Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
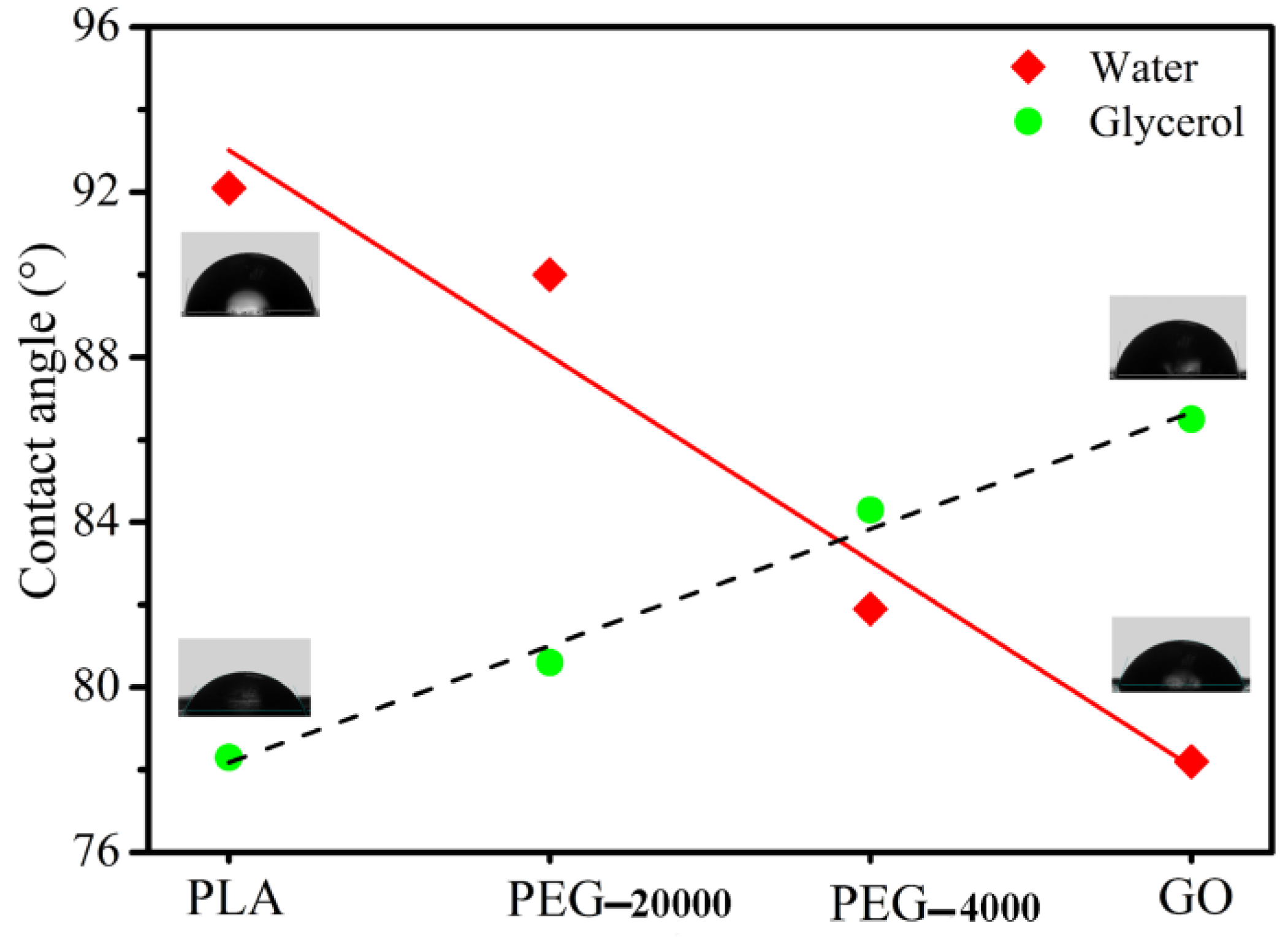
3.1. Contact Angle
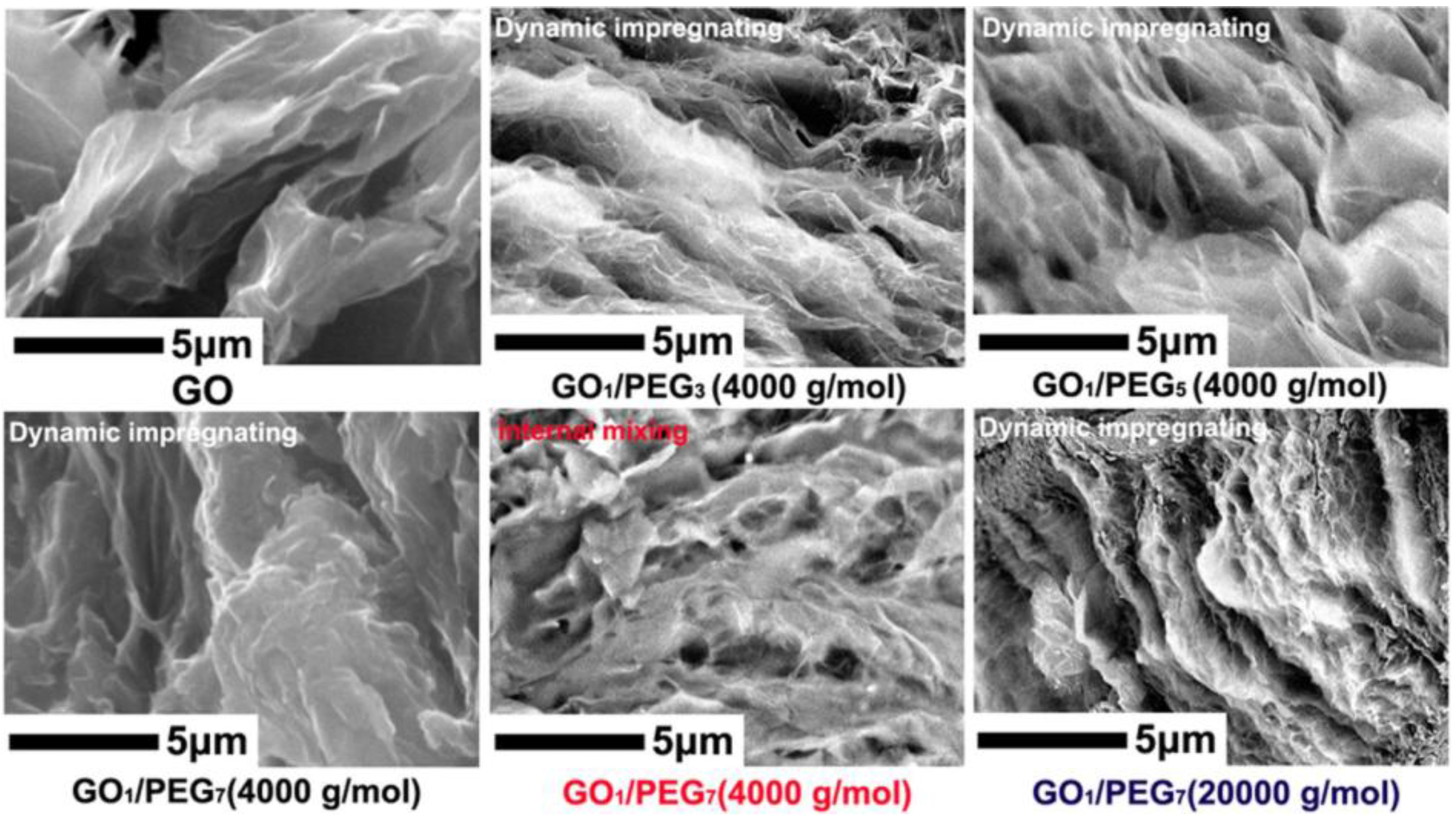
3.2. GO/PEG Morphology Analysis via SEM
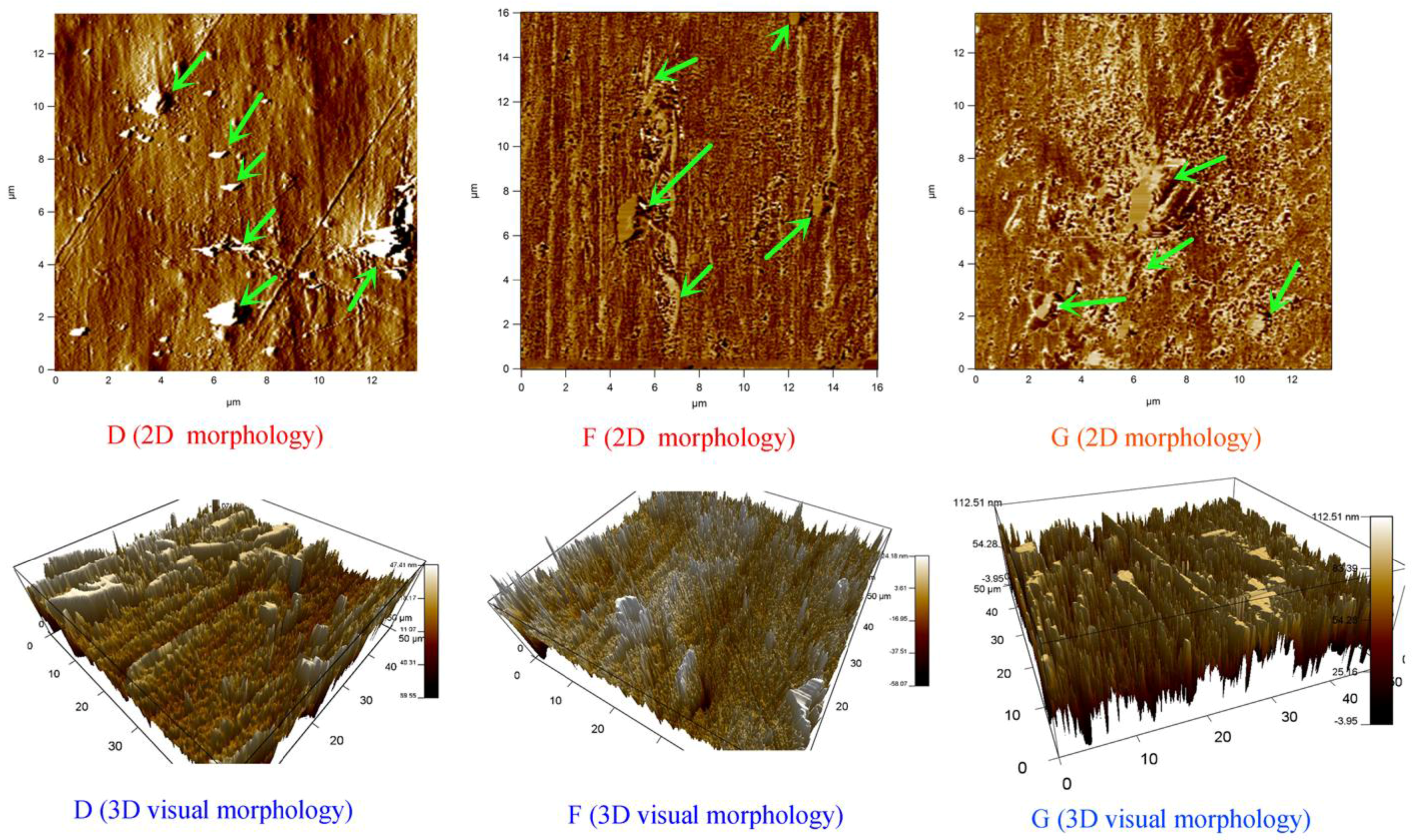
3.3. Composites Morphology Analysis via AFM
3.4. Composites Morphology Analysis via TEM
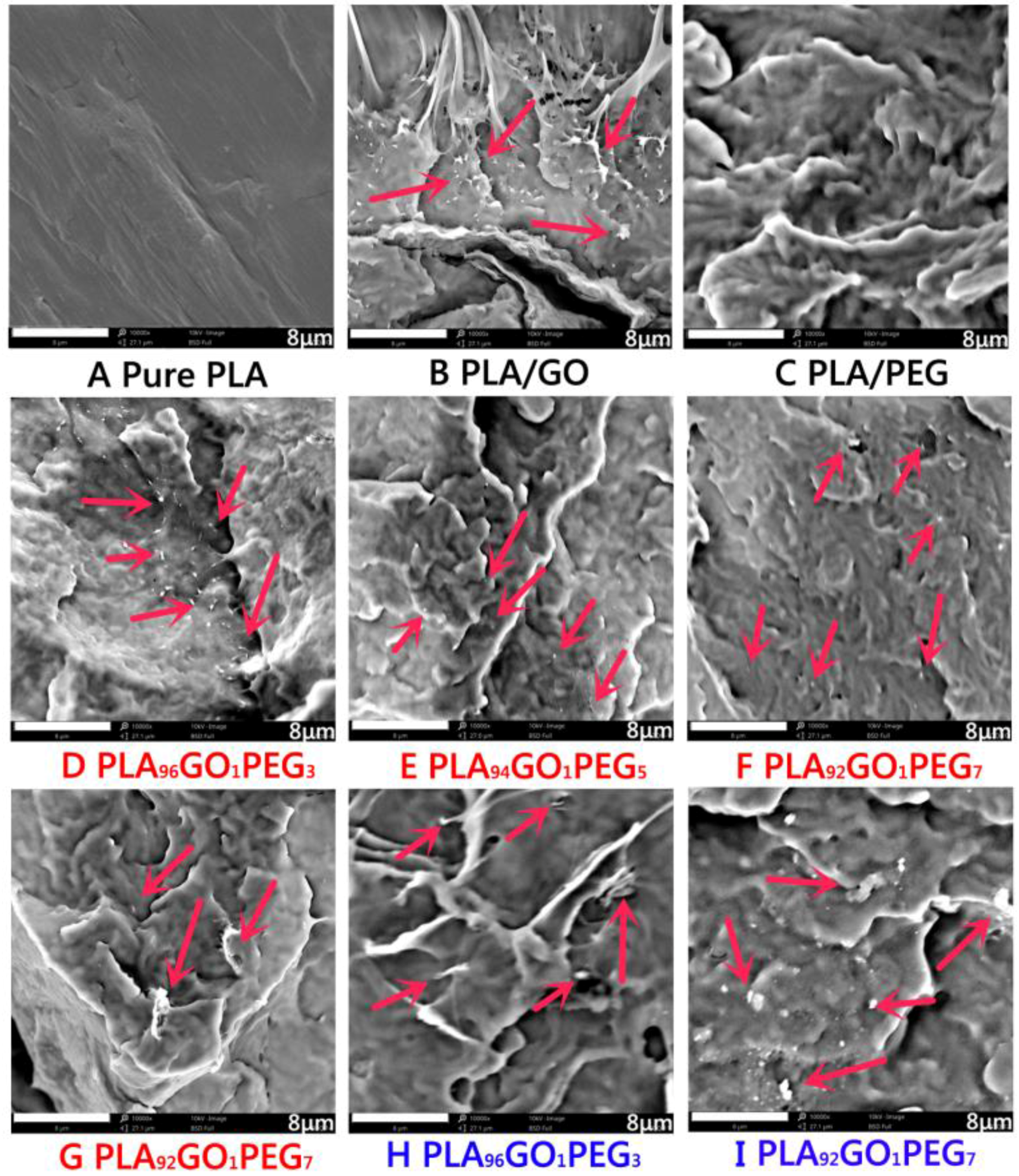
3.5. Composites Morphology Analysis via SEM
3.6. Composites Crystallization Behavior via WAXD
3.7. Composites Crystallization Behavior via DSC
3.8. Composites Crystallization Behavior via POM
3.9. Composites Thermal Behavior via DMA
3.10. Composites Thermal Behavior via HDT
3.11. Physical Model for Morphology and Crystallization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, L.; Qiu, X.; Xie, Z.; Hong, Z.; Sun, J.; Chen, X.; Jing, X. Poly(l-lactide)/starch blends compatibilized with poly(l-lactide)-g-starch copolymer. Carbohydr. Polym. 2006, 65, 75–80. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Effect of compounding approaches on fiber dispersion and performance of poly(lactic acid)/cellulose nanocrystal composite blown films. J. Appl. Polym. Sci. 2017, 134, 45212–45222. [Google Scholar] [CrossRef]
- Feng, C.S.; Piao, M.H.; Li, D. Stereocomplex-Reinforced PEGylated Polylactide Micelle for Optimized Drug Delivery. Polymers 2016, 8, 165. [Google Scholar] [CrossRef]
