Google Scholar] [CrossRef]
- Wu, T.Y.; Sheu, R.B.; Chen, Y. Synthesis and optically acid-sensory and electrochemical properties of novel polyoxadiazole derivatives. Macromolecules 2004, 37, 725–733. [Google Scholar] [CrossRef]
- Hung, C.C.; Wen, T.C.; Wei, Y. Site-selective deposition of ultra-fine Au nanoparticles on polyaniline nanofibers for H2O2 sensing. Mater. Chem. Phys. 2010, 122, 392–396. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, H.P.; Wu, T.Y.; Sun, I.W. Optical and electrochromic characterizations of four 2,5-dithienylpyrrole-based conducting polymer films. Electrochim. Acta 2014, 119, 225–235. [Google Scholar] [CrossRef]
- Cansu-Ergun, E.G.; Onal, A.M.; Cihaner, A. Propylenedioxy and benzimidazole based electrochromic polymers. J. Electrochem. Soc. 2016, 163, G53–G60. [Google Scholar] [CrossRef]
- Wu, T.Y.; Li, W.B.; Kuo, C.W.; Chou, C.F.; Liao, J.W.; Chen, H.R.; Tseng, C.G. Study of poly(methyl methacrylate)-based gel electrolyte for electrochromic device. Int. J. Electrochem. Sci. 2013, 8, 10720–10732. [Google Scholar]
- Gadgil, B.; Damlin, P.; Dmitrieva, E.; Ääritalo, T.; Kvarnström, C. ESR/UV–Vis-NIR spectroelectrochemical study and electrochromic contrast enhancement of a polythiophene derivative bearing a pendant viologen. RSC Adv. 2015, 5, 42242–42249. [Google Scholar] [CrossRef]
- Schottland, P.; Zong, K.; Gaupp, C.L.; Thompson, B.C.; Thomas, C.A.; Giurgiu, I.; Hickman, R.; Abboud, K.A.; Reynolds, J.R. Poly(3,4-alkylenedioxypyrrole)s: Highly stable electronically conducting and electrochromic polymers. Macromolecules 2000, 33, 7051–7061. [Google Scholar] [CrossRef]
