The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed
Abstract
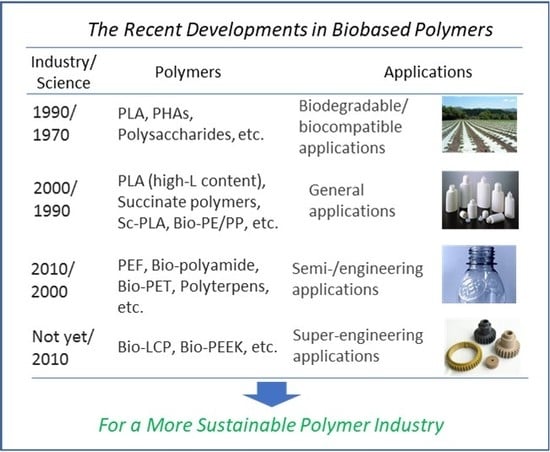
:1. Introduction
- 1st class; naturally derived biomass polymers: direct use of biomass as polymeric material including chemically modified ones such as cellulose, cellulose acetate, starches, chitin, modified starch, etc.;
- 2nd class; bio-engineered polymers: bio-synthesized by using microorganisms and plants such as poly(hydroxy alkanoates (PHAs), poly(glutamic acid), etc.;
2. Biobased Polymers: Upgraded from Biodegradable-Grade Polymers
2.1. Polylactide (PLA)
2.1.1. High l-Content PLA (PLLA)
2.1.2. Stereocomplexed PLA
2.1.3. Examples of PLA Applications
2.2. Poly(hydroxyalkanoates) (PHAs)
2.3. Polysaccharides
2.4. Succinate Polymers
3. Biobased Polymers Analogous to Conventional Petroleum-Derived Polymers
3.1. Biobased Polyethylene (Bio-PE)
3.2. Biobased Poly(Ethylene Terephthalate) (PET) and Poly(Trimethylene Terephthalate) (PTT)
3.3. Biobased Polyamides
4. Newly Developed Biobased Polymers
4.1. Poly(Ethylene 2,5-Furandicarboxylate) (PEF)
4.1.1. PEF from Condensation of Diol and FDCA
4.1.2. PEF from ROP
4.2. High-Performance PLA from Modified Lactides
4.3. Terpen-Derived Biobased Polymers
4.4. Other Noteworthy Biobased Polymers
5. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinbuechel, A. Biopolymers; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Domb, A.J.; Kost, J.; Wiseman, D.M. Handbook of Biodegradable Polymers; Harwood Academic Publishers: London, UK, 1997; ISBN 90-5702-153-6. [Google Scholar]
- Klass, D.L. Biomass for Renewable Energy, Fuels, and Chemicals; Academic Press: San Diego, CA, USA, 1998; ISBN 0-12-410950-0. [Google Scholar]
- Mohsenzadeh, A.; Zamani, A.; Taherzadeh, M.J. Bioethylene Production from Ethanol: A review and Techno-economical Evaluation. ChemBioEng Rev. 2017, 4, 75–91. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sust. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Im, S.S.; Kim, Y.H.; Yoon, J.S.; Chin, I.-J. Biobased-Polymers: Recent Progress; Wiley-VCH: New York, NY, USA, 2005; ISBN 3527313273. [Google Scholar]
- Kimura, Y. Molecular, Structural, and Material Design of Bio-Based Polymers. Polym. J. 2009, 41, 797–807. [Google Scholar] [CrossRef]
- Nakajima, H.; Kimura, Y. Chapter 1, General introduction: Overview of the current development of biobased polymers. In Bio-Based Polymers, 1st ed.; Kimura, Y., Ed.; CMC Publishing Co., Ltd.: Tokyo, Japan, 2013; pp. 1–23. ISBN 978-4-7813-0271-3. [Google Scholar]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef]
- Tsuji, H. Polylactide. In Biopolymers, Vol.4, Polyesters III; Steinbuchel, A., Doi, Y., Eds.; Wiley-VCH Verlag GmBH: Weinheim, Germany, 2002; pp. 129–177. ISBN 978-3-527-30225-3. [Google Scholar]
- Tsuji, H. Poly(lactide) Stereocomplexes: Formation, Structure, Properties, Degradation, and Applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Yoshiharu, K. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: Their formation, properties, and application. Polym. Int. 2006, 55, 626–642. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory Report. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 16 August 2017).