- Sarazin, P.; Li, G.; Orts, W.J.; Favis, B.D. Binary and ternary blends of polylactide, polycaprolactone and thermoplastic starch. Polymer 2008, 49, 599–609. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Savaris, M.; Santos, V.D.; Brandalise, R.N. Influence of different sterilization processes on the properties of commercial poly(lactic acid). Mater. Sci. Eng. C 2016, 69, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Fukui, I. Enhanced thermal stability of poly(lactide)s in the melt by enantiomeric polymer blending. Polymer 2003, 44, 2891–2896. [Google Scholar] [CrossRef]
- Jing, Z.X.; Shi, X.T.; Zhang, G.C. Competitive Stereocomplexation and Homocrystallization Behaviors in the Poly(lactide) Blends of PLLA and PDLA-PEG-PDLA with Controlled Block Length. Polymers 2017, 9, 107. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Wu, L.; Xu, B.; Zhang, Y.; Zhang, M. Comparison between isothermal cold and melt crystallization of polylactide/clay nanocomposites. J. Nanosci. Nanotechnol. 2008, 8, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Garskaite, E.; Alinauskas, L.; Drienovsky, M.; Krajcovic, J.; Cicka, R.; Palcut, M.; Jonusauskas, L.; Malinauskas, M.; Stankeviciute, Z.; Kareiva, A. Fabrication of a composite of nanocrystalline carbonated hydroxyapatite (cHAP) with polylactic acid (PLA) and its surface topographical structuring with direct laser writing (DLW). RSC Adv. 2016, 76, 72733–72743. [Google Scholar] [CrossRef]
- Zafar, M.T.; Maiti, S.N.; Ghosh, A.K. Effect of surface treatments of jute fibers on the microstructural and mechanical responses of poly(lactic acid)/jute fiber biocomposites. RSC Adv. 2016, 6, 73373–73382. [Google Scholar] [CrossRef]