- Deutschmann, T.; Oesterschulze, E. Integrated electrochromic iris device for low power and space-limited applications. J. Opt. 2014, 16. [Google Scholar] [CrossRef]
- Deutschmann, T.; Oesterschulze, E. Micro-structured electrochromic device based on poly(3,4-ethylenedioxythiophene). J. Micromech. Microeng. 2013, 23. [Google Scholar] [CrossRef]
- Roth, S.; Ignatowitz, M.; Muller, P.; Monch, W.; Oesterschulze, E. Non-mechanical variable apertures based on poly(3,4-ethylenedioxythiophene) (PEDOT). Microelectron. Eng. 2011, 88, 2349–2351. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsueh, J.C. Electrochemical synthesis and electrochromic properties of new conjugated polycarbazoles from di(carbazol-9-yl)-substituted triphenylamine and N-phenylcarbazole derivatives. J. Electroanal. Chem. 2015, 758, 100–110. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.Y.; Huang, M.W. Electrochromic characterizations of copolymers based on 4,4′-bis(N-carbazolyl)-1,1′-biphenyl and indole-6-carboxylic acid and their applications in electrochromic devices. J. Taiwan Inst. Chem. Eng. 2016, 68, 481–488. [Google Scholar] [CrossRef]
- Soyleyici, S.; Karakus, M.; Ak, M. Transparent-blue colored dual type electrochromic device: Switchable glass application of conducting organic-inorganic hybrid carbazole polymer. J. Electrochem. Soc. 2016, 163, H679–H683. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsai, C.J.; Tseng, L.Y.; Chen, S.J.; Hsieh, T.H.; Kuo, C.W. Nanocomposite of platinum particles embedded into nanosheets of polycarbazole for methanol oxidation. J. Chin. Chem. Soc. 2014, 61, 860–866. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, S.W. Electrochemical synthesis of electrochromic polycarbazole films from N-phenyl-3,6-bis(N-carbazolyl)carbazoles. Polym. Chem. 2016, 7, 198–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, M.; Liu, X.; Zhao, J. Low band gap donor–acceptor type polymers containing 2,3-bis(4-(decyloxy)phenyl)pyrido[4,3-b]pyrazine as acceptor and different thiophene derivatives as donors. Polymers 2016, 8, 377. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratio and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, B.K.; Li, W.B.; Tseng, L.Y.; Wu, T.Y.; Tseng, C.G.; Chen, H.R.; Huang, Y.C. Effects of supporting electrolytes on spectroelectrochemical and electrochromic properties of polyaniline-poly(styrene sulfonic acid) and poly(ethylenedioxythiophene)-poly(styrene sulfonic acid)-based electrochromic device. J. Chin. Chem. Soc. 2014, 61, 563–570. [Google Scholar] [CrossRef]
- Yigitsoy, B.; Varis, S.; Tanyeli, C.; Akhmedov, I.M.; Toppare, L. Electrochromic properties of a novel low band gap conductive copolymer. Electrochim. Acta 2007, 52, 6561–6568. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, L.C. Fluorescent and electrochromic polymers from 2,8-di(carbazol-9-yl)dibenzothiophene and its S, S-dioxide derivative. Dyes Pigments 2016, 134, 51–63. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chung, H.H. Applications of tris(4-(thiophen-2-yl)phenyl)amine- and dithienylpyrrole-based conjugated copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef]
- Koyuncu, S.; Gultekin, B.; Zafer, C.; Bilgili, H.; Can, M.; Demic, S.; Kaya, I.; Icli, S. Electrochemical and optical properties of biphenyl bridged-dicarbazole oligomer films: Electropolymerization and electrochromism. Electrochim. Acta 2009, 54, 5694–5702. [Google Scholar] [CrossRef]
- Udum, Y.A.; Gündoğdu Hızlıateş, C.; Ergün, Y.; Toppare, L. Electrosynthesis and characterization of an electrochromic material containing biscarbazole-oxadiazole units and its application in an electrochromic device. Thin Solid Films 2015, 595, 61–67. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Xiao, J.; Cui, C.; Liu, R. Synthesis and electropolymerization of 9H-carbazol-9-ylpyrene and its electrochromic properties and electrochromic device application. Int. J. Electrochem. Sci. 2012, 7, 2781–2795. [Google Scholar]
- Chen, S.; Gao, Q.; Zhao, J.; Cui, C.; Yang, W.; Zhang, X. Electrosyntheses, characterizations and electrochromic properties of a novel copolymer of 4,4′-di(N-carbazoyl)biphenyl with 4H-cyclopenta[2,1-b:3,4-b′]dithiophene. Int. J. Electrochem. Sci. 2012, 7, 5256–5272. [Google Scholar]