- PRO-BIP2009. Available online: https://www.uu.nl/sites/default/files/copernicus_probip2009_final_june_2009_revised_in_november_09.pdf (accessed on 15 July 2017).
- Bio-Based Chemicals. Available online: http://www.iea-bioenergy.task42-biorefineries.com/upload_mm/b/a/8/6d099772-d69d-46a3-bbf7-62378e37e1df_Biobased_Chemicals_Report_Total_IEABioenergyTask42.pdf (accessed on 16 August 2017).
- Corbion/Total Announcement. Available online: https://www.total-corbion.com/products/pla-polymers/ (accessed on 16 August 2017).
- Mochizuki, M. Application of Bio-based Polymers. In Bio-Based Polymers, 1st ed.; Kimura, Y., Ed.; CMC Publishing Co., Ltd.: Tokyo, Japan, 2013; Chapter 5; pp. 165–174. ISBN 978-4-7813-0271-3. [Google Scholar]
- Mochizuki, M. Biopolymers, Vol.4, Polyesters III; Wiley-VCH Verlag GmBH: Weinheim, Germany, 2002; pp. 1–23. ISBN 978-3-527-30225-3. [Google Scholar]
- Avantium Report. Renewable Chemicals into Bio-Based Materials: From Lignocellulose to PEF. Available online: http://biobasedperformancematerials.nl/upload_mm/3/5/7/651bed82-390b-4435-a006-7909570de736_BPM%202017%20-%20Speaker%2006%20-%20Ed%20de%20Jong%20-%20Renewable%20chemicals%20into%20bio-based%20materials%20-%20from%20lignocellulose%20to%20PEF.pdf (accessed on 16 August 2017).
- Avantium Report. PEF, a 100% Bio-Based Polyester: Synthesis, Properties & Sustainability. Available online: http://euronanoforum2015.eu/wp-content/uploads/2015/06/PlenaryII_PEF_a_100_bio-based_polyester_Gert-JanGruter_11062015_final.pdf (accessed on 16 August 2017).
- Kaku, M. Poly(trimethylene terephthalate, PTT). In Bio-Based Polymers, 1st ed.; Kimura, Y., Ed.; CMC Publishing Co., Ltd.: Tokyo, Japan, 2013; Chapter 3.4; pp. 86–94. ISBN 978-4-7813-0271-3. [Google Scholar]
- Mochizuki, M. Crystallization Behaviors of highly LLA-rich PLA Effects of D-isomer ratio of PLA on the rate of crystallization, crystallinity, and melting point. Sen’I Gakkaishi 2010, 66, 70–77. [Google Scholar] [CrossRef]
- Marega, C.; Marigo, A.; Noto, V.D.; Zannetti, R.; Martorana, A.; Paganetto, G. Structure and crystallization kinetics of poly(l-lactic acid). Macromol. Chem. Phys. 1992, 193, 1599–1606. [Google Scholar] [CrossRef]
- Sasaki, S.; Asakura, T. Helix Distortion and Crystal Structure of the α-Form of Poly(l-lactide). Macromolecules 2003, 36, 8385–8390. [Google Scholar] [CrossRef]
- Alemán, C.; Lotz, B.; Puiggali, J. Crystal Structure of the α-Form of Poly(l-lactide). Macromolecules 2001, 34, 4795–4801. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K. Crystal structure and disorder in poly(l-lactic acid) δ form (α′ form) and the phase transition mechanism to the ordered α form. Polymer 2011, 52, 6097–6109. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-Order Phase Transition and Multiple Melting Behavior of Poly(l-lactide) Investigated by Simultaneous Measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Sato, H.; Tsuji, H.; Noda, I.; Yan, S.; Ozaki, Y. Crystal Modifications and Thermal Behavior of Poly(l-lactic acid) Revealed by Infrared Spectroscopy. Macromolecules 2005, 38, 8012–8021. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, J.; Sato, H.; Zhang, J.; Tsuji, H.; Ozaki, Y.; Yan, S. Molecular Weight Dependence of the Poly(l-lactide)/Poly(d-lactide) Stereocomplex at the Air−Water Interface. Biomacromolecules 2006, 7, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Yamashita, H.; Fujiwara, T.; Kimura, Y.; Akashi, M. Stepwise Assembly of Enantiomeric Poly(lactide)s on Surfaces. Macromolecules 2001, 34, 1996–2001. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Brinke, G.T.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(l-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly(l-lactide) and poly(d-lactide). J. Macromol. Sci. Phys. 1991, 30, 119–140. [Google Scholar] [CrossRef]
- NatureWorks Website. Available online: http://www.natureworksllc.com/What-is-Ingeo (accessed on 16 August 2017).