- Shen, Y.; Jing, T.; Ren, W.; Zhang, J.; Jiang, Z.G.; Yu, Z.Z.; Dasari, A. Polylactide (PLA)-clay nanocomposites prepared by melt compounding in the presence of a chain extender. Compos. Sci. Technol. 2012, 72, 1430–1435. [Google Scholar] [CrossRef]
- Xu, H.; Wu, D.; Yang, X.; Xie, L.; Hakkarainen, M. Thermostable and Impermeable “Nano-Barrier Walls” Constructed by Poly(lactic acid) Stereocomplex Crystal Decorated Graphene Oxide Nanosheets. Macromolecules 2015, 48, 2127–2137. [Google Scholar] [CrossRef]
- Rostami, A.; Nazockdast, H.; Karimi, M. Graphene induced microstructural changes of PLA/MWCNT biodegradable nanocomposites: rheological, morphological, thermal and electrical properties. RSC Adv. 2016, 6, 49747–49759. [Google Scholar] [CrossRef]
- Tsuji, H.; Kawashima, Y.; Takikawa, H.; Tanaka, S. Poly(l-lactide)/nano-structured carbon composites: Conductivity, thermal properties, crystallization, and biodegradation. Polymer 2007, 48, 4213–4225. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.W.; Yun, H.; Kim, B.J. Impact of size control of graphene oxide nanosheets for enhancing electrical and mechanical properties of carbon nanotube–polymer composites. RSC Adv. 2017, 7, 30221–30228. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly(vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Woltornist, S.J.; Carrillo, J.M.Y.; Xu, T.O.; Dobrynin, A.V.; Adamson, D.H. Polymer/Pristine Graphene Based Composites: From Emulsions to Strong, Electrically Conducting Foams. Macromolecules 2015, 48, 687–693. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhang, Z.J.; Xu, H.; Chen, J.B.; Ran, R.; Li, Z.M. Highly Enhanced Crystallization Kinetics of Poly(l-lactic acid) by Poly(ethylene glycol) Grafted Graphene Oxide Simultaneously as Heterogeneous Nucleation Agent and Chain Mobility Promoter. Macromolecules 2015, 4, 4891–4900. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhai, T.; Wang, X.; Dan, Y.; Turng, L.S. The surface grafting of graphene oxide with poly(ethylene glycol) as a reinforcement for poly(lactic acid) nanocomposite scaffolds for potential tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2016, 53, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, C. Synthesis and Stereocomplex Crystallization of Poly(lactide)–Graphene Oxide Nanocomposites. ACS Macro Lett. 2012, 1, 709–713. [Google Scholar] [CrossRef]
- Tong, J.; Huang, H.X.; Wu, M. Facile green fabrication of well dispersed poly(vinylidene fluoride)/graphene oxide nanocomposites with improved properties. Compos. Sci. Technol. 2016, 129, 183–190. [Google Scholar] [CrossRef]
- Ji, X.; Cui, L.; Xu, Y.; Liu, J. Non-covalent interactions for synthesis of new graphene based composites. Compos. Sci. Technol. 2015, 106, 25–31. [Google Scholar] [CrossRef]