- Wang, B.; Zhao, J.; Liu, R.; Liu, J.; He, Q. Electrosyntheses, characterizations and electrochromic properties of a copolymer based on 4,4′-di(N-carbazoyl)biphenyl and 2,2′-bithiophene. Sol. Energy Mater. Sol. Cells 2011, 95, 1867–1874. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.L.; Lin, Y.C.; Chang, J.K.; Chen, H.R.; Wu, T.Y. Copolymers based on 1,3-bis(carbazol-9-yl)benzene and three 3,4-ethylenedioxythiophene derivatives as potential anodically coloring copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 368. [Google Scholar] [CrossRef]
- Deutschmann, T.; Kortz, C.; Walder, L.; Oesterschulze, E. High contrast electrochromic iris. Opt. Express 2015, 23, 31544–31549. [Google Scholar] [CrossRef] [PubMed]
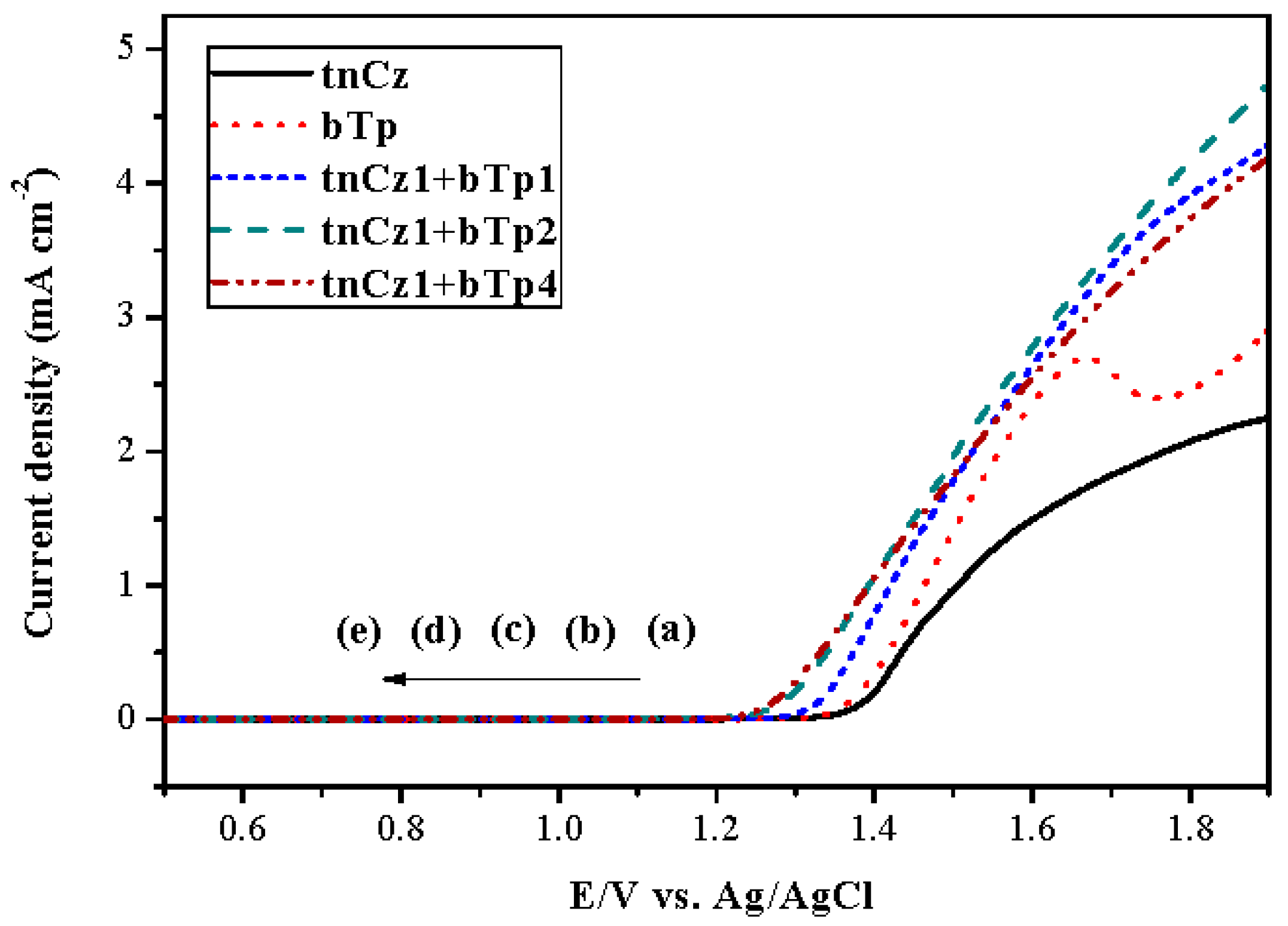
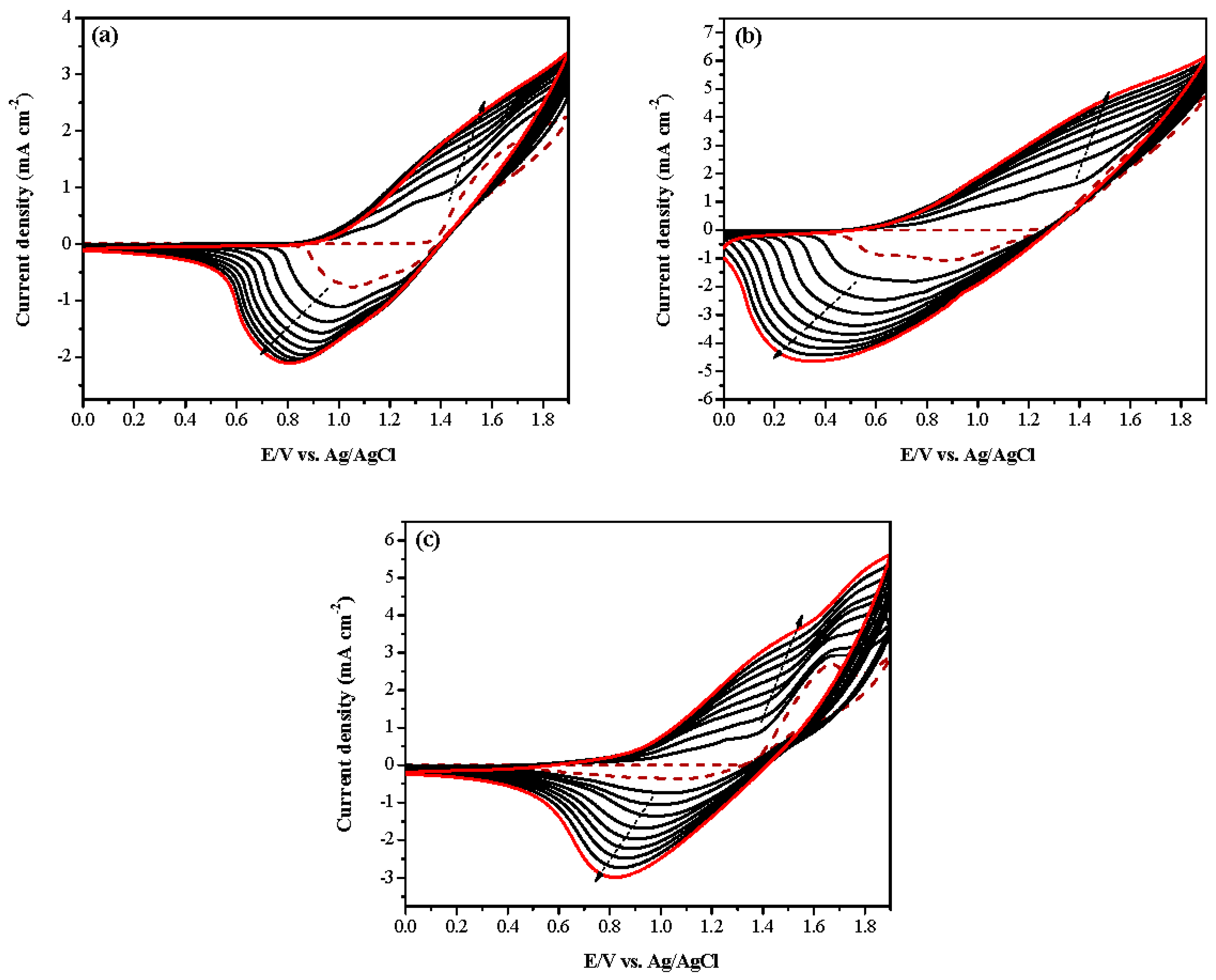
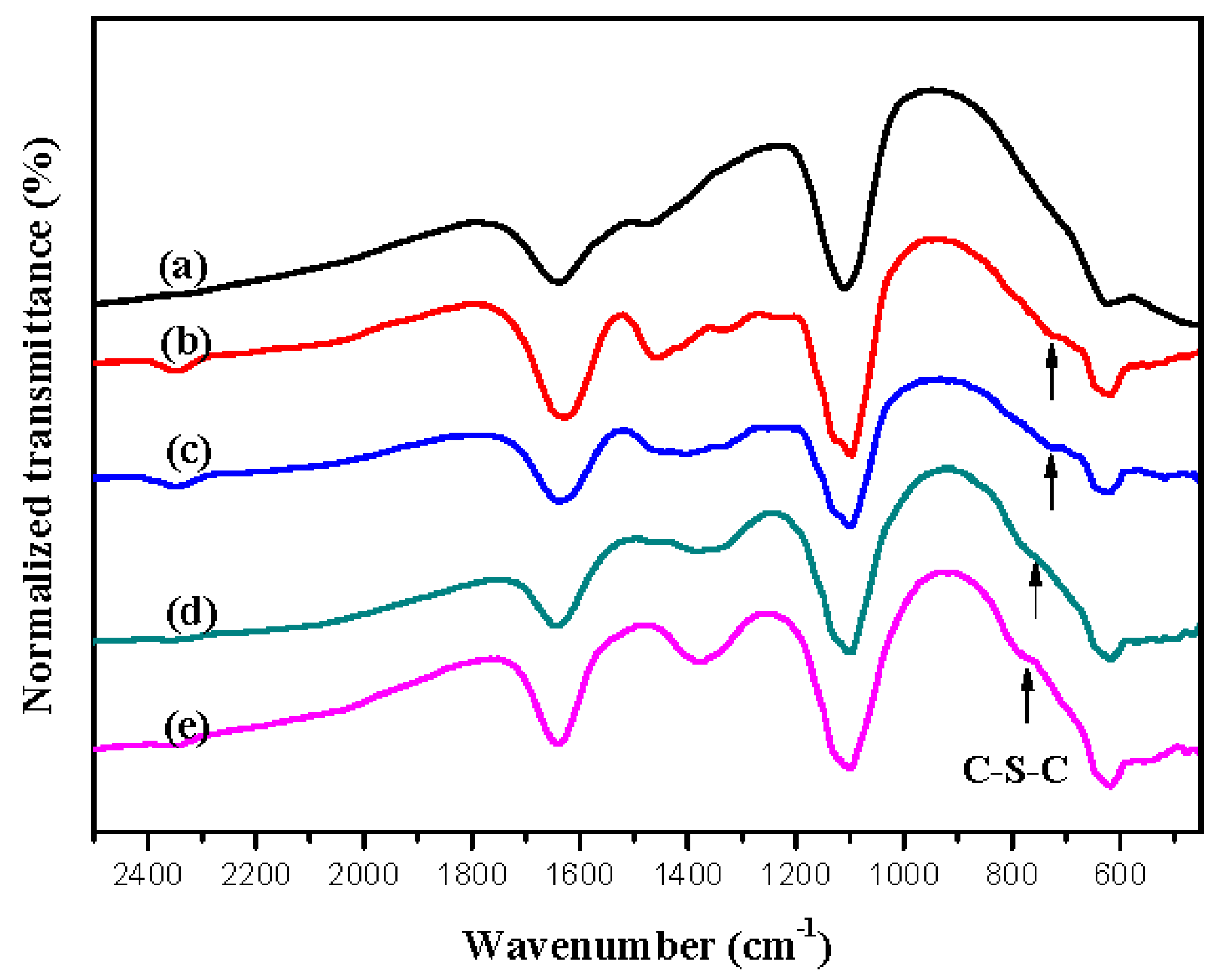
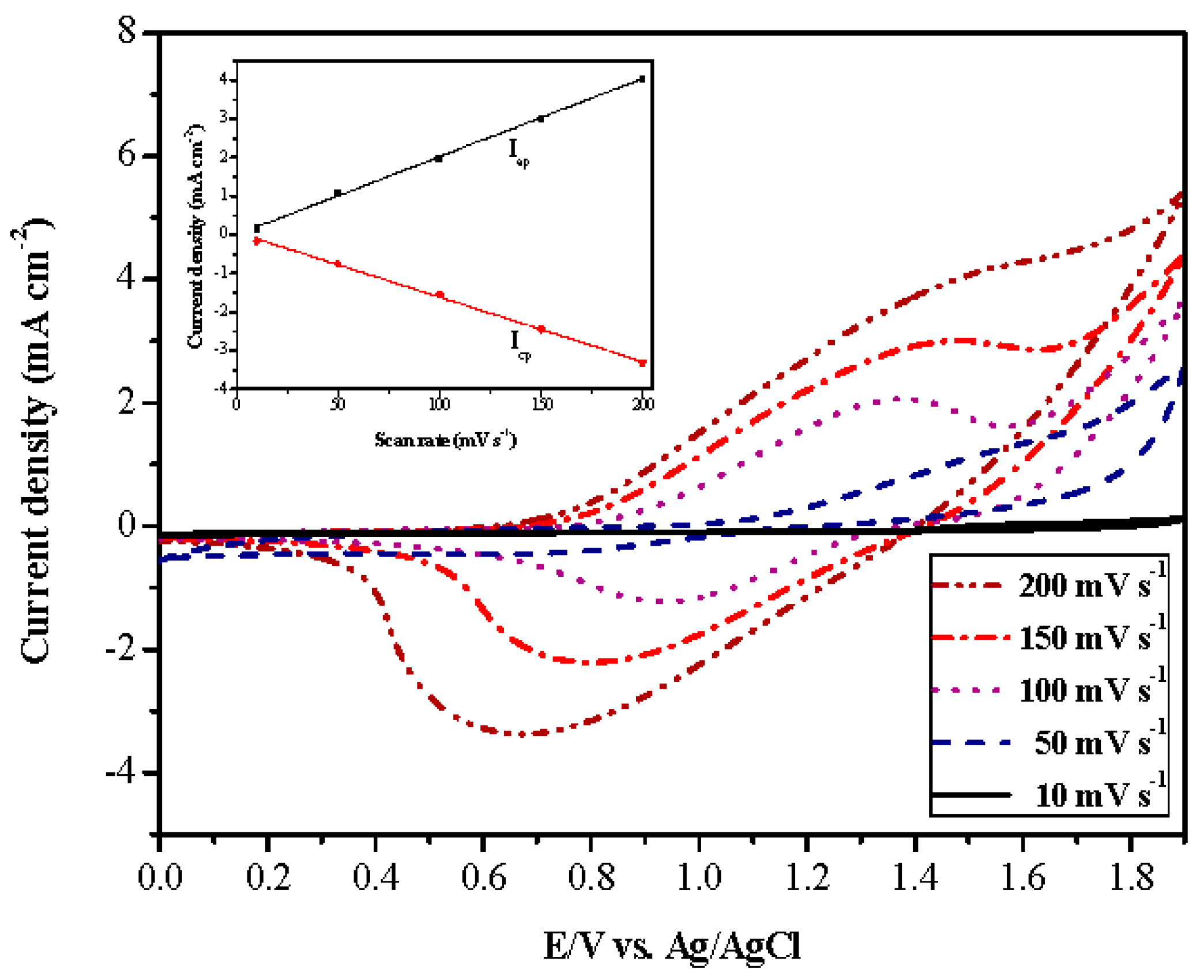
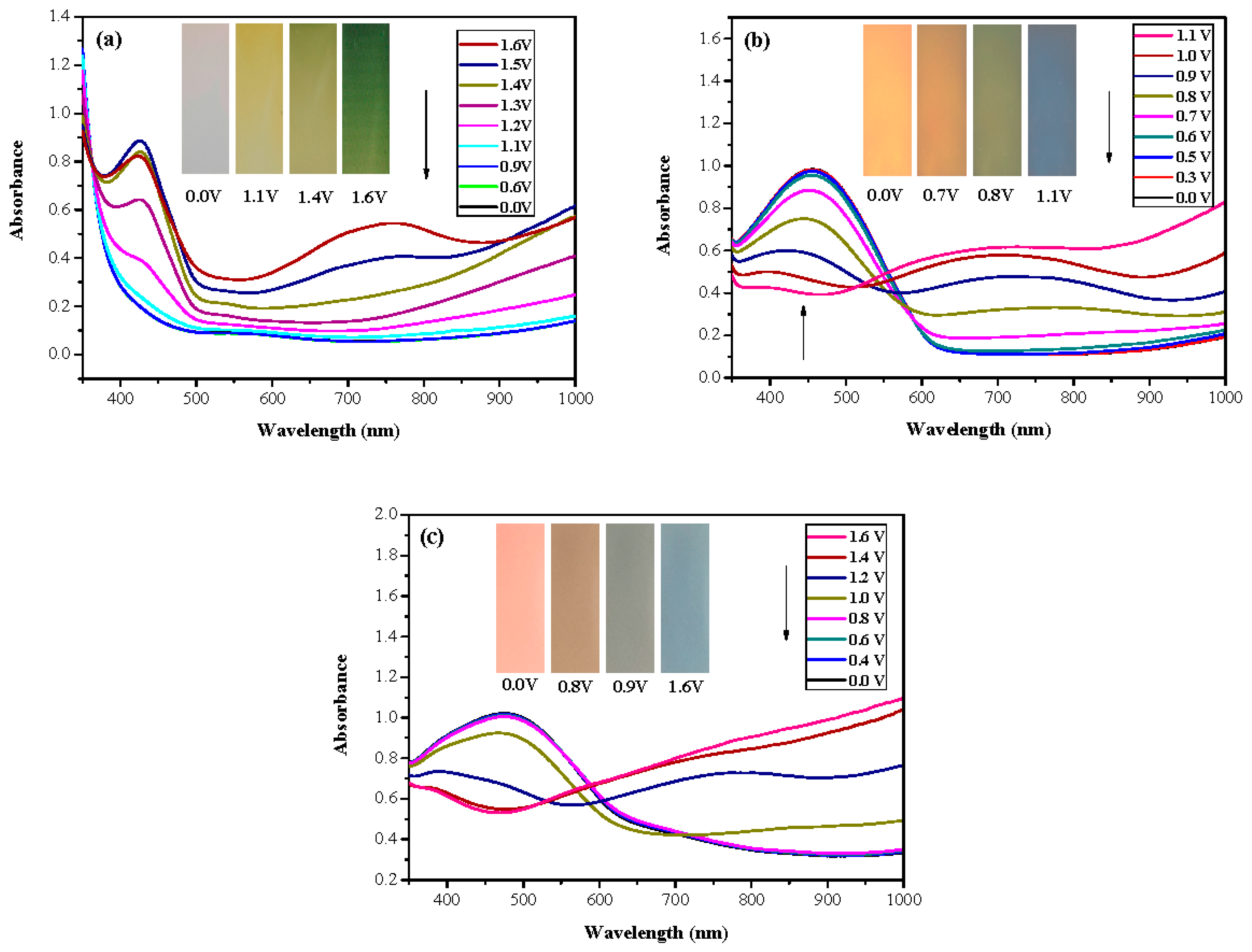
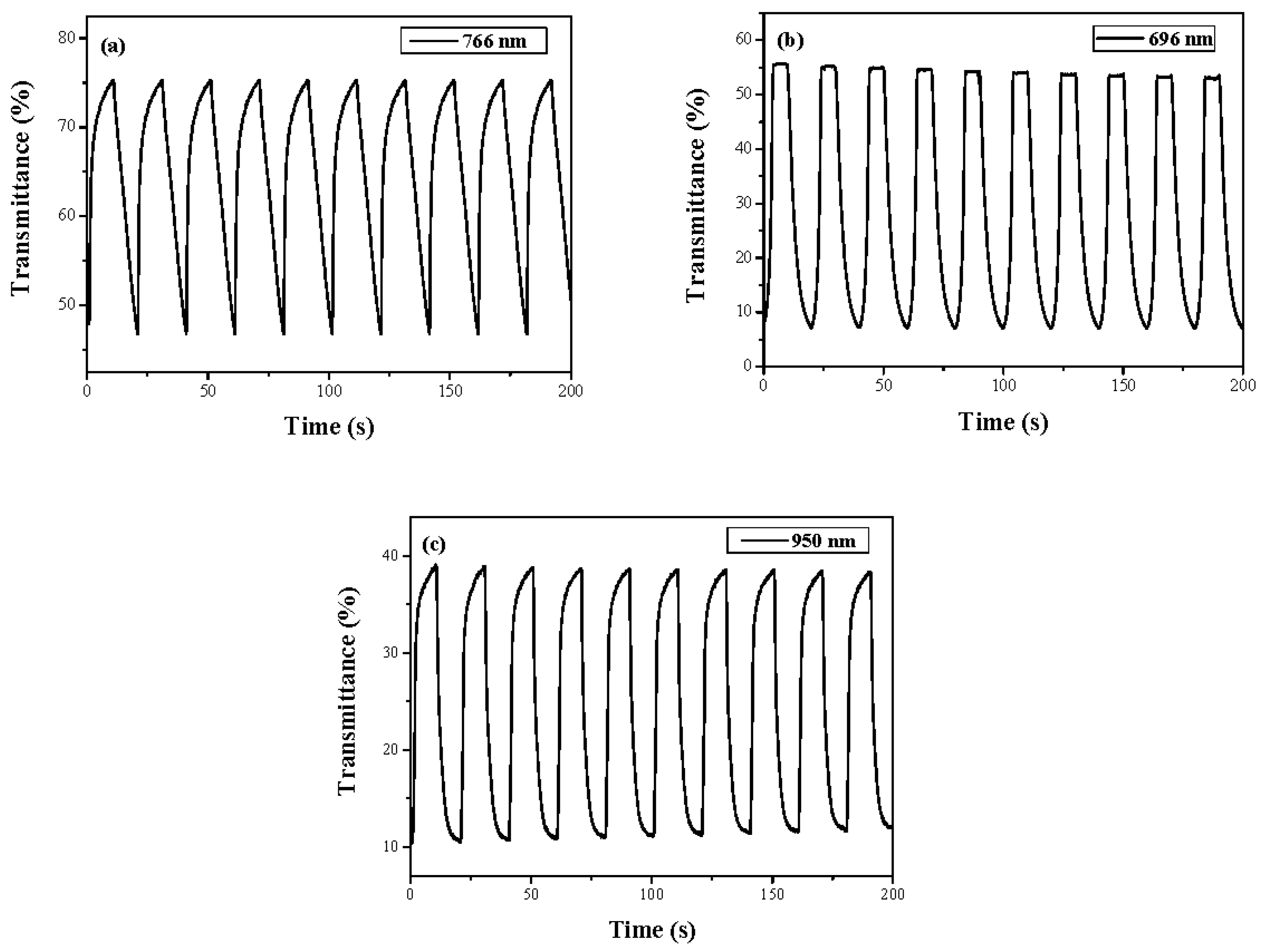
| Electrodes | Anodic Polymer | Feed Species of Anodic Polymer | Feed Molar Ratio of Anodic Polymer |
|---|---|---|---|
| (a) | PtnCz | 2 mM tnCz | Neat tnCz |
| (b) | P(tnCz1-bTp1) | 2 mM tnCz + 2 mM bTp | 1:1 |
| (c) | P(tnCz1-bTp2) | 2 mM tnCz + 4 mM bTp | 1:2 |
| (d) | P(tnCz1-bTp4) | 1 mM tnCz + 4 mM bTp | 1:4 |
| (e) | PbTp | 4 mM bTp | Neat bTp |
| Polymers | Potential (V) | L* | a* | b* | x | y | Diagrams |
|---|---|---|---|---|---|---|---|
| PtnCz | 0.0 | 92.77 | 1.22 | 7.05 | 0.4567 | 0.4125 |  |
| 1.1 | 91.69 | 0.96 | 9.62 | 0.4589 | 0.4152 | ||
| 1.2 | 89.86 | 0.48 | 19.25 | 0.4676 | 0.4243 | ||
| 1.3 | 87.25 | 0.00 | 31.83 | 0.4785 | 0.4356 | ||