- Niaounakis, M. Chapter 1, Definition of Terms and Types of Biopolymers. In Biopolymers: Applications and Trends, 1st ed.; Niaounakis, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–90. ISBN 9780323353991. [Google Scholar]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [PubMed]
- Morgan-Sagastume, F.; Valentino, F.; Hjort, M.; Cirne, D.; Karabegovic, L.; Gerardin, F.; Johansson, P.; Karlsson, A.; Magnusson, P.; Alexandersson, T.; et al. Polyhydroxyalkanoate (PHA) production from sludge and municipal wastewater treatment. Water Sci. Technol. 2014, 69, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Yuan, L. Directed evolution of metabolic pathways. Trends Biotechnol. 2006, 24, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Witholt, B.; Kessler, B. Perspectives of medium chain length poly(hydroxyalkanoates), a versatile set of bacterial bioplastics. Curr. Opin. Biotechnol. 1999, 10, 279–285. [Google Scholar] [CrossRef]
- Gerngross, T.U.; Martin, D.P. Enzyme-catalyzed synthesis of poly[(R)-(-)-3-hydroxybutyrate]: Formation of macroscopic granules in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 6279–6283. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Grubelnik, A.; Hoerler, M.; Ruth, K.; Hartmann, R.; Felber, H.; Zinn, M. Bacterial Poly(hydroxyalkanoates) as a Source of Chiral Hydroxyalkanoic Acids. Biomacromolecules 2005, 6, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Haywood, G.W.; Anderson, A.J.; Williams, D.R.; Dawes, E.A.; Ewing, D.F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int. J. Biol. Macromol. 1991, 13, 83–88. [Google Scholar] [CrossRef]
- Matsumoto, K.; Murata, T.; Nagao, R.; Nomura, C.T.; Arai, S.; Arai, Y.; Takase, K.; Nakashita, H.; Taguchi, S.; Shimada, H. Production of short-chain-length/medium-chain-length polyhydroxyalkanoate (PHA) copolymer in the plastid of Arabidopsis thaliana using an engineered 3-ketoacyl-acyl carrier protein synthase III. Biomacromolecules 2009, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Pollet, E.; Averous, L.; Plackett, D. Biopolymers: New Materials for Sustainable Films and Coatings; Wiley-VCH: New York, NY, USA, 2011; ISBN 9780470683415. [Google Scholar]
- Yokouchi, M.; Chatani, Y.; Tadokoro, H.; Teranishi, K.; Tani, H. Structural studies of polyesters: 5. Molecular and crystal structures of optically active and racemic poly (β-hydroxybutyrate). Polymer 1973, 14, 267–272. [Google Scholar] [CrossRef]
- Hoenich, N.A. Cellulose for medical applications: Past, present, and future. BioResources 2006, 1, 270–280. [Google Scholar]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Vshivkov, S.A.; Rusinova, E.V.; Galyas, A.G. Phase diagrams and rheological properties of cellulose ether solutions in magnetic field. Eur. Polym. J. 2014, 59, 326–332. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Khan, B.; Niazi, M.B.K.; Samin, G.; Jahan, Z. Thermoplastic Starch: A Possible Biodegradable Food Packaging Material—A Review. J. Food Proc. Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Halley, P.J.; Truss, R.W.; Markotsis, M.G.; Chaleat, C.; Russo, M.; Sargent, A.L.; Tan, I.; Sopade, P.A. A Review of Biodegradable Thermoplastic Starch Polymers. ACS Symp. Ser. 2007, 978, 287–300. [Google Scholar]
- Ahmadi-Abhari, S.; Woortman, A.J.; Hamer, R.J.; Loos, K. Rheological properties of wheat starch influenced by amylose–lysophosphatidylcholine complexation at different gelation phases. Carbohydr. Polym. 2015, 122, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Abhari, S.; Woortman, A.J.J.; Oudhuis, A.A.C.M.; Hamer, R.J.; Loos, K. The effect of temperature and time on the formation of amylose–lysophosphatidylcholine inclusion complexes. Starch 2014, 66, 251–259. [Google Scholar] [CrossRef]