- Lai, W.C.; Liau, W.B.; Lin, T.T. The effect of end groups of PEG on the crystallization behaviors of binary crystalline polymer blends PEG/PLLA. Polymer 2004, 45, 3073–3080. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Jiang, X.; Li, X.J.; Li, Y.; Zhang, Z.J.; Zhong, G.J.; Li, Z.M. Toward stronger transcrystalline layers in poly(l-lactic acid)/natural fiber biocomposites with the aid of an accelerator of chain mobility. J. Phys. Chem. B 2014, 118, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Shen, Y.; He, W.D.; Zhang, N.; Huang, T.; Zhang, J.H.; Wang, Y.J. Synergistic effect of poly(ethylene glycol) and graphene oxides on the crystallization behavior of poly(l-lactide). J. Appl. Polym. Sci. 2013, 130, 3498–3508. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.T.; Yang, L.; Zhang, N.; Jin, G.; Qu, J.P. In-situ thermal reduction and effective reinforcement of graphene nanosheet/poly (ethylene glycol)/poly (lactic acid) nanocomposites. Polym. Adv. Technol. 2014, 25, 1515–1522. [Google Scholar] [CrossRef]
- Liu, C.; Ye, S.; Feng, J. Promoting the dispersion of graphene and crystallization of poly (lactic acid) with a freezing-dried graphene/PEG masterbatch. Compos. Sci. Technol. 2017, 144, 215–222. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.X.; Yan, T.; Dai, Y.J.; Wang, R.Z. High performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage. Int. J. Heat Mass Transf. 2016, 102, 733–744. [Google Scholar] [CrossRef]
