Surface and Protein Adsorption Properties of 316L Stainless Steel Modified with Polycaprolactone Film
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- (1)
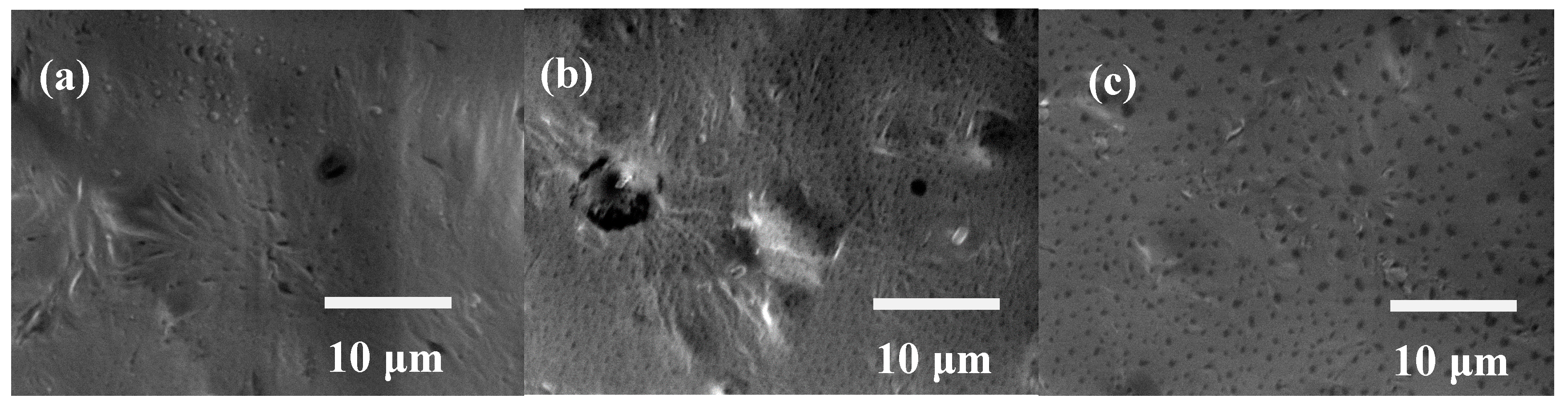
- Water contact angle measurement results showed that the wettability of the PCL films was comparable to that of bare 316L SS because the rough surface morphology of PCL counteracted the hydrophobicity.
- (2)
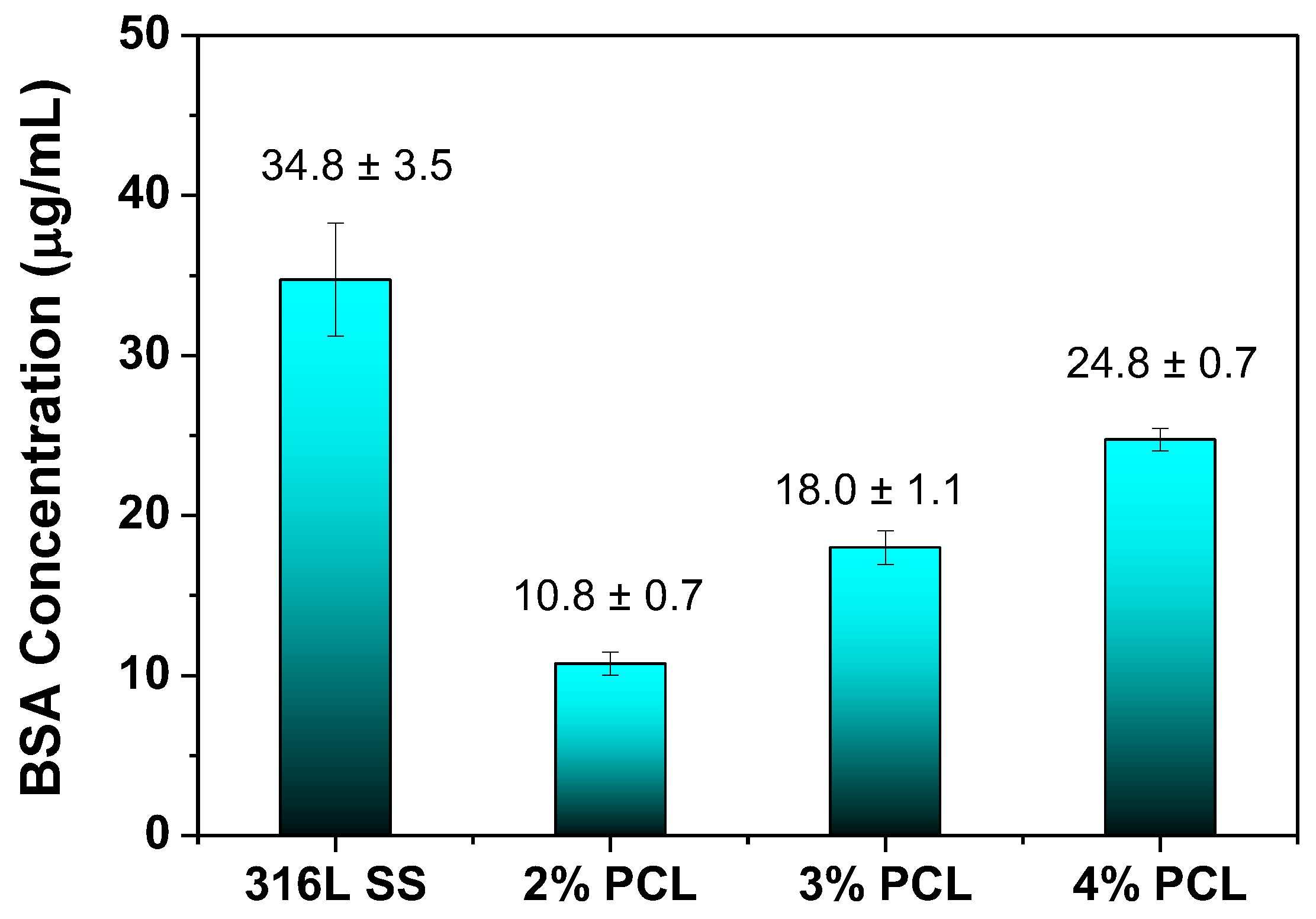
- BCA protein assay results showed that the 316L SS modified by PCL films all possessed lower adhered BSA concentrations than that of bare 316L SS, suggesting that the anticoagulant properties of the 316L SS surface could be improved by modification with PCL films. The BSA protein adsorption of the PCL films is slightly increased from the 2% to 4% PCL films because the higher-concentration PCL films exhibit rougher surfaces and more hydrogen bonding interactions between the carbonyl functional groups of the PCL films and the carboxylic acid of the BSA.
- (3)
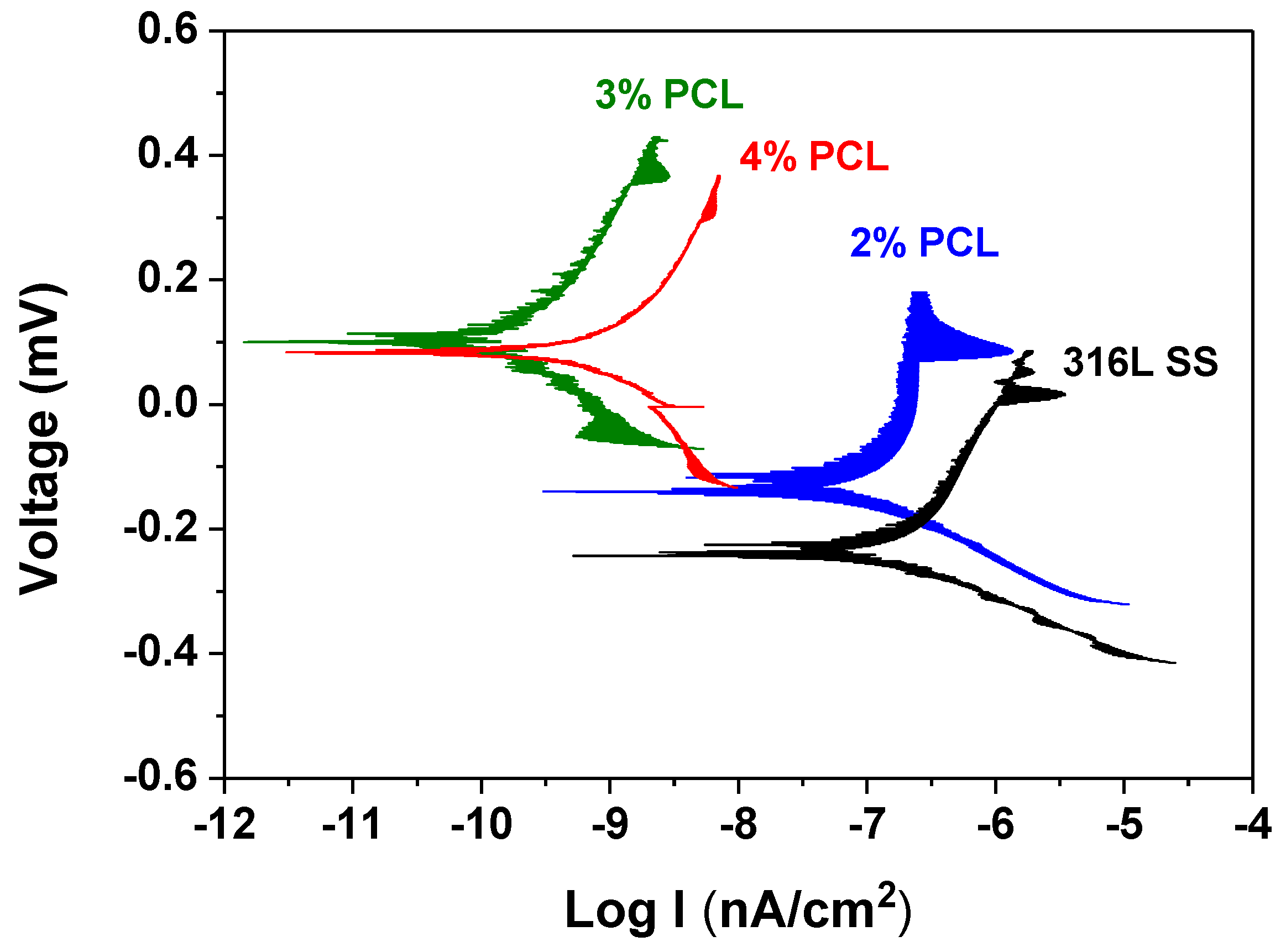
- Electrochemical tests revealed that the corrosion resistance of the 316L SS was effectively enhanced after coating with PCL films, which is favorable to prevent the undesirable metal ions from leaching from stainless steel.
- (4)
- PCL films possessed excellent corrosion resistance and good anticoagulant properties and showed promise for use in biomedical applications, particularly for the surface modification of implants.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Nickchi, T.; Alfantazi, A.M. Long-term corrosion investigation of AISI 316L, Co-28Cr-6Mo, and Ti-6Al-4V alloys in simulated body solutions. Appl. Surf. Sci. 2012, 258, 6087–6096. [Google Scholar] [CrossRef]