- Ahmadi-Abhari, S.; Woortman, A.J.J.; Hamer, R.J.; Loos, K. Assessment of the influence of amylose-LPC complexation on the extent of wheat starch digestibility by size-exclusion chromatography. Food Chem. 2013, 14, 4318–4323. [Google Scholar] [CrossRef] [PubMed]
- Thakker, C.; Martínez, I.; San, K.-Y.; Bennett, G.N. Succinate production in Escherichia coli. Biotechnol. J. 2012, 7, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zeikus, J.G.; Jain, M.K.; Elankovan, P. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 1999, 51, 545–552. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.-H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Niaounakis, M. Biopolymers: Applications and Trends, 1st ed.; William Andrew: New York, NY, USA, 2015; ISBN 9780323353991. [Google Scholar]
- Siracusa, V.; Lotti, N.; Munari, A.; Rosa, M.D. Poly(butylene succinate) and poly(butylene succinate-co-adipate) for food packaging applications: Gas barrier properties after stressed treatments. Polym. Degrad. Stab. 2015, 119, 35–45. [Google Scholar] [CrossRef]
- Luo, S.; Li, F.; Yu, J.; Cao, A. Synthesis of poly(butylene succinate-co-butylene terephthalate) (PBST) copolyesters with high molecular weights via direct esterification and polycondensation. J. Appl. Polym. Sci. 2010, 115, 2203–2211. [Google Scholar] [CrossRef]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, J.-M.; Dubois, P. High Molecular Weight Poly(butylene succinate-co-butylene furandicarboxylate) Copolyesters: From Catalyzed Polycondensation Reaction to Thermomechanical Properties. Biomacromolecules 2012, 13, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Morschbacker, A. Bio-Ethanol Based Ethylene. Polym. Rev. 2009, 49, 79–84. [Google Scholar] [CrossRef]
- Braskem report. Development of Bio-Based Olefins. Available online: http://www.inda.org/BIO/vision2014_659_PPT.pdf (accessed on 22 August 2017).
- Hess, G.; Johnson, J. Deconstructing Inherently Safer Technology. Chem. Eng. News 2014, 92, 11–16. [Google Scholar]
- The Coca Cola Company Website. Available online: http://www.coca-colacompany.com/plantbottle-technology (accessed on 19 August 2017).
- Gevo Report. Available online: http://www.gevo.com/wp-content/uploads/PDF/gevo-roadshow-2011-web.pdf (accessed on 19 August 2017).
- Carraher, J.M.; Pfennig, T.; Rao, R.G.; Shanks, B.H.; Tessonnier, J.-P. Cis,cis-Muconic acid isomerization and catalytic conversion to biobased cyclic-C6-1,4-diacid monomers. Green Chem. 2017, 19, 3042–3050. [Google Scholar] [CrossRef]
- Colonna, M.; Berti, C.; Fiorini, M.; Binassi, E.; Mazzacurati, M.; Vannini, M.; Karanam, S. Synthesis and radiocarbon evidence of terephthalate polyesters completely prepared from renewable resources. Green Chem. 2011, 13, 2543–2548. [Google Scholar] [CrossRef]
- Shiramizu, M.; Toste, F.D. On the Diels-alder Approach to Solely Biomass-derived Polyethylene terephthalate (PET): Conversion of 2,5-Dimethylfuran and Acrolein into p-Xylene. Chem. Eur. J. 2011, 17, 12452–12457. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates. Rev. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Tachibana, Y.; Kimura, S.; Kasuya, K. Synthesis and Verification of Biobased Terephthalic Acid from Furfural. Sci. Rep. 2015, 5, 8249. [Google Scholar] [CrossRef] [PubMed]
- Collias, D.I.; Harris, A.M.; Nagpal, V.; Cottrell, I.W.; Schultheis, M.W. Biobased Terephthalic Acid Technologies: A Literature Review. Ind. Biotech. 2014, 10, 91–105. [Google Scholar] [CrossRef]
- Schenk, N.J.; Biesbroek, A.; Heeres, A.; Heeres, H.J. Process for the Preparation of Aromatic Compounds. Patent WO 2,015,047,085 A1, 2 April 2015. [Google Scholar]
- DuPont Tate & Lyle BioProducts Report. Available online: http://www.cosmoschemicals.com/uploads/products/pdf/technical/susterra-propanediol-89.pdf (accessed on 01 October 2017).