- Khosrojerdi, M.; Mortazavi, S.M. Impregnation of a porous material with a PCM on a cotton fabric and the effect of vacuum on thermo-regulating textiles. J. Therm. Anal. Calorim. 2013, 114, 1111–1119. [Google Scholar] [CrossRef]
- Jia, S.K.; Zhu, Y.; Wang, Z.; Chen, L.G.; Fu, L. Improvement of shape stability and thermal properties of PCM using polyethylene glycol (PEG)/sisal fiber cellulose (SFC)/graphene oxide (GO). Fiber Polym. 2017, 18, 1171–1179. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.Y.; Jia, S.K.; Zhu, Y.; Chen, L.G.; Fu, L. Influences of dynamic impregnating on morphologies and thermal properties of polyethylene glycol-based composite as shape-stabilized PCMs. J. Therm. Anal. Calorim. 2017, 128, 1039–1048. [Google Scholar] [CrossRef]
- Xiu, H.; Bai, H.W.; Huang, C.M.; Xu, C.L.; Li, X.Y.; Fu, Q. Selective localization of titanium dioxide nanoparticles at the interface and its effect on the impact toughness of poly(l-lactide)/poly(ether)urethane blends. eXPRESS Polym. Lett. 2013, 7, 261–271. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Y.; Lin, D.; Zhou, W.; Zhang, M.; Yuan, L. Selective Localization of Nanofillers: Effect on Morphology and Crystallization of PLA/PCL Blends. Macromol. Chem. Phys. 2011, 212, 1700–1709. [Google Scholar] [CrossRef]
- Schwach, E.; Avérous, L. Starch-based biodegradable blends: Morphology and interface properties. Polym. Int. 2004, 53, 2115–2124. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Preparation and Characterization of Poly l-Lactide/Triclosan Nanoparticles for Specific Antibacterial and Medical Applications. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 497–508. [Google Scholar] [CrossRef]