- Williams, R.L.; Brown, S.A.; Merritt, K. Electrochemical studies on the influence of proteins on the corrosion of implant alloys. Biomaterials 1988, 9, 181–186. [Google Scholar] [CrossRef]
- Tang, Y.C.; Katsuma, S.; Fujimoto, S.; Hiromoto, S. Electrochemical study of Type 304 and 316L SS in simulated body fluids and cell cultures. Acta Biomater. 2006, 2, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Gotoh, E. Metal release from SS, Co–Cr–Mo–Ni–Fe and Ni–Ti alloys in vascular implants. Corros. Sci. 2008, 50, 3429–3438. [Google Scholar] [CrossRef]
- Takahashi, H.; Kinbara, M.; Sato, N.; Sasaki, K.; Sugawara, S.; Endo, Y. Nickel allergy-promoting effects of microbial or inflammatory substances at the sensitization step in mice. Int. Immunopharmacol. 2011, 11, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- D’Antò, V.; Eckhardt, A.; Hiller, K.A.; Spagnuolo, G.; Valletta, R.; Ambrosio, L.; Schmalz, G.; Schweikl, H. The influence of Ni(II) on surface antigen expression in murine macrophages. Biomaterials 2009, 30, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; He, X.; Hang, R.; Huang, X.; Tang, B. Preparation, biocompatibility and wear resistance of microstructured Zr and ZrO2 alloyed layers on 316L SS. Mater. Lett. 2017, 203, 24–27. [Google Scholar] [CrossRef]
- Al-Rashidy, Z.M.; Farag, M.M.; Abdel Ghany, N.A.; Ibrahim, A.M.; Abdel-Fattah, W.I. Aqueous electrophoretic deposition and corrosion protection of borate glass coatings on 316L SS for hard tissue fixation. Surf. Interfaces 2017, 7, 125–133. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, Y.; Hu, R.; Lin, C.; Sun, L.; Vogler, E.A. Reduced platelet adhesion and improved corrosion resistance of superhydrophobic TiO2-nanotube-coated 316L SS. Colloids Surf. B Biointerfaces 2014, 125, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sharifnabi, A.; Fathi, M.H.; Eftekhari Yekta, B.; Hossainalipour, M. The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316L SS human body implant. Appl. Surf. Sci. 2014, 288, 331–340. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Ding, M.H.; Zheng, H.; Zhang, H.S.; Zhang, B.; Li, X.Q.; Wu, G.Y. Surface modification of biomedical AISI 316L SS with zirconium carbonitride coatings. Appl. Surf. Sci. 2015, 340, 113–119. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, J.Z.; Hsiao, S.H.; Lin, G.W. Nanohardness, corrosion and protein adsorption properties of CuAlO2 films deposited on 316L SS for biomedical applications. Appl. Surf. Sci. 2014, 289, 455–461. [Google Scholar] [CrossRef]
- Cheng, I.C.; Chang, S.H.; Lin, G.W.; Chi, C.T.; Hsiao, S.H.; Chen, J.Z. Effect of Al/Cu ratios on the optical, electrical, and electrochemical properties of Cu-Al-Ca-O thin films. J. Alloy. Compd. 2014, 609, 111–115. [Google Scholar] [CrossRef]
- Chang, S.H.; Liou, J.S.; Huang, B.Y.; Chan, W.J.; Tsao, Y.T. Surface characteristics of the 316L SS modified by ethylene vinyl acetate/chitosan composite films. Surf. Coat. Technol. 2017, 320, 635–639. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.; Darvishi, S.; Torkaman, R.; Kharaziha, M.; Karbasi, M. Corrosion and bioactivity evaluation of nanocomposite PCL-forsterite coating applied on 316L SS. Surf. Coat. Technol. 2016, 307, 324–331. [Google Scholar] [CrossRef]
- Rubio, C.; Costa, D.; Bellon-Fontaine, M.N.; Relkin, P.; Pradier, C.M.; Marcus, P. Characterization of bovine serum albumin adsorption on chromium and AISI 304 stainless steel, consequences for the Pseudomonas fragi K1 adhesion. Colloids Surf. B Biointerfaces 2002, 24, 193–205. [Google Scholar] [CrossRef]
- Sakiyama, T.; Tomura, J.; Imamura, K.; Nakanishi, K. Adsorption characteristics of bovine serum albumin and its peptide fragments on a stainless steel surface. Colloids Surf. B Biointerfaces 2004, 33, 77–84. [Google Scholar] [CrossRef]