| 1.4 | 83.10 | –1.91 | 36.46 | 0.4802 | 0.4431 | ||
| 1.5 | 78.08 | –5.35 | 33.74 | 0.4728 | 0.4480 | ||
| 1.6 | 73.61 | –7.75 | 25.77 | 0.4616 | 0.4465 | ||
| P(tnCz1-bTp2) | 0.0 | 71.78 | 34.58 | 54.01 | 0.5661 | 0.3964 |  |
| 0.6 | 72.11 | 32.68 | 52.59 | 0.5614 | 0.3989 | ||
| 0.7 | 72.03 | 25.51 | 47.02 | 0.5445 | 0.4078 | ||
| 0.8 | 70.79 | 12.38 | 33.90 | 0.5098 | 0.4209 | ||
| 0.9 | 67.83 | –1.02 | 14.63 | 0.4640 | 0.4260 | ||
| 1.0 | 64.23 | –7.63 | –2.57 | 0.4274 | 0.4161 | ||
| 1.1 | 62.30 | –7.98 | –12.05 | 0.4119 | 0.4029 | ||
| PbTp | 0.0 | 51.45 | 21.71 | 23.34 | 0.5357 | 0.3929 |  |
| 0.6 | 51.01 | 20.62 | 21.90 | 0.5318 | 0.3936 | ||
| 0.8 | 51.01 | 20.34 | 21.58 | 0.5307 | 0.3939 | ||
| 0.9 | 55.79 | 19.12 | 24.19 | 0.5229 | 0.4000 | ||
| 1.2 | 57.79 | –2.40 | 8.42 | 0.4543 | 0.4231 | ||
| 1.4 | 55.13 | –7.32 | –7.38 | 0.4177 | 0.4089 | ||
| 1.6 | 54.83 | –7.08 | –9.69 | 0.4143 | 0.4047 |
| Electrodes | λ (nm) | Tox (%) | Tred (%) | ∆T (%) | Contrast (%) [23] | ∆OD | Qd (mC∙cm−2) | η (cm2∙C−1) | τc (s) | τb (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| PtnCz | 766 | 46.7 | 75.3 | 28.6 | 23.4 | 0.207 | 2.000 | 103.7 | 5.5 | 4.5 |
| P(tnCz1-bTp1) | 680 | 9.3 | 54.3 | 45.0 | 70.8 | 0.766 | 4.250 | 180.3 | 5.0 | 2.5 |
| P(tnCz1-bTp2) | 696 | 7.0 | 55.0 | 48.0 | 77.4 | 0.895 | 7.986 | 112.0 | 6.0 | 4.0 |
| P(tnCz1-bTp4) | 689 | 10.5 | 47.2 | 36.7 | 63.6 | 0.652 | 4.346 | 150.1 | 5.8 | 3.0 |
| PbTp | 950 | 10.7 | 38.8 | 28.1 | 56.8 | 0.559 | 6.696 | 83.5 | 5.5 | 4.5 |