- Bio-Based World News Report. Available online: https://www.biobasedworldnews.com/novamont-opens-worlds-first-plant-for-the-production-of-bio-based-butanediol-on-industrial-scale (accessed on 19 August 2017).
- Kawasaki, N.; Nakayama, A.; Yamano, N.; Takeda, S.; Kawata, Y.; Yamamoto, N.; Aiba, S. Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 2005, 46, 9987–9993. [Google Scholar] [CrossRef]
- Winnacker, M.; Rieger, B. Biobased Polyamides: Recent Advances in Basic and Applied Research. Macromol. Rapid Commun. 2016, 37, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.S.; Barthelon, A.B.; Pearsall, A.; Mittal, V.; Dorgan, J.R. Biorenewable blends of polyamide-4,10 and polyamide-6,10. J. Appl. Polym. Sci. 2016, 133, 43626. [Google Scholar] [CrossRef]
- Schouwer, F.D.; Claes, L.; Claes, N.; Bals, S.; Degrèvec, J.; Vos, D.E.D. Pd-catalyzed decarboxylation of glutamic acid and pyroglutamic acid to bio-based 2-pyrrolidone. Green Chem. 2015, 17, 2263–2270. [Google Scholar] [CrossRef]
- Winnacker, M.; Tischner, A.; Neumeier, M.; Rieger, B. New insights into synthesis and oligomerization of ε-lactams derived from the terpenoid ketone (−)-menthone. RSC. Adv. 2015, 5, 77699–77705. [Google Scholar] [CrossRef]
- Winnacker, M.; Vagin, S.; Auer, V.; Rieger, B. Synthesis of Novel Sustainable Oligoamides Via Ring-Opening Polymerization of Lactams Based on (−)-Menthone. Macromol. Chem. Phys. 2014, 215, 1654–1660. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly (ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Knoop, R.J.; Vogelzang, W.; Haveren, J.V.; Es, D.S.V. High molecular weight poly(ethylene-2,5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Avantium YXY Technology Website. Available online: https://www.avantium.com/yxy/yxy-technology/ (accessed on 19 August 2017).
- Avantium Report. Available online: https://www.coebbe.nl/sites/default/files/documenten/nieuwsbericht/491/PEF%20Polyester%20-%20Ed%20de%20Jong.pdf (accessed on 7 August 2017).
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2014, 112, 369–378. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, Q.; Zhang, Q.; Ye, C.; Zhou, G. A series of furan-aromatic polyesters synthesized via direct esterification method based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1026–1036. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Terzopoulou, Z.; Bikiaris, D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016, 83, 202–229. [Google Scholar] [CrossRef]
- Avantium Report. Furanics: Versatile Molecules Applicable for Biopolymers Applications. Available online: http://www.soci.org/-/media/Files/Conference-Downloads/2009/Bioplastic-Processing-Apr-09/Jong.ashx?la=en (accessed on 7 August 2017).