- Jia, S.K.; Zhu, Y.; Wang, Z.; Chen, L.G.; Fu, L. Influences of PP-g-MA on the surface free energy, morphologies and mechanical properties of thermoplastic polyurethane/polypropylene blends. J. Polym. Res. 2015, 22, 1–10. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]

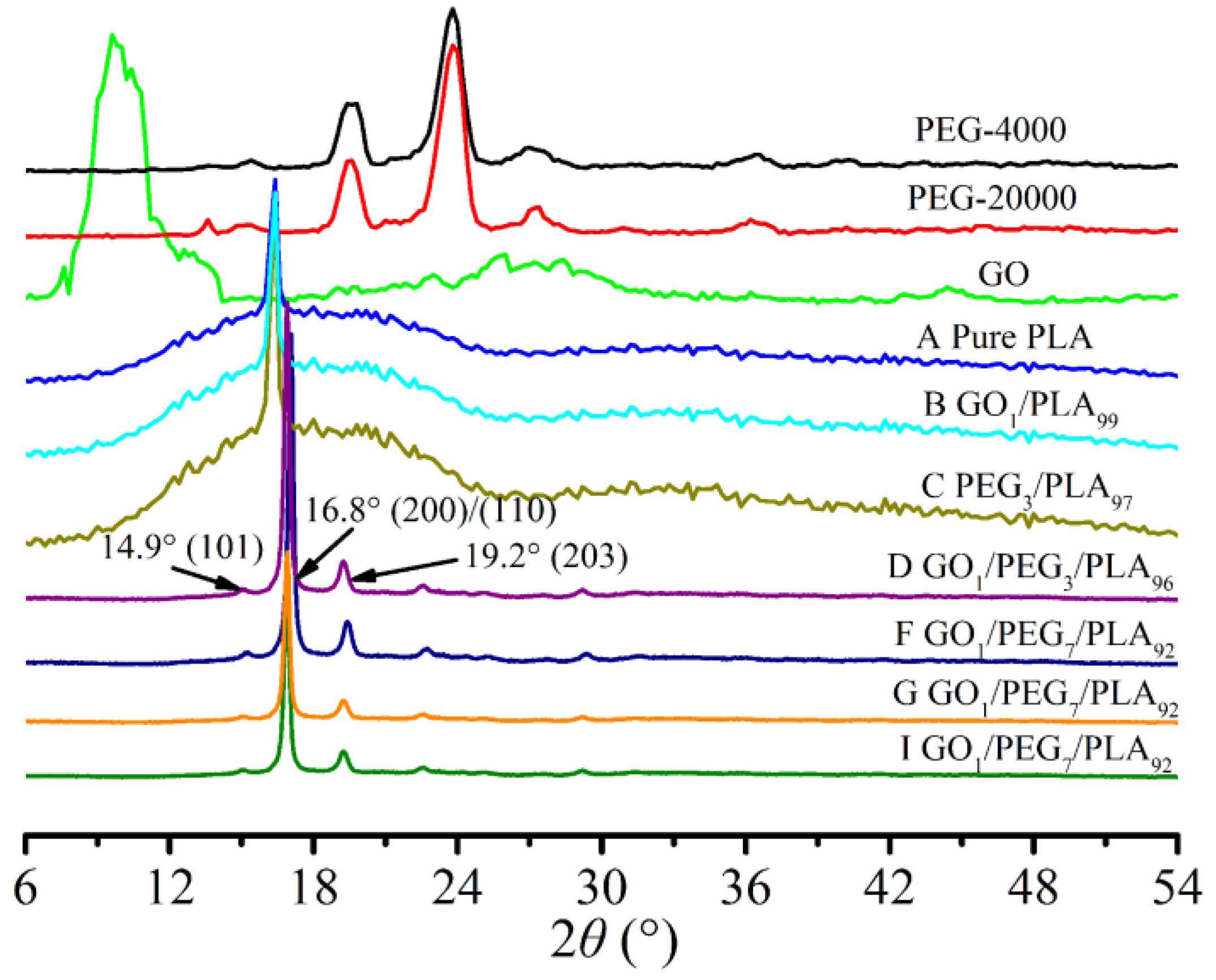


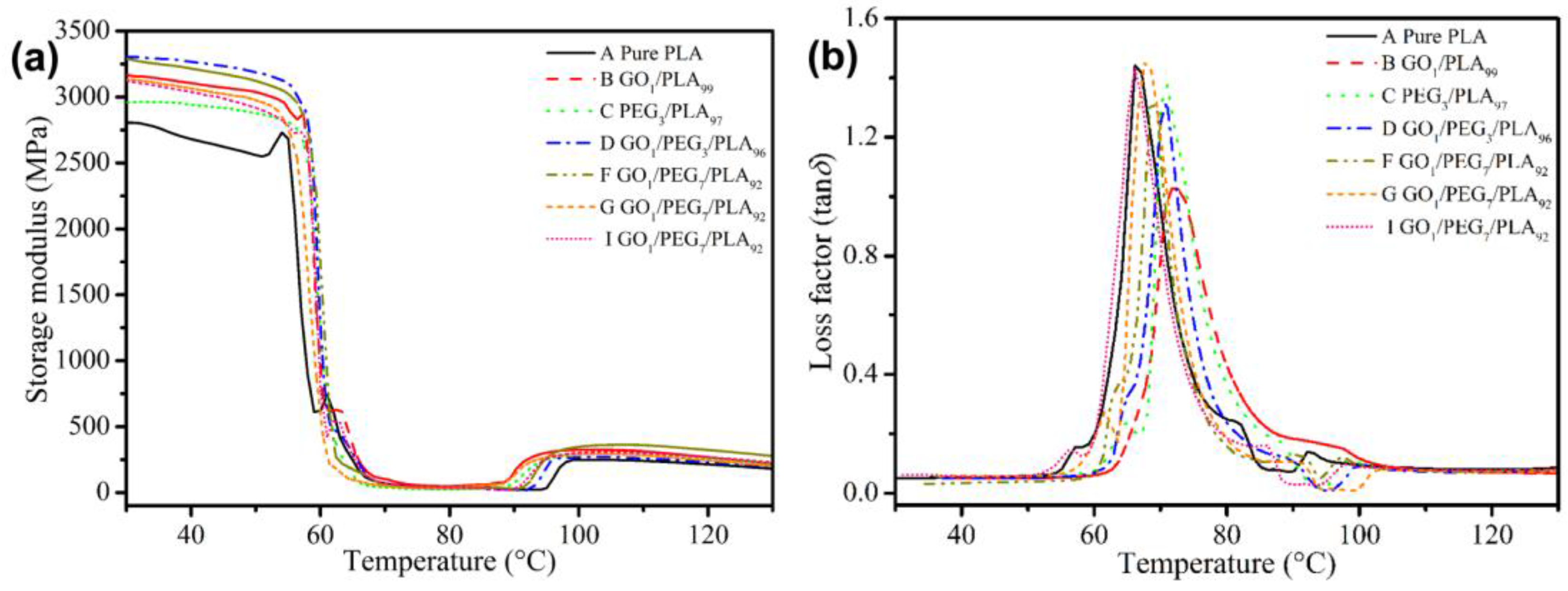
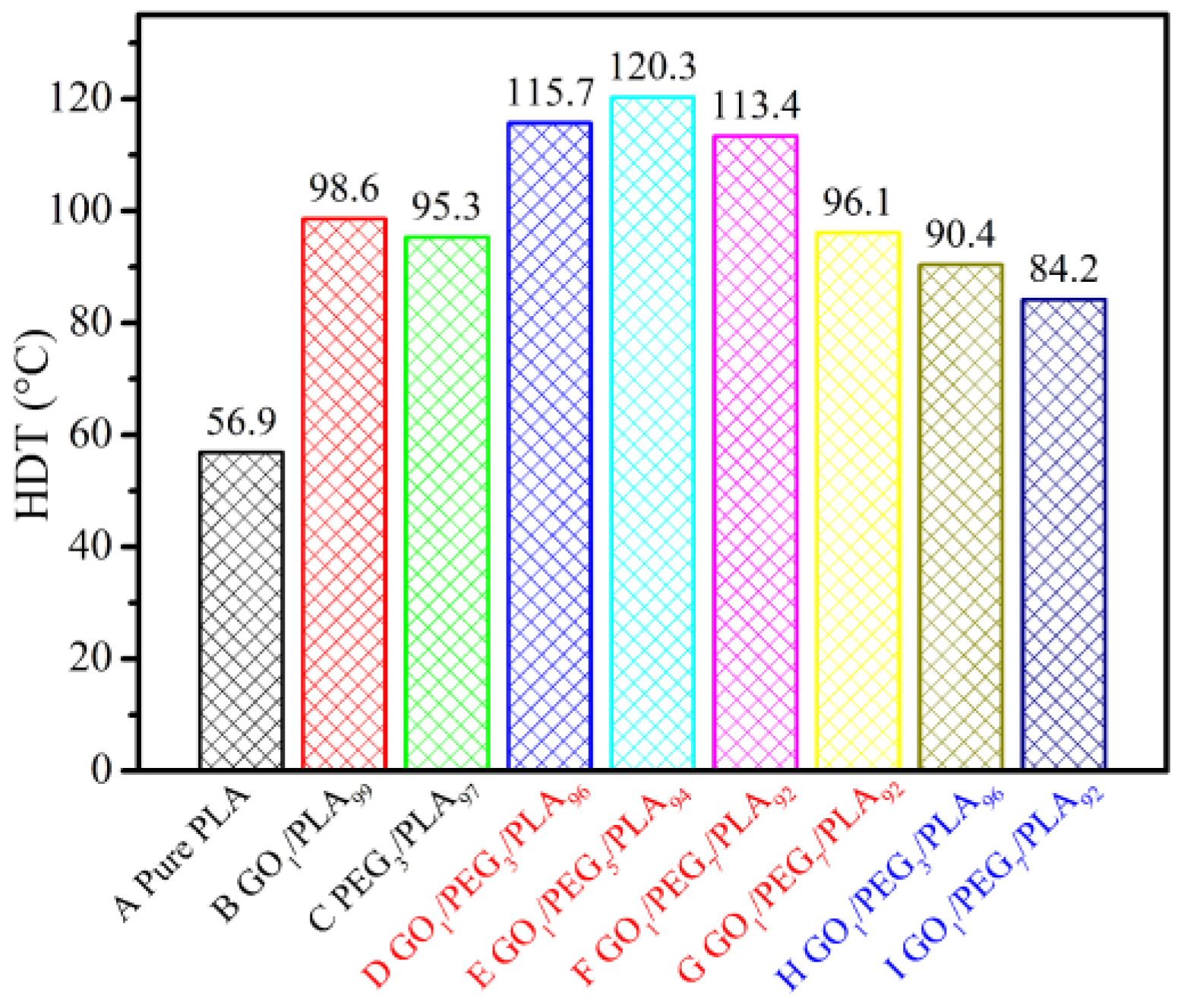
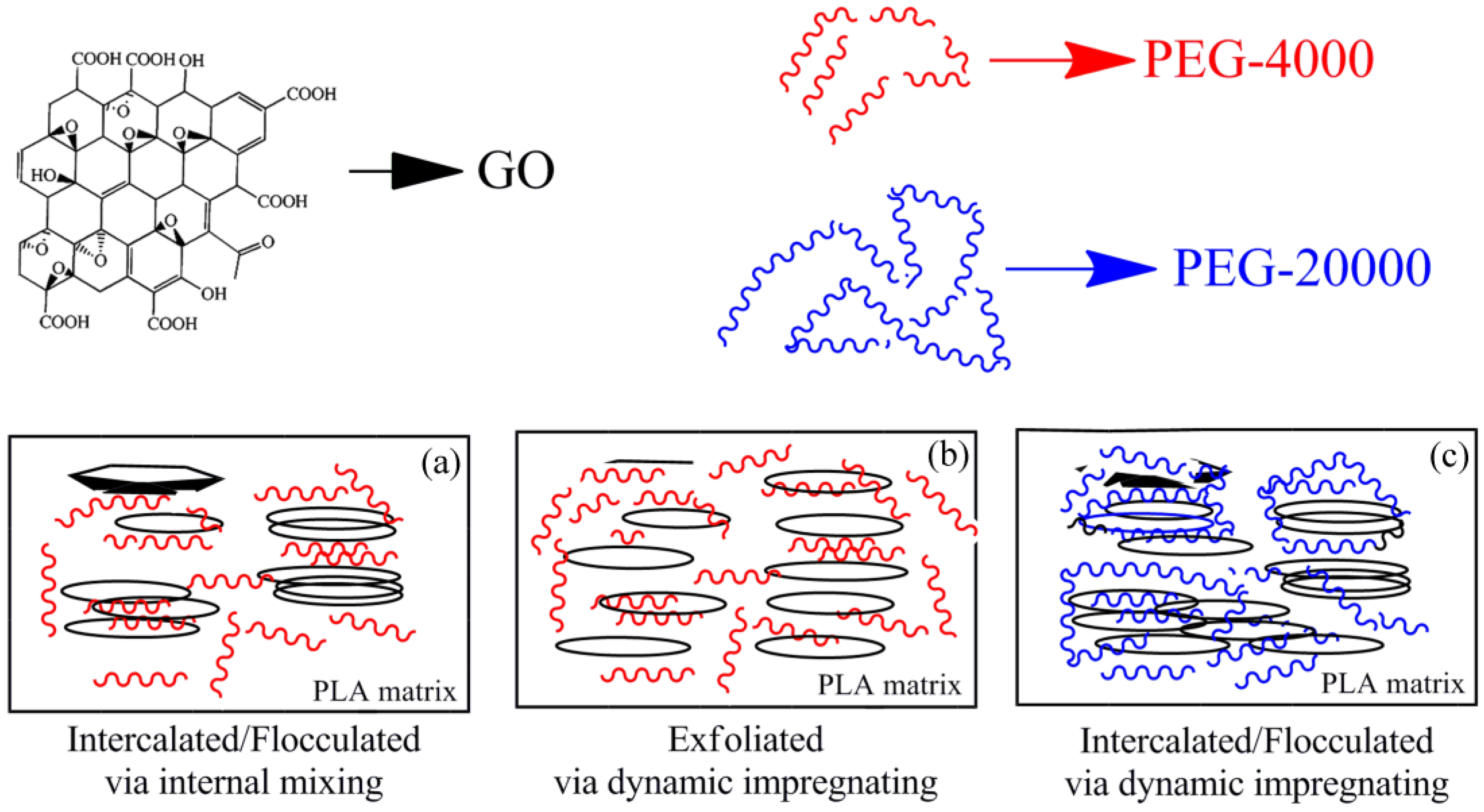
| Sample | GO (wt %) | PEG (wt %) | PLA (wt %) | Mw of PEG (g/mol) | Premixing Method for Hybrid GO/PEG |
|---|---|---|---|---|---|
| A | --- | --- | 100 | --- | --- |
| B | 1.0 | --- | 99 | --- | --- |
| C | --- | 3.0 | 97 | 4000 | --- |
| D | 1.0 | 3.0 | 96 | 4000 | Dynamic impregnating |
| E | 1.0 | 5.0 | 94 | 4000 | Dynamic impregnating |
| F | 1.0 | 7.0 | 92 | 4000 | Dynamic impregnating |
| G | 1.0 | 7.0 | 92 | 4000 | Internal mixing |
| H | 1.0 | 3.0 | 96 | 20,000 | Dynamic impregnating |
| I | 1.0 | 7.0 | 92 | 20,000 | Dynamic impregnating |
| Sample | Contact Angle (°) | Surface Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Water | Glycerol | ||||
| Water | --- | --- | 72.8 | 21.8 | 51.0 |
| Glycerol | --- | --- | 64.0 | 34.0 | 30.0 |
| PLA | 92.1 | 77.3 | 27.8 | 26.1 | 2.8 |
| PEG-20000 | 90 | 80.6 | 23.3 | 18.1 | 5.2 |
| PEG-4000 | 81.9 | 84.3 | 28.0 | 2.2 | 25.8 |