- Gispert, M.P.; Serro, A.P.; Colaço, R.; Saramago, B. Bovine serum albumin adsorption onto 316L stainless steel and alumina: A comparative study using depletion, protein radiolabeling, quartz crystal microbalance and atomic force microscopy. Surf. Interface Anal. 2008, 40, 1529–1537. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Killian, M.S.; Blomberg, E.; Virtanen, S.; Schmuki, P.; Odnevall Wallinder, I. Interaction of bovine serum albumin and lysozyme with stainless steel studied by time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy. Langmuir 2012, 28, 16306–16317. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.; Wang, X.; Hedberg, J.; Lundin, M.; Blomberg, E.; Odnevall Wallinder, I. Surface-protein interactions on different stainless steel grades: Effects of protein adsorption, surface changes and metal release. J. Mater. Sci. Mater. Med. 2013, 24, 1015–1033. [Google Scholar] [CrossRef] [PubMed]


| Specimens | Ecorr (mV) | icorr (nA/cm2) |
|---|---|---|
| Bare 316L SS | −196.2 | 97.5 |
| 2% PCL | −129.6 | 84.7 |
| 3% PCL | 96.0 | 0.1 |
| 4% PCL | 84.9 | 0.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-H.; Hsiao, Y.-C. Surface and Protein Adsorption Properties of 316L Stainless Steel Modified with Polycaprolactone Film. Polymers 2017, 9, 545. https://doi.org/10.3390/polym9100545
Chang S-H, Hsiao Y-C. Surface and Protein Adsorption Properties of 316L Stainless Steel Modified with Polycaprolactone Film. Polymers. 2017; 9(10):545. https://doi.org/10.3390/polym9100545
Chicago/Turabian StyleChang, Shih-Hang, and Yuan-Chien Hsiao. 2017. "Surface and Protein Adsorption Properties of 316L Stainless Steel Modified with Polycaprolactone Film" Polymers 9, no. 10: 545. https://doi.org/10.3390/polym9100545