- Storbeck, R.; Ballauff, M. Synthesis and properties of polyesters based on 2,5-furandicarboxylic acid and 1,4:3,6-dianhydrohexitols. Polymer 1993, 34, 5003–5006. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Ekensteina, G.O.R.A.V.; Loos, K. A biocatalytic approach towards sustainable furanic–aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Loos, K. Enzymatic synthesis of 2,5-furandicarboxylic acidbased semi-aromatic polyamides: Enzymatic polymerization kinetics, effect of diamine chain length and thermal properties. RSC Adv. 2016, 6, 67941–67953. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Ekensteina, G.O.R.A.V.; Petrović, D.M.; Loos, K. Enzymatic Synthesis of Biobased Polyesters Using 2,5-Bis(hydroxymethyl)furan as the Building Block. Biomacromolecules 2014, 15, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Storti, G.; Tancini, F.; Costa, L.I.; Morbidelli, M. Synthesis and Ring-Opening Polymerization of Cyclic Butylene 2,5-Furandicarboxylate. Macromol. Chem. Phys. 2015, 216, 2141–2146. [Google Scholar] [CrossRef]
- Morales-Huerta, J.C.; Ilarduya, A.M.D.; Munoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef]
- Liu, T.; Simmons, T.L.; Bohnsack, D.A.; Mackay, M.E.; Smith, M.R.; Baker, G.L. Synthesis of Polymandelide: A Degradable Polylactide Derivative with Polystyrene-like Properties. Macromolecules 2007, 40, 6040–6047. [Google Scholar] [CrossRef]
- Cairns, S.A.; Schultheiss, A.; Shaver, M.P. A broad scope of aliphatic polyesters prepared by elimination of small molecules from sustainable 1,3-dioxolan-4-ones. Polym. Chem. 2017, 8, 2990–2996. [Google Scholar] [CrossRef]
- Buchard, A.; Carbery, D.R.; Davidson, M.G.; Ivanova, P.K.; Jeffery, B.J.; Kociok-Kohn, G.I.; Lowe, J.P. Preparation of Stereoregular Isotactic Poly(mandelic acid) through Organocatalytic Ring-Opening Polymerization of a Cyclic O-Carboxyanhydride. Angew. Chem. Int. Ed. 2014, 53, 13858–13861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, F.; Hillmyer, M.A. Bifunctional Monomer Derived from Lactide for Toughening Polylactide. J. Am. Chem. Soc. 2008, 130, 13826–13827. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Baker, G.L. Preparation and Characterization of Substituted Polylactides. Macromolecules 1999, 32, 7711–7718. [Google Scholar] [CrossRef]
- Satoh, K.; Sugiyama, H.; Kamigaito, M. Biomass-derived heat-resistant alicyclic hydrocarbon polymers: Poly(terpenes) and their hydrogenated derivatives. Green Chem. 2006, 8, 878–882. [Google Scholar] [CrossRef]
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: Living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). Polym. Chem. 2014, 5, 3222–3230. [Google Scholar] [CrossRef]
- Li, A.-Y.; Sun, X.-D.; Zhang, H.-B.; Zhang, Y.-C.; Wang, B.; Shi, L.-Q. Cationic copolymerization of 1,3-pentadiene with α-pinene. J. Polym. Eng. 2014, 34, 583–589. [Google Scholar] [CrossRef]
- Miyaji, H.; Satoh, K.; Kamigaito, M. Bio-Based Polyketones by Selective Ring-Opening Radical Polymerization of a-Pinene-Derived Pinocarvone. Angew. Chem. Int. Ed. 2016, 55, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kamal, M. Synthesis and Characterization of Polylimonene: Polymer of an Optically Active Terpene. J. Appl. Polym. Sci. 2012, 125, 1456–1459. [Google Scholar] [CrossRef]
- Sharma, S.; Srivastava, A. Radical co-polymerization of limonene with N-vinyl pyrrolidone: Synthesis and characterization. Des. Monomer Polym. 2006, 9, 503–516. [Google Scholar] [CrossRef]