| ECDs | Potential (V) | Photographs | L* | a* | b* | x | y | Diagrams |
|---|---|---|---|---|---|---|---|---|
| PtnCz/PProdot-Me2 | −1.1 |  | 77.89 | −0.64 | −2.39 | 0.444 | 0.406 |  |
| 0.0 |  | 77.78 | −0.67 | −2.42 | 0.444 | 0.406 | ||
| 0.9 |  | 67.00 | 2.70 | 1.82 | 0.456 | 0.406 | ||
| 1.3 |  | 45.40 | 1.10 | −21.68 | 0.407 | 0.366 | ||
| 1.5 |  | 43.60 | 1.45 | −22.35 | 0.405 | 0.362 | ||
| P(tnCz1-bTp2)/PProdot-Me2 | −0.3 |  | 62.89 | 16.85 | 31.86 | 0.525 | 0.409 |  |
| 0.4 |  | 62.34 | 12.16 | 27.43 | 0.511 | 0.414 | ||
| 0.7 |  | 58.41 | 6.68 | 18.02 | 0.489 | 0.416 | ||
| 1.1 |  | 32.66 | 1.51 | −21.96 | 0.396 | 0.351 | ||
| 1.5 |  | 25.34 | 0.30 | −41.33 | 0.318 | 0.284 |
| ECDs | N | Tox (%) | Tred (%) | ∆T (%) | Contrast (%) | ∆OD | Qd (mC∙cm−2) | η (cm2∙C−1) | τc (s) | τb (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| PtnCz/PProdot-Me2 (580 nm) a | 3 | 18.2 | 51.4 | 33.2 | 47.7 | 0.450 | 0.859 | 523.9 | 2.5 | 0.5 |
| 50 | 19.5 | 44.0 | 24.5 | 38.6 | 0.353 | 0.620 | 569.4 | 1.0 | 1.0 | |
| P(tnCz1-bTp1)/PProdot-Me2 (582 nm) a | 3 | 7.1 | 42.1 | 35.0 | 71.1 | 0.773 | 1.596 | 484.3 | 0.5 | 0.5 |
| 50 | 7.5 | 39.0 | 31.5 | 67.7 | 0.716 | 1.252 | 571.8 | 2.0 | 2.0 | |
| P(tnCz1-bTp2)/PProdot-Me2 (630 nm) a | 3 | 8.0 | 48.0 | 40.0 | 71.4 | 0.778 | 1.500 | 518.8 | 0.8 | 0.3 |
| 50 | 7.4 | 46.0 | 38.6 | 72.3 | 0.793 | 1.470 | 539.4 | 0.7 | 0.5 | |
| P(tnCz1-bTp4)/PProdot-Me2 (624 nm) a | 3 | 11.5 | 39.2 | 27.7 | 54.6 | 0.532 | 0.953 | 558.7 | 0.5 | 0.5 |