| GO | 78.2 | 86.5 | 34.6 | 0.4 | 34.2 |
| Component Couple | (mJ·m−2) | (mJ·m−2) | (mJ·m−2) |
|---|---|---|---|
| PLA/GO | 50.5 | 36.4 | 25.0 |
| PLA/PEG-4000 | 37.6 | 23.6 | 32.2 |
| PLA/PEG-20000 | 34.7 | 26.2 | 27.9 |
| GO/PEG-4000 | 2.4 | 1.3 | 61.3 |
| GO/PEG-20000 | 38.3 | 57.9 | 32.1 |
| Samples | (°C) | (°C) | (°C) | (°C) | (J/g) | (J/g) | (J/g) | (%) |
|---|---|---|---|---|---|---|---|---|
| PEG-4000 | 58.2 | 61.9 | --- | 39.1 | 154.1 | --- | 149.6 | 72.3 |
| PEG-20,000 | --- | 68.3 | --- | 44.8 | 157.3 | --- | 153.5 | 73.9 |
| A | 161.3 | 168.5 | 110.1 | --- | 33.7 | 28.5 | 9.78 | 5.5 |
| B | 164.7 | 171.6 | --- | 99.6 | 29.8 | --- | 26.3 | 31.8 |
| C | --- | 169.4 | 100.3 | 103.2 | 34.1 | 15.9 | 14.6 | 19.4 |
| D | 163.8 | 170.1 | --- | 102.7 | 30.5 | --- | 15.3 | 32.6 |
| F | 164.2 | 170.2 | --- | 102.9 | 36.8 | --- | 24.3 | 39.3 |
| G | 163.9 | 172.7 | --- | 104.4 | 28.7 | --- | 13.5 | 30.6 |
| I | 163.1 | 170.3 | --- | 108.3 | 25.4 | --- | 11.8 | 27.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers 2017, 9, 528. https://doi.org/10.3390/polym9100528
Jia S, Yu D, Zhu Y, Wang Z, Chen L, Fu L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers. 2017; 9(10):528. https://doi.org/10.3390/polym9100528
Chicago/Turabian StyleJia, Shikui, Demei Yu, Yan Zhu, Zhong Wang, Ligui Chen, and Lei Fu. 2017. "Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating" Polymers 9, no. 10: 528. https://doi.org/10.3390/polym9100528