- Martín, C.; Kleij, A.W. Terpolymers Derived from Limonene Oxide and Carbon Dioxide: Access to Cross-Linked Polycarbonates with Improved Thermal Properties. Macromolecules 2016, 49, 6285–6295. [Google Scholar] [CrossRef]
- Byrne, C.; Allen, S.; Lobkovsky, E.; Coates, W.G. Alternating Copolymerization of Limonene Oxide and Carbon Dioxide. J. Am. Chem. Soc. 2004, 126, 11404–11405. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, F.; Rosa, C.D.; Caprio, M.R.D.; Girolamo, R.D.; Ellis, W.C.; Coates, G.W. Stereocomplexed Poly(Limonene Carbonate): A Unique Example of the Cocrystallization of Amorphous Enantiomeric Polymers. Angew. Chem. Int. Ed. 2015, 54, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, F.; Rosa, C.D.; Caprio, M.R.D.; Girolamo, R.D.; Coates, G.W. Crystallization of Alternating Limonene Oxide/Carbon Dioxide Copolymers: Determination of the Crystal Structure of Stereocomplex Poly(limonene carbonate). Macromolecules 2015, 48, 2534–2550. [Google Scholar] [CrossRef]
- Kobayashi, S.; Cheng, L.; Hoye, T.; Hillmyer, M.A. Controlled Polymerization of a Cyclic Diene Prepared from the Ring-Closing Metathesis of a Naturally Occurring Monoterpene. J. Am. Chem. Soc. 2009, 131, 7960–7961. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Bhowmick, A.K. Green Approach toward Sustainable Polymer: Synthesis and Characterization of Poly(myrcene-co-dibutyl itaconate). ACS Sustain. Chem. Eng. 2016, 4, 2129–2141. [Google Scholar] [CrossRef]
- Kaneko, T.; Matsusaki, M.; Hang, T.T.; Akashi, M. Thermotropic Liquid-Crystalline Polymer Derived from Natural Cinnamoyl Biomonomers. Macromol. Rapid. Commun. 2004, 25, 673–677. [Google Scholar] [CrossRef]
- Kaneko, T.; Thi, T.H.; Dong, J.-S.; Akashi, M. Environmentally degradable, high-performance thermoplastics from phenolic phytomonomers. Nat. Mater. 2006, 5, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Tateyama, S.; Masuo, S.; Suvannasara, P.; Oka, Y.; Miyazato, A.; Yasaki, K.; Teerawatananond, T.; Muangsin, N.; Zhou, S.; Kawasaki, Y.; et al. Ultrastrong, Transparent Polytruxillamides Derived from Microbial Photodimers. Macromolecules 2016, 49, 3336–3342. [Google Scholar] [CrossRef]
- Puanglek, S.; Kimura, S.; Enomoto-Rogers, Y.; Kabe, T.; Yoshida, M.; Wada, M.; Iwata, T. In vitro synthesis of linear α-1,3-glucan and chemical modification to ester derivatives exhibiting outstanding thermal properties. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kanetaka, Y.; Yamazaki, S.; Kimura, K. Preparation of Poly(ether ketone)s Derived from 2,5-Furandicarboxylic Acid via Nucleophilic Aromatic Substitution Polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3094–3101. [Google Scholar] [CrossRef]











| Petroleum-derived polymers | Biobased polymers | ||
|---|---|---|---|
| Industry | Industrial approach | Scientific approach | |
| Super-engineering applications | since 1960 | not yet | since 2010 |
| PEEK, PSU, PES, PPS, PEI, PAI, LCP | bio-LCP, bio-PEEK (new generation) | ||
| Engineering/semi-engineering applications | since 1950 | since 2010 | since 2000 |
| Polyamide, POM, PC, PPO, PET, PTT, PBT, ultra-high MW PE, HIPS | bio-PET, bio-PTT, bio-PBT, bio-polyamide (analogous to petroleum-derived ones) | polyterpenes, PEF, bio-polyamide, sc-PLA (high Tm), sb-PLA (high Tm) (new generation) | |
| General applications | since 1930 | since 2000 | since 1990 |
| PE, PP, PS, PMMA, PVC, ABS | PLLA (high-l content) reinforced PHAs, PHAs blends, succinate polymers, bio-PE/PP | sc-PLA (low Tm), PHAs (super high MW), succinate polymers (upgrading from biodegradable polymers) | |