| 50 | 9.6 | 32.3 | 22.7 | 54.2 | 0.526 | 0.981 | 537.1 | 0.5 | 0.7 | |
| PbTp/PProdot-Me2 (580 nm) a | 3 | 8.2 | 32.6 | 24.4 | 59.8 | 0.599 | 1.106 | 541.6 | 0.5 | 0.5 |
| 50 | 8.3 | 28.9 | 20.6 | 55.4 | 0.542 | 0.908 | 596.6 | 0.5 | 0.5 |
| ECD Configuration | ΔTmax (%) | ηmax (cm2∙C−1) | Reference |
|---|---|---|---|
| PdNCz/PEDOT | 19 (550 nm) | — | [36] |
| PbmCz/PEDOT | 35 (620 nm) | — | [37] |
| PHCz/PEDOT | 23 (623 nm) | 290 | [38] |
| P(dNCz-Hcp)/PEDOT | 39.8 (628 nm) | 319.98 | [39] |
| P(dNCz-bT)/PEDOT | 28.6 (700 nm) | 234 | [40] |
| P(Cz4-6CIn1)/PProDOT-Me2 | 32 (575 nm) | 372.7 | [41] |
| P(BCz-ProDOTme)/PEDOT-PSS | 41 (642 nm) | 417 | [42] |
| P(tnCz1-bTp2)/PProdot-Me2 | 40 (630 nm) | 539 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-W.; Lee, P.-Y. Electrosynthesis of Copolymers Based on 1,3,5-Tris(N-Carbazolyl)Benzene and 2,2′-Bithiophene and Their Applications in Electrochromic Devices. Polymers 2017, 9, 518. https://doi.org/10.3390/polym9100518
Kuo C-W, Lee P-Y. Electrosynthesis of Copolymers Based on 1,3,5-Tris(N-Carbazolyl)Benzene and 2,2′-Bithiophene and Their Applications in Electrochromic Devices. Polymers. 2017; 9(10):518. https://doi.org/10.3390/polym9100518
Chicago/Turabian StyleKuo, Chung-Wen, and Po-Ying Lee. 2017. "Electrosynthesis of Copolymers Based on 1,3,5-Tris(N-Carbazolyl)Benzene and 2,2′-Bithiophene and Their Applications in Electrochromic Devices" Polymers 9, no. 10: 518. https://doi.org/10.3390/polym9100518