| Biodegradable/biocompatible applications | since 1970 | since 1990 | since 1970 |
| PCL, PEG | PLLA (low-l content) PBS, PHAs, PGA, polysaccharides | PLA, PHAs, succinate polymers | |
| l-Purity (%) | Mw (×105) | Approximate value of growth rate of spherulite (μm/min) 1 | ts (min) 2 | t1/2 (min) 3 | te (min) 4 | Crystallinity (%) 5 |
|---|---|---|---|---|---|---|
| 99.75 | 1.39 | 5.2 | 0.97 | 3.02 | 8.12 | 37.8 |
| 98.82 | 1.55 | 4.2 | 2.47 | 8.04 | 16.48 | 31.9 |
| 97.79 | 1.42 | 2.4 | 5.19 | 14.2 | 28.69 | 23.7 |
| Amorphous (cm−1) | α’-Form (cm−1) | α-Form (cm−1) | |
|---|---|---|---|
| νas (CH3) | 2995 | 2997 | 2997 |
| 3006 | |||
| νs (CH3) | 2945 | 2946 | 2946 |
| 2964 | |||
| ν (C=O) | 1757 | 1761 | 1759 |
| 1749 | |||
| δas (CH3) | 1454 | 1457 | 1457 |
| 1444 | |||
| δs (CH3) | 1387 | 1386 | 1386 |
| 1382 |
| Ingeo type | Application | MFR (g/10 min, 210 °C/2.16 kg) | Tm (°C) | Tg (°C) |
|---|---|---|---|---|
| 2003D | extrusion, injection | 6 | 145–160 | 55–60 |
| 3001D | 22 | 155–170 | 55–60 | |
| 3251D | 80 | 155–170 | 55–60 | |
| 3801X | 155–170 | 45 | ||
| 4032D | film, sheet | 7 | 155–170 | 55–60 |
| 4060D | 10 | - | 55–60 | |
| 6060D | fiber, non-woven | 8 | 122–135 | 55–60 |
| 6252D | 80 | 155–170 | 55–60 | |
| 6752D | 14 | 145–160 | 55–60 |
| P3HB | P(3HB-co-20% 3HV) | P(3HB-co-12% 3HH) | Poly(4-hydroxybutyrate) (P4HB) | P(3HB-co-16% 4HB) | |
|---|---|---|---|---|---|
| Tm (°C) | 177 | 145 | 61 | 60 | 152 |
| Tg (°C) | 4 | −1 | −35 | −50 | −8 |
| Tensile (MPa) | 40 | 32 | 9 | 104 | 26 |
| Elongation at break (%) | 6 | 50 | 380 | 1000 | 444 |
| Source | Chemical structure | Examples of commercial suppliers | Tm (°C) | Modulus (GPa) |
|---|---|---|---|---|
| Biobased | Polyamide 4 | N.A. | 265 | |
| Polyamide 4.6 | DSM | 295 | ||
| Polyamide 4.10 | DSM | 250 | 1.3 | |
| Polyamide 6.10 | Evonik | 206 | 2.1 | |
| Polyamide 10.10 | Arkema, Evonik | 191 | 1.8 | |
| Polyamide 11 | Arkema | 185 | 1.0 | |
| Polyamide 12 | Evonik | 178 | 1.6 | |
| Petroleum derived | Polyamide 6 | Chemical companies | 218 | 3.0 |
| Polyamide 6.6 | Chemical companies | 258 | 2.5 |
| PEF | PET | |
|---|---|---|
| Density (g/cm3) | 1.43 | 1.36 |
| O2 permeability | 0.0107 | 0.114 |
| CO2 permeability | 0.026 | 0.46 |
| Tg (°C) | 88 | 76 |
| Tm (°C) | 210–230 | 250–270 |
| E-modulus (GPa) | 3.1–3.3 | 2.1–2.2 |
| Yield stress (MPa) | 90–100 | 50–60 |
| Quiescent crystallization time (min) | 20–30 | 2–3 |
| Modified lactide | Tg of Polymers (°C) |
|---|---|
| Glycolide | 40 |
| methyl glycolide(lactide) | 66 |
| ethylglycolide | 15 |
| hexyl glycolide | −37 |
| isobutyl glycolide | 22 |
| cyclohexyl glycolide (meso) | 96 |
| cyclohexyl glycolide (iso) | 104 |
| meso-mandelide | 100 |
| Norbornene | 192 |
| Polymerization | Tg (°C) | |
|---|---|---|
| α-pinene | free radical | 162 |
| β-pinene | cationic | 132 |
| β-pinene | cationic | 90 |
| cationic | 130 | |
| limonene oxide | trans-carbonation | 95 |
| trans-carbonation | 114 | |
| limonene oxide/phthalic anhydride | ROP, ester | 82 |
| Myrcene/styrene | emulsion | −61 |
| myrcene(3-methylenecyclopentene) | cationic polymerization | 11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. https://doi.org/10.3390/polym9100523
Nakajima H, Dijkstra P, Loos K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers. 2017; 9(10):523. https://doi.org/10.3390/polym9100523
Chicago/Turabian StyleNakajima, Hajime, Peter Dijkstra, and Katja Loos. 2017. "The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed" Polymers 9, no. 10: 523. https://doi.org/10.3390/polym9100523